Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography–Time-of-Flight Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Determination of Fatty Acid Composition by Gas Chromatography
2.3. Determination of Lipid Composition Using UPLC-TOF-MS/MS [16]
2.4. Analytical Conditions for UPLC–MS
2.5. Data Processing
3. Results and Discussion
3.1. Analysis of Fatty Acid Composition in Pitaya Seed Oil
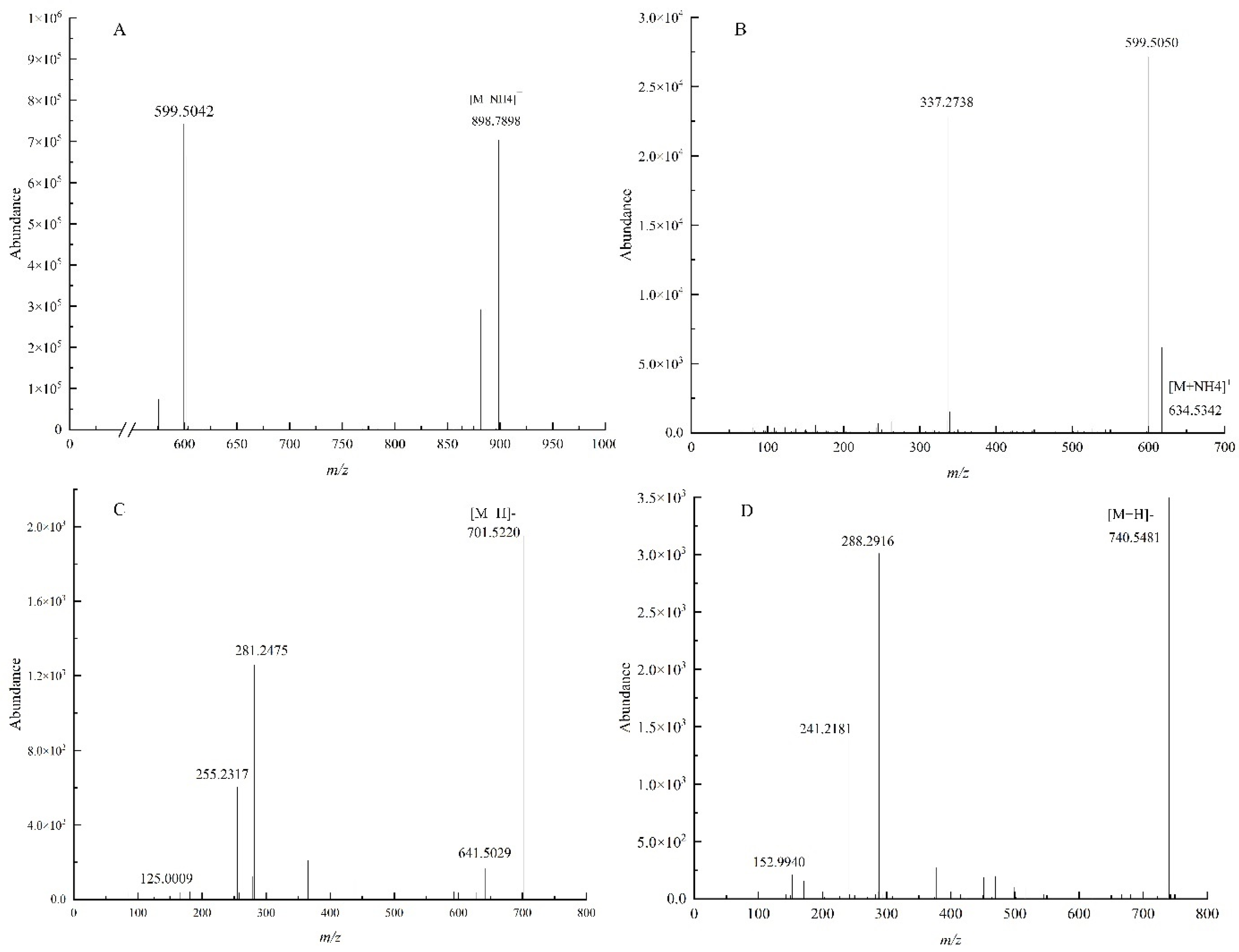
3.2. Identification of Lipids
3.3. Analysis of Lipid Composition in Pitaya Seed Oil
3.4. Analysis of the Lipid Content of Pitaya Seed Oil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, F.; Jiang, Z.B.; Xu, J.; Bai, X.P.; Liang, Q.Y.; Fu, Z.H. Optimized extraction of phenolic antioxidants from red pitaya (Hylocereus polyrhizus) seeds by subcritical water extraction using response surface methodology. J. Food Manag. Charact. 2020, 16, 2240–2258. [Google Scholar] [CrossRef]
- Hoa, T.T.; Clark, C.J.; Waddell, B.C.; Woolf, A.B. Postharvest quality of Dragon fruit (Hylocereus undatus) following disinfesting hot air treatments. Postharvest Biol. Technol. 2006, 41, 62–69. [Google Scholar] [CrossRef]
- Chen, G.L.; Deng, X.T.; Hu, K.; Gao, Y.Q. The nutritional value, biological activity and utilization of pitaya. Mod. Prev. Med. 2013, 40, 2030–2033. [Google Scholar]
- Wu, D.F.; Pang, D.X.; Lin, Q.S. On the extraction and antioxidation of total flavonoids and polysaccharides from pitaya peel (Hylocereus undatus ‘Foo-Lon’). J. South China Norm. Univ. (Nat. Sci. Ed.) 2021, 53, 68–75. [Google Scholar] [CrossRef]
- Zhao, N.J.; Wang, Y.L.; Zhu, Y.; Jin, X. Analysis of anthocyanin content and protective enzyme differences among different pitaya varieties. Anhui Agric. Sci. Bull. 2022, 28, 16–17. [Google Scholar] [CrossRef]
- Ariffin, A.A.; Bakar, J.; Tan, C.P.; Rahman, R.A.; Karim, R.; Loi, C.C. Essential fatty acids of pitaya (dragon fruit) seed oil. Food Chem. 2008, 114, 561–564. [Google Scholar] [CrossRef]
- Pan, Y.L.; Rui, H.M.; Lin, C.P. Analysis of the nutritive composition in the seed kernel of white pitaya. Acta Nutr. Sin. 2004, 06, 497–498. [Google Scholar] [CrossRef]
- Pan, Y.L.; Rui, H.M.; Lin, C.P. Analysis of fatty acid and amino acid in the seed kernel of white pitaya. Guangzhou Food Sci. Technol. 2004, 1, 83–84. [Google Scholar] [CrossRef]
- Pan, Y.L.; Rui, H.M.; Lin, C.P. Determination and evaluation of the nutritive composition in the seed kernel of white pitaya. J. South China Univ. Technol. (Nat. Sci. Ed.) 2004, 3, 41–43. [Google Scholar] [CrossRef]
- Jiang, X.; Cao, J.; Bai, X.P.; Li, C.; Cao, Z.Y.; Feng, Z. Nutritional composition analysis of red pitaya seeds. Nat. Prod. Res. Dev. 2018, 30, 232–238. [Google Scholar] [CrossRef]
- Li, S.F.; Chen, W.D.; Xiao, G.S.; Xu, Y.J. Study on the fatty acids composition of hylocereus seed oil. Fujian Fruits 2006, 2, 4–5. [Google Scholar] [CrossRef]
- Villalobos-Gutiérrez, M.G.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Chemical characterization of Central American pitaya (Hylocereus sp.) seeds and seed oil. CyTA J. Food 2012, 10, 78–83. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, C.P.; Karim, R.; Ariffin, A.A.; Bakar, J. Chemical composition and DSC thermal properties of two species of Hylocereus cacti seed oil: Hylocereus undatus and Hylocereus polyrhizus. Food Chem. 2010, 119, 1326–1331. [Google Scholar] [CrossRef]
- Lim, H.K.; Tan, C.P.; Bakar, J.; Ng, S.P. Effects of different wall materials on the physicochemical properties and oxidative stability of spray-dried microencapsulated red-fleshed pitaya (Hylocereus polyrhizus) seed oil. Food Bioprocess Technol. 2012, 5, 1220–1227. [Google Scholar] [CrossRef]
- Hao, M.Z.; Xi, J.R.; Zhao, Z.Y.; Che, H.L. Identification of allergens in white- and red-fleshed pitaya (Selenicereus undatus and Selenicereus costaricensis) seeds using bottom-up proteomics coupled with immunoinformatics. Nutrients 2022, 14, 1962. [Google Scholar] [CrossRef]
- Thilakarathna, R.C.N.; Siow, L.F.; Tang, T.K.; Lee, Y.Y. A review on application of ultrasound and ultrasound assisted technology for seed oil extraction. J. Food Sci. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- DiSarli, P.V.O.; Oliveira, S.L.; Naciuk, C.B.V.; Guedes, T.A. Baru (Dipteryx alata Vogel) Oil Extraction by Supercritical-CO2: Improved Composition by Using Water as Cosolvent. J. Oleo Sci. 2022, 71, 201–213. [Google Scholar] [CrossRef]
- Zeng, J.P.; Xiao, T.; Ni, X.G.; Wei, T.; Liu, X.R.; Deng, Z.Y.; Li, J. The comparative analysis of different oil extraction methods based on the quality of flaxseed oil. J. Food Compos. Anal. 2021, 107, 104373. [Google Scholar] [CrossRef]
- Liu, Y.J.; Gong, X.; Jing, W.; Lin, L.J.; Zhou, W.; He, J.N.; Li, J.H. Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds. Open Chem. 2021, 19, 367–376. [Google Scholar] [CrossRef]
- Wang, Q.L.; Mo, J.G.; Xie, Y.X. Optimization of supercritical C02 extraction of pitaya seed oil by response surface methodology. Food Sci. 2012, 33, 92–97. [Google Scholar]
- Wang, Q.L. The Research of Pitaya Seeds; Guangxi University: Nanning, China, 2012. [Google Scholar]
- Deng, C.J.; Liu, S.C.; Tao, L.Q.; Wu, X.P. Optimization of extraction process of pitaya seed oil by supercritical carbon dioxide based on artificial neural network. Food Res. Dev. 2014, 35, 58–63. [Google Scholar] [CrossRef]
- Wu, G.; Xu, T.T. Study on process of white pitaya seed kernel oil by microwave extraction. Food Sci. Technol. 2010, 35, 235–237. [Google Scholar] [CrossRef]
- Deng, C.J.; Liu, S.C.; Tao, L.Q.; Wu, X.P. Process optimization of dragon fruit seed oil extraction by solvent method and its physicochemical properties. Food Ind. Technol. 2013, 34, 184–189. [Google Scholar] [CrossRef]
- Xu, F.; Dong, D.D.; Zhou, Z.Q.; Wang, H.F.; Shao, X.F.; Li, H.S. The antiproliferative properties of Hylocereus undatus britt seed oil. J. Chin. Cereals Oils Assoc. 2015, 30, 84–87. [Google Scholar] [CrossRef]
- Huang, Y.F.; Ruan, H.J.; Li, S.H. Progress in nutritional characteristics of oil and fat and its influence on intestinal health. China Oils Fats 2022, 47, 97–102. [Google Scholar] [CrossRef]
- Tao, J.M.; Liu, L.J.; Ma, Q.; Ma, K.Y.; Chen, Z.Y.; Ye, F.Y.; Lei, L.; Zhao, G.H. Effect of γ-oryzanol on oxygen consumption and fatty acids changes of canola oil. LWT 2022, 160, 113275. [Google Scholar] [CrossRef]
- Liu, C.; Chen, F.S.; Xia, Y.M.; Liu, B. Physicochemical and rheological properties of peanut oil body following alkaline pH treatment. LWT 2022, 154, 112590. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liang, Y.; Liang, J.; Deng, D.; Li, J. Comparative study on the physicochemical characteristics and fatty acid composition of cashew nuts and other three tropical fruits. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052011. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Deng, D.; Liang, J.; Chen, W.; Chen, X.; Li, J. Study on extracting avocado oil from avocado pulp by aqueous extraction. IOP Conf. Ser. Earth Environ. Sci. 2019, 330, 042027. [Google Scholar] [CrossRef]
- Liu, Y.J.; Chen, M.; Li, Y.M.; Feng, X.Q.; Chen, Y.L.; Lin, L.J. Analysis of lipids in green coffee by ultra-performance liquid chromatography–time-of-flight tandem mass spectrometry. Molecules 2022, 27, 5271. [Google Scholar] [CrossRef]
- Madhu, S.V.; Bhardwaj, S.; Mishra, B.K.; Aslam, M. Total cholesterol and postprandial triglyceride levels as early markers of GDM in Asian Indian women. Int. J. Diabetes Dev. Ctries. 2022, 1–6. [Google Scholar] [CrossRef]
- Sushil, B.; Kumar, B.S.; Rupesh, K. Study of triglyceride glucose index and total cholesterol/HDLc for assessment of cardiovascular outcomes in patients with diabetes and hypertension. Am. Heart J. 2021, 242, 148. [Google Scholar] [CrossRef]
- Zhu, C.; Dane, A.; Spijksma, G.; Wang, M.; Greef, J.; Luo, G.; Hankemeier, T.; Vreeken, R.J. An efficient hydrophilic interaction liquid chromatography separation of 7 phospholipid classes based on a diol column. J. Chromatogr. A 2012, 1220, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, F.; Xu, S.L.; Wu, B.F.; Zheng, C.; Lv, X.; Wu, Z.Y.; Chen, H.; Huang, F.H. Profiling and quantification of lipids in cold-pressed rapeseed oils based on direct infusion electrospray ionization tandem mass spectrometry. Food Chem. 2019, 285, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.P.; Wei, F.; Huang, F.H.; Xie, Y.; Wu, B.F.; Lv, X.; Chen, H. Comprehensive and high-coverage lipidomic analysis of oilseeds based on ultrahigh-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2021, 69, 8964–8980. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Sun, W.C.; Luo, Y.H. Research progress in detection and function of sphingomyelin in food. Food Res. Dev. 2020, 41, 211–218. [Google Scholar] [CrossRef]
- Yuan, L.P.; Liu, B.; Xiong, B.; Peng, S.H.; Li, M.F. Progress in preparation, function, and application of soy lecithin. China Brew. 2013, 32, 13–15. [Google Scholar] [CrossRef]


| Fatty Acid | Red Pitaya Seed | White Pitaya Seed | ||
|---|---|---|---|---|
| Cold Pressing Method | Chloroform–Methanol Extraction [10] | Soxhlet Extraction [10] | Soxhlet Extraction [6] | |
| C12:0 | ND | ND | 0.13 ± 0.01 | ND |
| C13:0 | ND | ND | 0.04 ± 0.02 | ND |
| C14:0 | 0.12 ± 0.00 | 0.22 ± 0.01 | 0.39 ± 0.13 | 0.30 ± 0.01 |
| C16:0 | 16.66 ± 0.02 | 15.85 ± 0.12 | 16.64 ± 0.68 | 17.10 ± 0.78 |
| C16:1 | 0.66 ± 0.00 | 0.82 ± 0.04 | 1.01 ± 0.22 | 0.61 ± 0.01 |
| C17:0 | ND | 0.08 ± 0.00 | 0.10 ± 0.02 | ND |
| C18:0 | 4.78 ± 0.02 | 5.24 ± 0.36 | 6.05 ± 0.09 | 4.37 ± 0.24 |
| C18:1 | 27.29 ± 0.07 | 26.66 ± 0.27 | 26.63 ± 0.09 | 23.80 ± 0.14 |
| C18:2 n6t | 4.15 ± 0.05 | 3.91 ± 0.30 | 3.97 ± 0.12 | 2.81 ± 0.10 |
| C18:2 n6c | 42.78 ± 0.05 | 43.12 ± 1.16 | 39.76 ± 1.23 | 50.10 ± 0.35 |
| C18:3 n3 | 0.29 ± 0.00 | 0.43 ± 0.04 | 0.59 ± 0.19 | 0.98 ± 0.10 |
| C20:0 | 1.48 ± 0.01 | 1.39 ± 0.07 | 1.44 ± 0.10 | ND |
| C20:1 | 0.25 ± 0.09 | 0.29 ± 0.01 | 0.37 ± 0.08 | ND |
| C22:0 | 1.54 ± 0.03 | 1.25 ± 0.05 | 1.35 ± 0.09 | ND |
| C23:0 | ND | 0.48 ± 0.04 | 1.12 ± 0.15 | ND |
| C24:0 | ND | 0.26 ± 0.01 | 0.41 ± 0.02 | ND |
| Saturated fatty acids | 24.57 ± 0.08 | 24.77 ± 0.57 | 27.67 ± 0.75 | 21.70 ± 1.03 |
| Monounsaturated fatty acids | 32.36 ± 0.21 | 31.39 ± 0.57 | 31.61 ± 0.20 | 27.20 ± 0.25 |
| Polyunsaturated fatty acids | 43.07 ± 0.05 | 43.84 ± 1.12 | 40.72 ± 0.95 | 51.10 ± 0.45 |
| Total unsaturated fatty acids | 75.43 ± 0.36 | 75.23 ± 1.69 | 72.33 ± 1.15 | 78.30 ± 0.70 |
| Oil extraction rate (g/100 g seeds) | 19.48 ± 1.04 | 19.35 ± 1.36 | 31.79 ± 1.19 | 32.00 ± 1.40 |
| No | Lipid Species | Fatty Acid Composition (Carbon Number: Double Bond Number) | No | Lipid Species | Fatty Acid Composition (Carbon Number: Double Bond Number) |
|---|---|---|---|---|---|
| 1 | PE | PE 34:2|PE 17:1_17:1 | 25 | DG | DG 36:5|DG 18:2_18:3 |
| 2 | PE | PE 36:2|PE 18:0_18:2 | 26 | DG | DG 38:1|DG 20:0_18:1 |
| 3 | PE | PE 39:4|PE 18:1_21:3 | 27 | DG | DG 38:2|DG 20:0_18:2 |
| 4 | PE | PE 42:1; O|PE 16:1_26:0; O | 28 | DG | DG 38:3|DG 20:1_18:2 |
| 5 | PEtOH | PEtOH 34:1|PEtOH 16:0_18:1 | 29 | DG | DG 40:1|DG 22:0_18:1 |
| 6 | PEtOH | PEtOH 36:1|PEtOH 18:0_18:1 | 30 | DG | DG 40:2|DG 22:0_18:2 |
| 7 | PEtOH | PEtOH 36:2|PEtOH 18:1_18:1 | 31 | DG | DG 40:3|DG 22:1_18:2 |
| 8 | PEtOH | PEtOH 36:4|PEtOH 18:2_18:2 | 32 | EtherDG | DG O-34:2|DG O-17:0_17:2 |
| 9 | PG | PG 29:1|PG 10:0_19:1 | 33 | EtherDG | DG O-36:3|DG O-19:1_17:2 |
| 10 | PG | PG 33:1; O|PG 16:0_17:1; O | 34 | EtherDG | DG O-36:4|DG O-19:2_17:2 |
| 11 | PG | PG 34:2|PG 16:0_18:2 | 35 | TG_EST | TG 71:6; O2|TG 15:0_18:2 22:3; O (FA 16:0) |
| 12 | PG | PG 35:0|PG 16:0_19:0 | 36 | TG | TG 34:0|TG 8:0_10:0_16:0 |
| 13 | PG | PG 42:4|PG 21:2_21:2 | 37 | TG | TG 36:0|TG 10:0_12:0_14:0 |
| 14 | PMeOH | PMeOH 16:0|PMeOH 8:0_8:0 | 38 | TG | TG 36:1|TG 8:0_10:0_18:1 |
| 15 | PMeOH | PMeOH 32:0|PMeOH 16:0_16:0 | 39 | TG | TG 38:0|TG 8:0_14:0_16:0 |
| 16 | PMeOH | PMeOH 34:0|PMeOH 15:0_19:0 | 40 | TG | TG 38:1|TG 10:0_10:0_18:1 |
| 17 | DG | DG 32:0|DG 16:0_16:0 | 41 | TG | TG 40:0|TG 10:0_14:0_16:0 |
| 18 | DG | DG 34:0|DG 16:0_18:0 | 42 | TG | TG 40:1|TG 8:0_14:0_18:1 |
| 19 | DG | DG 34:1|DG 16:0_18:1 | 43 | TG | TG 42:0|TG 10:0_16:0_16:0 |
| 20 | DG | DG 34:3|DG 16:1_18:2 | 44 | TG | TG 42:1|TG 8:0_16:0_18:1 |
| 21 | DG | DG 36:1|DG 18:0_18:1 | 45 | TG | TG 42:2|TG 8:0_16:0_18:2 |
| 22 | DG | DG 36:2|DG 18:1_18:1 | 46 | TG | TG 44:0|TG 10:0_16:0_18:0 |
| 23 | DG | DG 36:3|DG 18:1_18:2 | 47 | TG | TG 44:1|TG 10:0_16:0_18:1 |
| 24 | DG | DG 36:4|DG 18:2_18:2 | 48 | TG | TG 44:2|TG 8:0_18:1_18:1 |
| 49 | TG | TG 46:0|TG 14:0_16:0_16:0 | 89 | TG | TG 56:6|TG 18:2_18:2_20:2 |
| 50 | TG | TG 46:1|TG 12:0_16:0_18:1/TG 14:0_16:0_16:1 | 90 | TG | TG 57:1|TG 16:0_23:0_18:1 |
| 51 | TG | TG 46:2|TG 10:0_18:0_18:2 | 91 | TG | TG 57:2|TG 16:0_23:0_18:2 |
| 52 | TG | TG 48:0|TG 16:0_16:0_16:0 | 92 | TG | TG 57:3|TG 21:0_18:1_18:2 |
| 53 | TG | TG 48:1|TG 14:0_16:0_18:1/TG 16:0_16:0_16:1 | 93 | TG | TG 58:1|TG 18:0_22:0_18:1/TG 16:0_24:0_18:1 |
| 54 | TG | TG 48:2|TG 14:0_16:0_18:2 | 94 | TG | TG 58:2|TG 22:0_18:1_18:1 |
| 55 | TG | TG 48:3|TG 14:0_16:1_18:2/TG 12:0_18:1_18:2/TG 16:1_16:1_16:1 | 95 | TG | TG 58:4|TG 22:0_18:2_18:2 |
| 56 | TG | TG 50:0|TG 16:0_16:0_18:0 | 96 | TG | TG 58:5|TG 22:1_18:2_18:2 |
| 57 | TG | TG 50:1|TG 16:0_16:0_18:1 | 97 | TG | TG 58:6|TG 22:1_18:2_18:3 |
| 58 | TG | TG 50:2|TG 16:0_16:0_18:2 | 98 | TG | TG 59:1|TG 16:0_25:0_18:1 |
| 59 | TG | TG 50:3|TG 16:0_16:1_18:2/TG 14:0_18:1_18:2 | 99 | TG | TG 59:2|TG 23:0_18:1_18:1/TG 16:0_25:0_18:2 |
| 60 | TG | TG 50:4|TG 14:0_18:2_18:2/TG 16:1_16:1_18:2 | 100 | TG | TG 59:3|TG 23:0_18:1_18:2 |
| 61 | TG | TG 51:1|TG 16:0_17:0_18:1 | 101 | TG | TG 59:4|TG 23:0_18:2_18:2 |
| 62 | TG | TG 51:2|TG 16:0_17:0_18:2 | 102 | TG | TG 60:1|TG 18:0_24:0_18:1/TG 16:0_26:0_18:1 |
| 63 | TG | TG 51:3|TG 16:0_17:1_18:2 | 103 | TG | TG 60:2|TG 24:0_18:1_18:1 |
| 64 | TG | TG 51:4|TG 16:0_17:2_18:2/TG 15:0_18:2_18:2 | 104 | TG | TG 60:3|TG 24:0_18:1_18:2 |
| 65 | TG | TG 52:0|TG 16:0_18:0_18:0/TG 16:0_16:0_20:0 | 105 | TG | TG 60:4|TG 24:0_18:2_18:2 |
| 66 | TG | TG 52:1|TG 16:0_18:0_18:1 | 106 | TG | TG 60:5|TG 24:1_18:2_18:2 |
| 67 | TG | TG 52:2|TG 16:0_18:1_18:1 | 107 | TG | TG 62:2|TG 26:0_18:1_18:1/TG 22:0_22:0_18:2 |
| 68 | TG | TG 52:3|TG 16:0_18:1_18:2 | 108 | TG | TG 62:3|TG 26:0_18:1_18:2 |
| 69 | TG | TG 52:4|TG 16:0_18:2_18:2 | 109 | TG | TG 62:4|TG 26:0_18:2_18:2 |
| 70 | TG | TG 52:5|TG 16:1_18:2_18:2 | 110 | TG | TG 62:5|TG 26:1_18:2_18:2 |
| 71 | TG | TG 52:6|TG 16:2_18:2_18:2 | 111 | OxTG | TG 43:2;2O|TG 16:0_15:2_12:0;2O |
| 72 | TG | TG 53:2|TG 17:0_18:1_18:1/TG 16:0_18:1_19:1 | 112 | OxTG | TG 45:2;2O|TG 18:0_15:2_12:0;2O |
| 73 | TG | TG 53:3|TG 17:0_18:1_18:2 | 113 | OxTG | TG 45:3;2O|TG 18:1_17:2_10:0;2O |
| 74 | TG | TG 53:4|TG 17:0_18:2_18:2 | 114 | OxTG | TG 52:2;2O|TG 16:0_18:1_18:1;2O |
| 75 | TG | TG 53:5|TG 17:1_18:2_18:2 | 115 | OxTG | TG 52:3;2O|TG 16:0_18:1_18:2;2O |
| 76 | TG | TG 54:0|TG 16:0_18:0_20:0/TG 16:0_16:0_22:0 | 116 | OxTG | TG 52:4;2O|TG 16:0_18:2_18:2;2O |
| 77 | TG | TG 54:1|TG 16:0_20:0_18:1 | 117 | OxTG | TG 54:2;2O|TG 16:0_19:2_19:0;2O |
| 78 | TG | TG 54:2|TG 18:0_18:1_18:1/TG 20:0_16:1_18:1 | 118 | OxTG | TG 54:3;2O|TG 18:1_18:1_18:1;2O |
| 79 | TG | TG 54:3|TG 18:1_18:1_18:1 | 119 | OxTG | TG 54:5;2O|TG 18:1_18:2_18:2;2O |
| 80 | TG | TG 54:4|TG 18:1_18:1_18:2 | 120 | OxTG | TG 54:6;2O|TG 18:2_18:2_18:2;2O |
| 121 | OxTG | TG 56:2;2O|TG 16:0_22:0_18:2;2O | |||
| 81 | TG | TG 54:5|TG 18:1_18:2_18:2 | 122 | OxTG | TG 56:4;2O|TG 21:0_16:3_19:1;2O |
| 82 | TG | TG 54:6|TG 18:2_18:2_18:2 | 123 | OxTG | TG 58:4;2O|TG 22:0_19:2_17:2;2O |
| 83 | TG | TG 54:7|TG 18:2_18:2_18:3 | 124 | OxTG | TG 52:3;3O|TG 16:0_18:2_18:1;3O |
| 84 | TG | TG 56:1|TG 16:0_22:0_18:1/TG 18:0_20:0_18:1 | 125 | OxTG | TG 54:4;3O|TG 18:1_18:2_18:1;3O |
| 85 | TG | TG 56:2|TG 16:0_22:0_18:2/TG 20:0_18:1_18:1 | 126 | OxTG | TG 54:5;3O|TG 18:2_18:2_18:1;3O |
| 86 | TG | TG 56:3|TG 20:0_18:1_18:2 | 127 | OxTG | TG 41:1;1O|TG 10:0_16:0_15:1;1O |
| 87 | TG | TG 56:4|TG 20:0_18:2_18:2 | 128 | OxTG | TG 43:1;1O|TG 10:0_18:0_15:1;1O |
| 88 | TG | TG 56:5|TG 20:1_18:2_18:2 | 129 | OxTG | TG 43:2;1O|TG 10:0_16:0_17:2;1O |
| 130 | OxTG | TG 43:3;1O|TG 10:0_18:2_15:1;1O | 142 | OxTG | TG 54:3;1O|TG 18:1_18:1_18:1;1O |
| 131 | OxTG | TG 45:2;1O|TG 10:0_18:1_17:1;1O | 143 | OxTG | TG 54:4;1O|TG 18:1_18:1_18:2;1O |
| 132 | OxTG | TG 45:3;1O|TG 10:0_18:1_17:2;1O | 144 | OxTG | TG 54:5;1O|TG 18:1_18:2_18:2;1O |
| 133 | OxTG | TG 45:4;1O|TG 10:0_18:1_17:3;1O | 145 | OxTG | TG 54:6;1O|TG 18:2_18:2_18:2;1O |
| 134 | OxTG | TG 45:5;1O|TG 10:0_18:2_17:3;1O | 146 | OxTG | TG 56:2;1O|TG 20:0_18:1_18:1;1O |
| 135 | OxTG | TG 50:1;1O|TG 16:0_16:0_18:1;1O | 147 | OxTG | TG 56:3;1O|TG 20:0_18:1_18:2;1O |
| 136 | OxTG | TG 50:2;1O|TG 16:0_16:0_18:2;1O | 148 | OxTG | TG 56:4;1O|TG 19:1_19:1_18:2;1O |
| 137 | OxTG | TG 50:3;1O|TG 16:0_18:2_16:1;1O | 149 | OxTG | TG 58:2;1O|TG 22:0_19:1_17:1;1O |
| 138 | OxTG | TG 52:2;1O|TG 16:0_18:1_18:1;1O | 150 | OxTG | TG 58:4;1O|TG 22:0_18:2_18:2;1O |
| 139 | OxTG | TG 52:3;1O|TG 16:0_18:1_18:2;1O | 151 | EtherTG | TG O-55:5|TG O-19:1_18:2_18:2/TG O-19:2_18:1_18:2 |
| 140 | OxTG | TG 52:4;1O|TG 16:0_18:2_18:2;1O | 152 | EtherTG | TG O-59:3|TG O-19:1_18:2_22:0/TG O-19:2_18:1_22:0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tu, X.; Lin, L.; Du, L.; Feng, X. Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography–Time-of-Flight Tandem Mass Spectrometry. Foods 2022, 11, 2988. https://doi.org/10.3390/foods11192988
Liu Y, Tu X, Lin L, Du L, Feng X. Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography–Time-of-Flight Tandem Mass Spectrometry. Foods. 2022; 11(19):2988. https://doi.org/10.3390/foods11192988
Chicago/Turabian StyleLiu, Yijun, Xinghao Tu, Lijing Lin, Liqing Du, and Xingqin Feng. 2022. "Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography–Time-of-Flight Tandem Mass Spectrometry" Foods 11, no. 19: 2988. https://doi.org/10.3390/foods11192988
APA StyleLiu, Y., Tu, X., Lin, L., Du, L., & Feng, X. (2022). Analysis of Lipids in Pitaya Seed Oil by Ultra-Performance Liquid Chromatography–Time-of-Flight Tandem Mass Spectrometry. Foods, 11(19), 2988. https://doi.org/10.3390/foods11192988







