Abstract
This study aimed to investigate whether bacterial lysates (BLs) extracted from Pediococcus acidilactici reduce Listeria monocytogenes biofilm formation, as well as adhesion to and invasion of human intestinal epithelial cells. Pretreatment with P. acidilactici BLs (20, 40, and 80 μg/mL) significantly inhibited L. monocytogenes biofilm formation on the surface of polystyrene (p < 0.05). Fluorescence and scanning-electron-microscopic analyses indicated that L. monocytogenes biofilm comprised a much less dense layer of more-dispersed cells in the presence of P. acidilactici BLs. Moreover, biofilm-associated genes, such as flaA, fliG, flgE, motB, degU, agrA, and prfA, were significantly downregulated in the presence of P. acidilactici BLs (p < 0.05), suggesting that P. acidilactici BLs prevent L. monocytogenes biofilm development by suppressing biofilm-associated genes. Although P. acidilactici BLs did not dose-dependently inhibit L. monocytogenes adhesion to and invasion of intestinal epithelial cells, the BLs effectively inhibited adhesion and invasion at 40 and 80 μg/mL (p < 0.05). Supporting these findings, P. acidilactici BLs significantly downregulated L. monocytogenes transcription of genes related to adhesion and invasion, specifically fbpA, ctaP, actA, lapB, ami, and inlA. Collectively, these results suggest that P. acidilactici BLs have the potential to reduce health risks from L. monocytogenes.
1. Introduction
Listeria monocytogenes is frequently found in soil, vegetation, and animals and causes serious diseases, including listeriosis [1]. Although listeriosis is less common than other foodborne diseases, more than 30% of mortality has been reported in vulnerable groups, such as pregnant women, newborns, and the elderly [2]. L. monocytogenes can survive in acidic or salty conditions and multiply slowly at the low temperatures typical of food-processing environments. Consequently, listeriosis is often caused by ingestion of contaminated food sources, such as dairy products, vegetables, meat products, and ready-to-eat foods [3].
Many foodborne pathogenic bacteria, including L. monocytogenes, are known to form biofilms on food-contact surfaces such as polystyrene, glass, and stainless steel, as well as on biotic surfaces [4]. Biofilms consist of a structured community of bacterial cells embedded in the matrix of extracellular polymeric substances produced by bacteria [5]. The molecular mechanisms underlying the regulation of biofilm formation of L. monocytogenes have been widely studied. For instance, the ability of L. monocytogenes to adhere to surfaces in the early period of biofilm formation is facilitated by flagellin [6]. Genes involved in flagellar synthesis and motility that enable the adhesion of L. monocytogenes to surfaces include flaA, fliG, flgE, motB, and degU [7,8]. In biofilm formation of L. monocytogenes, genes related to quorum sensing and virulence, such as agrA and prfA, are also involved [9,10].
Compared to their planktonic counterparts, bacterial cells in a biofilm are highly resistant to chemical and physical stresses, such as heat, desiccation, acidic conditions, and antibiotics [11]. Although biofilm formation of L. monocytogenes on food-contact surfaces is considered a critical pathway for pathogenic resistance, inhibiting or eradicating L. monocytogenes biofilm is difficult during food processing. For instance, L. monocytogenes biofilm that was repeatedly exposed to sanitizers developed resistance to those sanitizing agent [12].
Once ingested, L. monocytogenes binds to receptors on the surface of intestinal epithelial cells and disseminates to various tissues via the bloodstream [13]. Adhesion of L. monocytogenes to intestinal epithelial cells is required for initiating infection and promoting bacterial dissemination to extraintestinal sites [14]. Bacterial adhesion is also a prerequisite for biofilm development [15]. It has been known that several factors of L. monocytogenes, including fibronectin binding protein, are associated with the bacterial adhesion to intestinal epithelial cells. In addition, other proteins, including CtaP, ActA, LapB, and Ami, are also attributed to the adhesion of L. monocytogenes to the intestinal epithelial cells [16].
Numerous efforts have been carried out to find natural antimicrobials that can act as alternatives to chemical additives in preserving food and preventing infection. Among the natural antimicrobials, probiotic lactic acid bacteria have attracted strong interest for their ability to inhibit foodborne pathogenic bacteria. Since lactic acid bacteria are considered “generally recognized as safe” and produce various antimicrobial substances, their antimicrobial activity has been widely examined [17]. However, live probiotic microorganisms have certain limitations, such as antibiotic resistance and the potential for infection in immunocompromised individuals [18]. Currently, postbiotics, such as metabolites from cell-free supernatants, exopolysaccharides, cell-wall fragments, and bacterial lysates (BLs), have emerged as a promising alternative to probiotics. Pediococcus acidilactici is recognized as a potential probiotic bacterium with antimicrobial activities [19]. Many probiotic lactobacilli and their postbiotic compounds have shown antagonistic activities against the growth and biofilm formation of L. monocytogenes [20,21,22]. In addition, it has been widely documented that bacteriocins produced by P. acidilactici suppress the growth of L. monocytogenes [23,24,25]. However, the antibiofilm, antiadhesion, and anti-invasion activities of P. acidilactici BLs against L. monocytogenes have not been elucidated. Therefore, in this study, we investigated the inhibitory effects of BLs extracted from two P. acidilactici strains, K10 and HW01, on L. monocytogenes mediated biofilm formation on an abiotic surface, as well as L. monocytogenes adhesion to and invasion of human intestinal epithelial cells.
2. Materials and Methods
2.1. Bacterial Culture and BL Preparation
Two strains of P. acidilactici (K10 and HW01) were cultured in Man−Rogosa−Sharpe broth (Neogen, Lansing, MI, USA) at 37 °C. To prepare P. acidilactici BLs, bacterial pellets were obtained from overnight cultures of P. acidilactici K10 and P. acidilactici HW01 that were centrifugated at 8000 × g for 10 min and vigorously washed with phosphate-buffered saline (PBS). The bacterial pellets were then resuspended in an extraction buffer (50 mM Trizma base, 0.1 mM EDTA, and 1 mM 2-mercaptoethanol, pH 7.5) and homogenized in a tube containing zirconium beads (Benchmark Scientific, Sayreville, NJ, USA) at 4 °C for 100 s using a benchtop homogenizer (BeadBugTM 3, Benchmark Scientific, Sayreville, NJ, USA). After centrifugation at 20,000 × g for 30 min, P. acidilactici BLs were obtained and their concentration was measured using a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL, USA). L. monocytogenes KCTC 3569 was purchased from the Korean Collection for Type Cultures (Jeongeup, Korea) and cultured in Brain Heart Infusion (BHI) broth (BD Biosciences, Franklin Lakes, NJ, USA) at 37 °C. For subsequent experiments, L. monocytogenes cultures were diluted to 1 × 107 colony-forming units (CFU) per mL, corresponding to 0.1 at 600 nm of optical density (OD) in BHI broth.
2.2. Inhibition of L. monocytogenes Biofilm Formation
BLs extracted from P. acidilactici K10 (K10 BL) or P. acidilactici HW01 (HW01 BL) (20, 40, or 80 μg/mL) were added to wells of a 96-well culture plate and pre-incubated for 3 h at 37 °C. L. monocytogenes (1 × 107 CFU/mL) was then added to wells of the culture plate and incubated for an additional 24 h. After incubation, planktonic L. monocytogenes was removed, and L. monocytogenes biofilm was gently washed with PBS. The biofilm was stained with 0.1% crystal violet for 30 min at room temperature and washed with PBS twice to remove excess stain. The adherent stain was dissolved with 0.1% acetic acid and 95% ethanol, after which the biofilm was quantified by measuring the OD at 595 nm using a microplate reader (AMR-100, Allsheng, Hangzhou, China).
2.3. Microscopic Analysis
For confocal laser scanning microscopy (CLSM), K10 BL or HW01 BL (20, 40, or 80 μg/mL) was added to cover glass-bottom dishes (SPL Life Science, Pocheon, Korea) and preincubated at 37 °C for 3 h. L. monocytogenes (1 × 107 CFU/mL) was then added and incubated at 37 °C for an additional 24 h. Planktonic L. monocytogenes was removed by washing with PBS, and L. monocytogenes biofilm was stained using a LIVE/DEAD BacLight bacterial viability kit (Molecular Probes, Eugene, OR, USA) according to the manufacturer’s instructions. The biofilm was visualized with a confocal laser scanning microscope (Eclipse Ti-E, Nikon, Tokyo, Japan). To analyze the L. monocytogenes biofilm with a scanning electron microscope (SEM), K10 BL or HW01 BL (20, 40, or 80 μg/mL) was added to a 12 mm coverslip (SPL Life Science) in a 24-well culture plate and preincubated at 37 °C for 3 h, followed by incubation with L. monocytogenes (1 × 107 CFU/mL) at 37 °C for an additional 24 h. After incubation, the L. monocytogenes biofilm was gently washed with PBS and fixed with 2.5% glutaraldehyde and 2% paraformaldehyde in PBS at 4 °C overnight. The biofilm was then rinsed with PBS and dehydrated by washing with different concentrations of ethanol (70, 80, 90, 95, and 99%, each for 15 min). After drying with hexamethyldisilazane for 30 min, the coverslip was coated with ion sputter. The L. monocytogenes biofilm was examined using a scanning electron microscope (Carl Zeiss, Oberkochen, Germany).
2.4. Viability of Planktonic L. monocytogenes
To measure the viability of planktonic L. monocytogenes, L. monocytogenes was cultured in the presence or absence of K10 BL or HW01 BL (20, 40, or 80 μg/mL) in a tube at 37 °C for 24 h. After the serial dilution, the viability of planktonic L. monocytogenes was determined by plating samples on BHI agar.
2.5. Adhesion and Invasion Assay
To investigate the inhibitory effects of P. acidilactici BLs on the adhesion and invasion of L. monocytogenes to HT-29 cells, a human-intestinal-epithelial cell line, HT-29 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Welgene, Gyeongsan, Korea) supplemented with 10% fetal-bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (HyClone, Logan, UT, USA) at 37 °C in a 5% CO2 humidified incubator. To conduct the adhesion assay, HT-29 cells (5 × 105 cells/mL) were plated in a 12-well culture plate and incubated until the cells were fully confluent. After washing the cell monolayers with PBS, the cells were pretreated with K10 BL or HW01 BL (20, 40, or 80 μg/mL) at 37 °C for 3 h and incubated with L. monocytogenes (1 × 107 CFU/mL) for 1 h in antibiotic-free DMEM. The control cells were incubated with L. monocytogenes without the pretreatment with K10 BL or HW01 BL. HT-29 cells were gently washed with PBS to remove nonadherent L. monocytogenes and lysed with 0.2% Triton X-100 at 4 °C for 20 min. The lysed HT-29 cells were serially diluted, and the adherent L. monocytogenes was enumerated by plating samples on BHI agar. For the invasion assay, HT-29 cells were cultured in DMEM supplemented with 10% fetal-bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (HyClone), as described above. The cell monolayers were gently washed with PBS. The cells were pretreated with K10 BL or HW01 BL (20, 40, or 80 μg/mL) at 37 °C for 3 h and incubated with L. monocytogenes (1 × 107 CFU/mL) for 1 h in antibiotic-free DMEM. After incubation with L. monocytogenes, HT-29 cells were gently washed with PBS and incubated in DMEM supplemented with gentamicin (100 μg/mL) at 37 °C for 1 h to kill the remaining extracellular L. monocytogenes. Then, HT-29 cells were lysed with 0.2% Triton X-100, and the extent of the L. monocytogenes invasion was determined by plating samples on BHI agar.
2.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
To examine the inhibitory effect of P. acidilactici BLs on L. monocytogenes biofilm, the expression of genes responsible for biofilm formation was determined by qRT-PCR. After K10 BL or HW01 BL (80 μg/mL) was incubated in a 6-well culture plate for 3 h, L. monocytogenes (1 × 107 CFU/mL) was added to each well and incubated at 37 ℃ for an additional 24 h to allow for biofilm formation. The L. monocytogenes biofilm was washed with PBS and incubated with 1.5 mg/mL lysozyme (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 30 min. Total RNA was extracted from L. monocytogenes biofilm cells using TRIzol® Max™ Bacterial RNA Isolation Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Then, the total RNA was reverse-transcribed to complementary DNA (cDNA) using random hexamers and reverse transcriptase (Promega, Madison, WI, USA). The cDNA was amplified by qRT-PCR with SYBR Green Real-Time PCR master mix (Toyobo, Osaka, Japan) using the StepOnePlusTM real-time PCR system (Applied Biosystems, Foster City, CA, USA). The cycling conditions of qRT-PCR consisted of an initial denaturation step at 95 °C for 10 s, followed by amplification for 40 cycles at 95 °C for 5 s and at 60 °C for 31 s. The primer sequences used for genes responsible for biofilm formation are as follows: flaA, forward, 5′-CTGGTATGAGTCGCCTTAG-3′ and reverse, 5′-CATTTGCGGTGTTTGGTTTG-3′; fliG, forward, 5′-CCGCCCTTATTATTTGGAGC-3′ and reverse, 5′-CGAGTTTAGCAATTCCTCCTG-3′; flgE, forward, 5′-AATGCCAACACGACAGGATA-3′ and reverse, 5′-TTTGTTCCAGCGTAAAGTCC-3′; motB, forward, 5′-TGCAAAAAAATTCGAACAAATGG-3′ and reverse, 5′-CTGCCGCGCCTTCCT-3′; degU, forward, 5′-ACGCATAGAGAGTGCGA GGTATT-3′ and reverse, 5′-CCCAATTCCGCGGTTACTT-3′; agrA, forward, 5′-ATGAAGCAAGCGGAAGAAC-3′ and reverse, 5′-TACGACCTGTGACAACGATAAA-3′; and prfA, forward, 5′-ATGAACGCTCAAGCAGAAGA-3′ and reverse, 5′-CGAAAGCACCTTTGTAGTATTG-3′. In a separate experiment, genes related to adhesion and invasion in L. monocytogenes, specifically fbpA, ctaP, actA, lapB, ami, and inlA, were amplified as described above. The primer sequences used are as follows: fbpA, forward, 5′- AAGCGACTTTACCTGCTCCA-3′ and reverse, 5′- AACCAGGCAAATCTTTCACG-3′; ctaP, forward, 5′- GCAGACTACTCTATCGCACTAAATGG-3′ and reverse, 5′- GATTTCTTGACGTTCTTTGTCGTCAGC-3′; actA, forward, 5′- CGACCGACCARCTMTRCAAGTG-3′ and reverse, 5′- TCCGCKGCGCTATCC-3′; lapB, forward, 5′- TGGAGTGGGCACGTGTTGT-3′ and reverse, 5′- TTGTCAGCTGCATATTGTGAATTG-3′; ami, forward, 5′- TGGGGAGCAGGACAATATGC-3′ and reverse, 5′- CAGTATGGGTTGTTCCGCCT-3′; and inlA, forward, 5′-ACTTGGCAGTGGAGTATGGA-3′ and reverse, 5′-CTGAAGCGTCGTAACTTGGTC-3′. An internal standard for all qRT-PCR, 16s rRNA, was amplified using the following primer sequence: forward, 5′-ACCGTCAAGGGACAAGCA-3′ and reverse, 5′-GGGAGGCAGCAGTAGGGA-3′. The 2−ΔΔCt method was used to normalize relative gene expression to 16 s rRNA.
2.7. Statistical Analysis
All data presented in this study were obtained from three independent experiments. Statistical significance was determined in comparison to the appropriate control by performing a one-way analysis of variance (ANOVA) using IBM SPSS Statistics 25 software (IBM, Armonk, NY, USA) or by performing an unpaired two-tailed t-test using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) when p < 0.05.
3. Results
3.1. P. acidilactici BLs Inhibit L. monocytogenes Biofilm Formation
As shown in Figure 1, pretreatment with either K10 BL or HW01 BL inhibited L. monocytogenes biofilm formation. K10 BL significantly inhibited L. monocytogenes biofilm formation compared to the untreated control (p < 0.05). A reduction of 53, 78, and 82% relative to the untreated control was determined at 20, 40, and 80 μg/mL of K10 BL, respectively (Figure 1A). Similarly, HW01 BL significantly reduced L. monocytogenes biofilm development (p < 0.05). HW01 BL at a concentration of 20 μg/mL was able to reduce L. monocytogenes biofilm formation to 42% of untreated L. monocytogenes biofilm formation. Furthermore, 40 and 80 μg/mL of HW01 BL markedly inhibited L. monocytogenes biofilm formation by 66 and 76%, respectively (Figure 1B). To further characterize the effect of P. acidilactici BLs on L. monocytogenes biofilm formation, glass coverslips were pretreated with 20, 40, or 80 μg/mL of K10 BL or HW01 BL, and L. monocytogenes was allowed to form a biofilm on the surface of glass coverslips. CLSM analysis revealed a dense, thick, and fully established biofilm in untreated L. monocytogenes samples, while L. monocytogenes biofilm formed in the presence of K10 BL was much less dense. At the highest K10 BL concentration (80 μg/mL), negligible biofilm mass was detected (Figure 2A). Similar to the K10 BL pretreatment, the HW01 BL pretreatment caused L. monocytogenes biofilm to become thinner and more dispersed (Figure 2B). Further, SEM images showed that K10 BL and HW01 BL inhibited L. monocytogenes biofilm formation at concentrations of 20, 40, and 80 μg/mL (Figure 2C and Figure 2D, respectively). Upon pretreatment with K10 BL or HW01 BL, L. monocytogenes biofilm appeared as a monolayer of dispersed cells scattered on the surface. These results demonstrate that P. acidilactici BLs have a remarkable effect on the inhibition of L. monocytogenes biofilm formation.
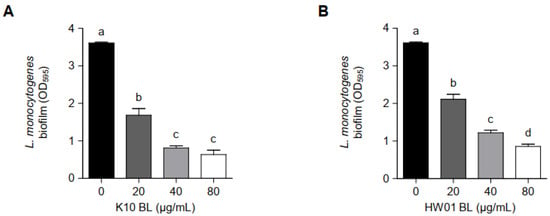
Figure 1.
Inhibitory effect of P. acidilactici BLs on L. monocytogenes biofilm formation. K10 BL (A) or HW01 BL (B) (0, 20, 40, and 80 μg/mL) was preincubated at 37 °C for 3 h in a 96-well culture plate, after which L. monocytogenes (1 × 107 CFU/mL) was added and incubated at 37 °C for an additional 24 h. L. monocytogenes biofilm formation was assessed by crystal violet staining. The results are represented as the means ± standard deviations from three independent experiments (p < 0.05). Significant differences between treatment with K10 BL or HW01 BL are expressed with different letters (a–d).
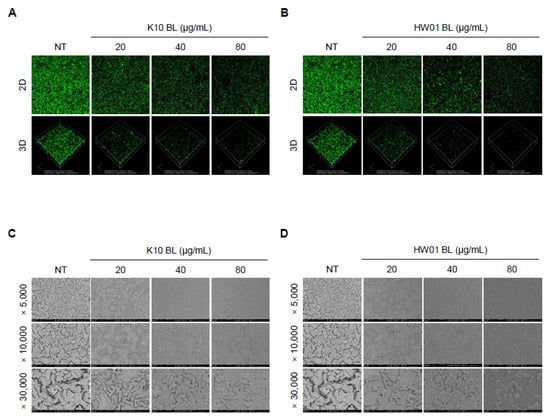
Figure 2.
Disruption of L. monocytogenes biofilm formation by P. acidilactici BLs. K10 BL or HW01 BL (0, 20, 40, and 80 μg/mL) was preincubated at 37 °C for 3 h on cover glasses, after which L. monocytogenes (1 × 107 CFU/mL) was added and incubated at 37 °C for an additional 24 h. Biofilm formation was then analyzed using confocal laser scanning microscopy (A,B). For scanning-electron-microscopic analysis, K10 BL or HW01 BL (0, 20, 40, and 80 μg/mL) was preincubated at 37 °C for 3 h on coverslips, and L. monocytogenes (1 × 107 CFU/mL) was then added and incubated at 37 °C for an additional 24 h. Samples were then analyzed using scanning electron microscopy at 5000, 10,000, and 30,000× magnifications (C,D). All images taken from one of three similar results are shown.
3.2. Antibiofilm Activity of P. acidilactici BL Is Not Affected by the Viability of L. monocytogenes
In order to examine whether P. acidilactici BLs inhibit L. monocytogenes biofilm formation by decreasing bacterial viability at planktonic states, planktonic L. monocytogenes cultured with different concentrations of P. acidilactici BLs were enumerated. As shown in Figure 3A, the viability rates of planktonic L. monocytogenes cultured with 20 and 40 μg/mL of K10 BL showed no significant difference compared to the control without K10 BL. However, a higher concentration of K10 BL (80 μg/mL) decreased the viability of planktonic L. monocytogenes by 83%. HW01 BL gradually decreased the viability of planktonic L. monocytogenes; however, the viability rates, which were 93, 82, and 73% at the concentrations of 20, 40, and 80 μg/mL of HW01 BL, respectively, did not decrease dose-dependently (Figure 3B). These results suggest that P. acidilactici BLs did not inhibit L. monocytogenes biofilm formation by killing bacteria in the planktonic state.
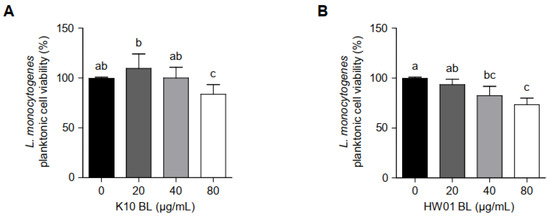
Figure 3.
The viability of planktonic L. monocytogenes in the presence or absence of P. acidilactici BLs. Planktonic L. monocytogenes was incubated with K10 BL (A) or HW01 BL (B) (0, 20, 40, and 80 μg/mL) at 37 °C for 24 h. After incubation, L. monocytogenes was enumerated by plating samples on BHI agar. The results are represented as the means ± standard deviations from three independent experiments (p < 0.05). Significant differences between treatment with K10 BL or HW01 BL are expressed with different letters (a–d).
3.3. P. acidilactici BLs Downregulate the Expression of Genes Critical for L. monocytogenes Biofilm Formation
Since several genes are related to biofilm formation and virulence of L. monocytogenes, mRNA expression in response to treatment with BLs was determined. As compared to the untreated control, the expressions of all genes considered in the current study were significantly downregulated in the presence of K10 BL and HW01 BL (80 μg/mL) (Figure 4A and Figure 4B, respectively). Genes involved in flagellar synthesis and motility that enable L. monocytogenes attachment, such as flaA, fliG, flgE, motB, and degU, were significantly downregulated by K10 BL and HW01 BL (p < 0.05). Particularly, expression of flaA decreased by more than 50% in the presence of both P. acidilactici BLs. Thus, it is likely that P. acidilactici BLs inhibited L. monocytogenes biofilm formation by interfering with bacterial attachment to the surface. The expression of agrA is associated with the L. monocytogenes quorum-sensing system, which plays a critical role in biofilm formation [26]. As shown in Figure 4A,B, both K10 BL and HW01 BL also significantly downregulated the expression of agrA (p < 0.05). The reduction of agrA transcription by K10 BL exceeded 60%, whereas HW01 BL inhibited agrA gene expression by 40%. Both K10 BL and HW01 BL significantly reduced expression of prfA, which is closely associated with L. monocytogenes biofilm formation, compared to the untreated control (p < 0.05). These results suggest that P. acidilactici BLs regulate the expression of genes that play a critical role in biofilm initiation and development in L. monocytogenes.
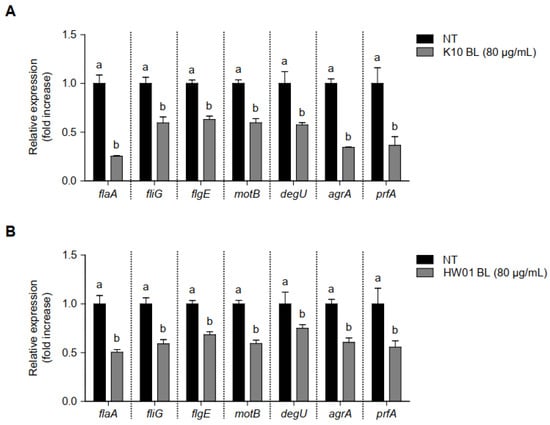
Figure 4.
Regulation of gene transcription associated with L. monocytogenes biofilm formation in the presence or absence of P. acidilactici BLs. K10 BL (A) or HW01 BL (B) (80 μg/mL) was preincubated for 3 h in a culture plate, after which L. monocytogenes was added and cultured at 37 °C for an additional 24 h. The expression levels of flaA, fliG, flgE, motB, degU, agrA, and prfA were measured using qRT-PCR. The results are represented as the means ± standard deviations from three independent experiments (p < 0.05). Significant differences between treatment with K10 BL or HW01 BL are expressed with different letters (a and b).
3.4. P. acidilactici BLs Reduce L. monocytogenes Adhesion to and Invasion of HT-29 Cells
L. monocytogenes adhesion to and invasion of intestinal epithelial cells are responsible for listeriosis. Thus, we examined whether P. acidilactici BLs interfere L. monocytogenes adhesion to and invasion of HT-29 cells. As shown in Figure 5A, adhesion of L. monocytogenes to HT-29 cells was not significantly reduced by K10 BL at 20 μg/mL (p > 0.05), although the same concentration of HW01 BL (20 μg/mL) significantly inhibited the adhesion of L. monocytogenes to HT-29 cells (p < 0.05) (Figure 5B). Further, treatment with higher concentrations (40 μg/mL) of K10 BL and HW01 BL significantly reduced L. monocytogenes adhesion by 19%. L. monocytogenes adhesion was further inhibited by 29% and 25% when the cells were treated with 80 μg/mL of K10 BL and HW01 BL, respectively (Figure 5A,B). Figure 5C displays that the invasion rates of L. monocytogenes were reduced to 8, 14, and 16% of those of the control, at 20, 40, and 80 μg/mL of K10 BL, respectively. Similarly, HW01 pretreatment at 20, 40, and 80 μg/mL reduced the invasion of L. monocytogenes at rates of 19, 20, and 25% of those of the control, respectively (Figure 5D).
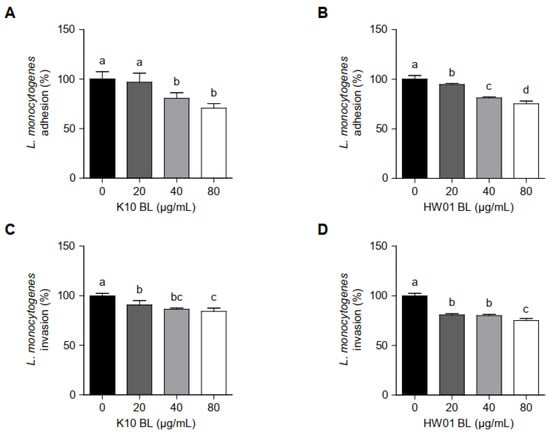
Figure 5.
L. monocytogenes adhesion to and invasion of HT-29 cells in the presence or absence of P. acidilactici BLs. HT-29 cells were pretreated with K10 BL (A,C) or HW01 BL (B,D) (0, 20, 40, and 80 μg/mL) for 3 h, after which L. monocytogenes (1 × 107 CFU/mL) was added and incubated for 1 h. Adhesive and invasive abilities of L. monocytogenes were determined by plating samples on BHI agar. The results are represented as the means ± standard deviations from three independent experiments (p < 0.05). Significant differences between treatment with K10 BL or HW01 BL are expressed with different letters (a–d).
3.5. P. acidilactici BLs Downregulate Genes Related to the Adhesion and Invasion in L. monocytogenes
To examine gene regulation responsible for adhesion and invasion in L. monocytogenes in the presence or absence of P. acidilactici BLs, the transcriptional profiles of fbpA, ctaP, actA, lapB, ami, and inlA, which are associated with adhesion and invasion of L. monocytogenes, were investigated. L. monocytogenes treated with K10 BL (80 μg/mL) significantly decreased the expression all of genes tested in this study (p < 0.05), compared to the untreated control (Figure 6A). Similar results were observed when L. monocytogenes was treated with HW01 BL (80 μg/mL); in this case, fbpA, ctaP, actA, lapB, ami, and inlA were significantly downregulated (p < 0.05). These results suggest that P. acidilactici BLs regulate the expression of genes responsible for adhesion and invasion and thus may inhibit L. monocytogenes adhesion to and invasion of HT-29 cells.
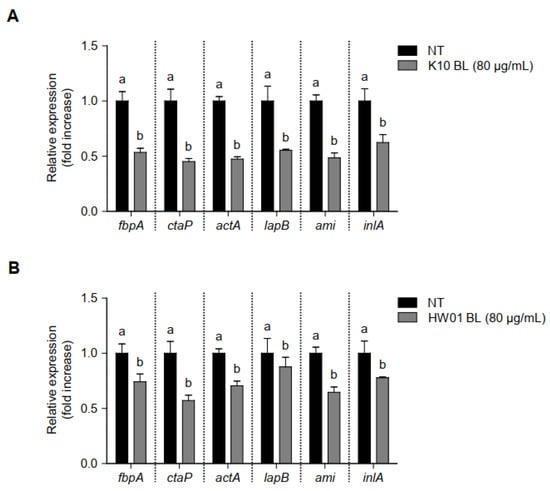
Figure 6.
Regulation of gene transcription associated with L. monocytogenes adhesion and invasion in the presence or absence of P. acidilactici BLs. K10 BL (A) or HW01 BL (B) (80 μg/mL) was preincubated for 3 h in a culture plate, after which L. monocytogenes was added and cultured at 37 °C for an additional 24 h. The expression levels of fbpA, ctaP, actA, lapB, ami, and inlA were measured using qRT-PCR. The results are represented as the means ± standard deviations from three independent experiments (p < 0.05). Significant differences between treatment with K10 BL or HW01 BL are expressed with different letters (a and b).
4. Discussion
L. monocytogenes is a well-known invasive bacterium that causes life-threatening foodborne diseases in humans [27]. Bacterial adhesion is a crucial step in the process of biofilm formation and invasion of host cells, facilitating infection [28]. Many lactic acid bacteria, particularly Lactobacillus spp., have shown an ability to control L. monocytogenes by inhibiting its growth and biofilm formation [20,29,30]. Moreover, it has been also reported that bacteriocin-producing P. acidilactici inhibited the growth of L. monocytogenes [25,31]. However, the role of P. acidilactici BLs in controlling biofilm formation, adhesion, and invasion of L. monocytogenes has not been elucidated. In the present study, P. acidilactici BLs markedly inhibited L. monocytogenes biofilm formation as well as its adhesion to and invasion of intestinal epithelial cells. These results suggest that P. acidilactici BLs have the potential to act as biocontrol agents against L. monocytogenes.
The ability of probiotics and their metabolites to reduce L. monocytogenes biofilm formation has been widely studied. For example, Lactobacillus sakei CRL1862 reduced the biofilm formation of L. monocytogenes by forming its own biofilm on abiotic surfaces [20]. Some Lactobacillus spp. have been reported to reduce L. monocytogenes biofilm development on the surfaces of lettuce and stainless steel in co-incubations of Lactobacillus spp. and L. monocytogenes [21]. In addition, bacteriocins derived from Latilactobacillus curvatus inhibited biofilm formation and motility of L. monocytogenes by regulating genes responsible for biofilm formation [22]. Moreover, the inhibitory effect of P. acidilactici on the growth of L. monocytogenes has been documented. Bacteriocins and cell-free culture supernatants of P. acidilactici suppressed the growth of L. monocytogenes [32,33,34]. However, the effect of P. acidilactici itself or its intracellular derivatives on the inhibition of biofilm development, adhesion, and invasion of L. monocytogenes has not been elucidated. Postbiotics have been recognized as favorable and promising alternatives to probiotics [18]. Postbiotics containing biological active factors frequently show health benefits for the host [35]. For instance, postbiotics derived from lactic acid bacteria exhibited the antagonistic activity against L. monocytogenes biofilm [36]. A similar report has also shown the antilisterial activity of cell-free supernatants from Lactobacillus spp. [37]. Although postbiotics derived from lactobacilli exhibited the inhibitory activity against L. monocytogenes, the antagonistic effect of postbiotic compounds, such as BLs, derived from pediococci has not been well documented. Here, our study demonstrated that P. acidilactici BLs noticeably reduced biofilm formation in L. monocytogenes and also inhibited its ability to adhere to and invade intestinal epithelial cells.
Although the antibiofilm mechanism of BLs has not been established, some have speculated that probiotics’ production of substances with antagonistic properties, which could be present in the lysates of probiotics, could modify quorum sensing [38]. Moreover, during biofilm formation, several genes are involved in flagellar motility, such as flaA, fliG, and flgE [8]. Paenibacterin produced by Paenibacillus thiaminolyticus significantly reduced these biofilm-associated genes, consequently leading to the inhibition of L. monocytogenes biofilm formation [39]. In line with this possibility, we found that the expression of agrA, which is associated with virulence regulation via activation of the quorum-sensing mechanism [40], was downregulated by P. acidilactici BLs. This result suggests that specific substance(s) in P. acidilactici BLs could regulate the quorum-sensing mechanism of L. monocytogenes, ultimately reducing biofilm formation. Additionally, we also observed the decreased expressions of other genes, such as flaA, fliG, flgE, and prfA, involved in L. monocytogenes biofilm formation, suggesting that P. acidilactici BLs inhibits L. monocytogenes biofilm by affecting quorum sensing and flagellar motility. Furthermore, previous reports have shown that biofilm-inhibiting peptides contain positively charged amino acids, such as lysine [41,42]. The peptides rich in lysine form a charge clamp that enhances the proper association of biofilm-inhibiting peptides with the microbial cell membrane of target bacteria [43]. In the current study, K10 BL and HW01 BL did not effectively eradicate the planktonic L. monocytogenes viability. However, P. acidilactici BLs regulated genes responsible for biofilm formation in L. monocytogenes. A previous report showed that a synthetic cationic peptide did not affect the bacterial growth of Pseudomonas aeruginosa but effectively reduced P. aeruginosa biofilm formation [44]. Moreover, a recent study demonstrated that tryptophan-containing peptides modified quorum sensing and decreased biofilm development of P. aeruginosa [45]. These results indicate that substances such as peptides with antagonistic properties in P. acidilactici BLs may regulate genes involved in biofilm formation of L. monocytogenes.
In addition, the current study demonstrated that P. acidilactici BLs can also inhibit the adhesion and invasion of L. monocytogenes. Secreted proteins from Lactobacillus plantarum BMCM12 has been reported to participate in the inhibition of pathogen adhesion to mucin layers [46]. Moreover, a soluble protein HM0539 derived from L. rhamnosus GG enhanced tight junction and increased mucus secretion in intestinal epithelial cells, which probably attenuated pathogen invasion by promoting intestinal integrity [47]. Thus, P. acidilactici BLs containing proteins or peptides could play a role in the inhibition of L. monocytogenes adhesion to and invasion of intestinal epithelial cells. Moreover, L. monocytogenes adhesion is the initial step in biofilm development, and it is critical for the establishment of infection [14]. Several proteins, including FbpA, CtaP, ActA, LapB, and Ami, are closely associated with adhesion to and invasion of host cells [14,48]. Although it has been well studied that many probiotics can inhibit L. monocytogenes biofilm formation on and adhesion to host cells [49,50], the role of probiotics in regulating genes responsible for the adhesion and invasion of L. monocytogenes has not been revealed. Our observations indicate that critical genes underlying adhesion and invasion in L. monocytogenes were markedly downregulated by P. acidilactici BLs. Among the proteins, fbpA gene-encoding fibronectin-binding protein is a cell-wall-anchored protein that is distributed in Gram-positive bacteria, including L. monocytogenes, and plays a role in the interaction between the bacterial and the host cells [51]. Adhesion of ΔfbpA L. monocytogenes to host cells is decreased, suggesting that fbpA is an important factor in the pathogenesis of L. monocytogenes [52]. Moreover, a reduction of invasion capability in Caco-2 cells was observed in ΔinlA L. monocytogenes [53]. These findings are in agreement with our results of the importance of fbpA and inlA in L. monocytogenes adhesion and invasion. In addition, although a transcription factor, prfA is involved in L. monocytogenes biofilm formation as described above, it is also associated with the regulation of genes, such as inlA and actA [54]. Therefore, we speculate that P. acidilactici BLs downregulated prfA genes, consequently resulting in a decrease of inlA and actA gene expression.
In conclusion, the present study showed the antagonistic activities of P. acidilactici BLs against L. monocytogenes biofilm formation. P. acidilactici BLs also inhibited L. monocytogenes adhesion to and invasion of intestinal epithelial cells. Although probiotic lactobacilli and their postbiotic compounds have widely shown the antagonistic activity against the growth and biofilm formation of L. monocytogenes, P. acidilactici BLs as postbiotic compounds also inhibit biofilm formation of L. monocytogenes and its adhesion to and invasion of intestinal epithelial cells. These inhibitory effects are possibly affected by downregulating genes responsible for biofilm formation as well as adhesive and invasive abilities in L. monocytogenes. Although further studies are needed to confirm specific peptides or proteins of P. acidilactici BLs that are associated with antagonistic activities against L. monocytogenes, these results suggest that P. acidilactici BLs could reduce health risks posed by L. monocytogenes.
Author Contributions
Conceptualization, H.B.L. and S.-S.K.; methodology, H.B.L. and S.-S.K.; validation, H.B.L., K.H.K., and G.A.K.; formal analysis, H.B.L., K.H.K., and G.A.K.; investigation, H.B.L., K.H.K., and G.A.K.; writing—original draft preparation, H.B.L., K.-G.L., and K.H.K.; writing—review and editing, H.B.L., K.-G.L., and S.-S.K.; supervision, H.B.L. and S.-S.K.; project administration, H.B.L. and S.-S.K.; funding acquisition, S.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2020R1A2C1010010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that are presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Jeyaletchumi, P.; Tunung, R.; Selina, P.M.; Chai, L.; Radu, S.; Farinazleen, M.; Cheah, Y.; Mitsuaki, N.; Yoshitsugu, N.; Kumar, M.P. Assessment of Listeria monocytogenes in salad vegetables through kitchen simulation study. J. Trop. Agric. Food Sci. 2012, 40, 55–62. [Google Scholar]
- Hamidiyan, N.; Salehi-Abargouei, A.; Rezaei, Z.; Dehghani-Tafti, R.; Akrami-Mohajeri, F. The prevalence of Listeria spp. food contamination in Iran: A systematic review and meta-analysis. Food Res. Int. 2018, 107, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Di Bonaventura, G.; Piccolomini, R.; Paludi, D.; D’orio, V.; Vergara, A.; Conter, M.; Ianieri, A. Influence of temperature on biofilm formation by Listeria monocytogenes on various food-contact surfaces: Relationship with motility and cell surface hydrophobicity. J. Appl. Microbiol. 2008, 104, 1552–1561. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.P.; Higgins, D.E.; Kolter, R. Flagellar motility is critical for Listeria monocytogenes biofilm formation. J. Bacteriol. 2007, 189, 4418–4424. [Google Scholar] [CrossRef]
- Williams, T.; Joseph, B.; Beier, D.; Goebel, W.; Kuhn, M. Response regulator DegU of Listeria monocytogenes regulates the expression of flagella-specific genes. FEMS Microbiol. Lett. 2005, 252, 287–298. [Google Scholar] [CrossRef]
- Chang, Y.; Gu, W.; Fischer, N.; McLandsborough, L. Identification of genes involved in Listeria monocytogenes biofilm formation by mariner-based transposon mutagenesis. Appl. Microbiol. Biotechnol. 2012, 93, 2051–2062. [Google Scholar] [CrossRef]
- Zetzmann, M.; Sanchez-Kopper, A.; Waidmann, M.S.; Blombach, B.; Riedel, C.U. Identification of the agr peptide of Listeria monocytogenes. Front. Microbiol. 2016, 7, 989. [Google Scholar] [CrossRef]
- Lemon, K.P.; Freitag, N.E.; Kolter, R. The virulence regulator PrfA promotes biofilm formation by Listeria monocytogenes. J. Bacteriol. 2010, 192, 3969–3976. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef]
- Pan, Y.; Breidt, F., Jr.; Kathariou, S. Resistance of Listeria monocytogenes biofilms to sanitizing agents in a simulated food processing environment. Appl. Environ. Microbiol. 2006, 72, 7711–7717. [Google Scholar] [CrossRef] [PubMed]
- Donovan, S. Listeriosis: A rare but deadly disease. Clin. Microbiol. Newsl. 2015, 37, 135–140. [Google Scholar] [CrossRef]
- Burkholder, K.M.; Bhunia, A.K. Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60. Infect. Immun. 2010, 78, 5062–5073. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Dramsi, S.; Bourdichon, F.; Cabanes, D.; Lecuit, M.; Fsihi, H.; Cossart, P. FbpA, a novel multifunctional Listeria monocytogenes virulence factor. Mol. Microbiol. 2004, 53, 639–649. [Google Scholar] [CrossRef]
- Reis, J.; Paula, A.; Casarotti, S.; Penna, A. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, B.S.; Kang, S.S. Bacteriocin of Pediococcus acidilactici HW01 inhibits biofilm formation and virulence factor production by Pseudomonas aeruginosa. Probiotics Antimicrob. Proteins 2020, 12, 73–81. [Google Scholar] [CrossRef]
- Pérez-Ibarreche, M.; Castellano, P.; Leclercq, A.; Vignolo, G. Control of Listeria monocytogenes biofilms on industrial surfaces by the bacteriocin-producing Lactobacillus sakei CRL1862. FEMS Microbiol. Lett. 2016, 363, fnw118. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Ashrafudoulla, M.; Nahar, S.; Joo, H.-J.; Jahid, I.K.; Park, S.H.; Kim, K.-S.; Ha, S.-D. Inhibitory effects of probiotic potential lactic acid bacteria isolated from kimchi against Listeria monocytogenes biofilm on lettuce, stainless-steel surfaces, and MBEC™ biofilm device. LWT-Food Sci. Technol. 2020, 118, 108864. [Google Scholar] [CrossRef]
- Melian, C.; Bentencourt, E.; Castellano, P.; Ploper, D.; Vignolo, G.; Mendoza, L.M. Biofilm genes expression of Listeria monocytogenes exposed to Latilactobacillus curvatus bacteriocins at 10 °C. Int. J. Food Microbiol. 2022, 370, 109648. [Google Scholar] [CrossRef] [PubMed]
- Rodrıguez, E.; Calzada, J.; Arqués, J.; Rodrıguez, J.; Nunez, M.; Medina, M. Antimicrobial activity of pediocin-producing Lactococcus lactis on Listeria monocytogenes, Staphylococcus aureus and Escherichia coli O157: H7 in cheese. Int. Dairy J. 2005, 15, 51–57. [Google Scholar] [CrossRef]
- Berry, E.D.; Hutkins, R.W.; Mandigo, R.W. The use of bacteriocin-producing Pediococcus acidilactici to control postprocessing Listeria monocytogenes contamination of frankfurters. J. Food Protect. 1991, 54, 681–686. [Google Scholar] [CrossRef]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Pelaez-Martinez Mdel, C.; Hardisson de la Torre, A. Effect of a bacteriocin produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens on Spanish raw meat. Meat Sci. 2006, 72, 57–61. [Google Scholar] [CrossRef][Green Version]
- Rieu, A.; Weidmann, S.; Garmyn, D.; Piveteau, P.; Guzzo, J. Agr system of Listeria monocytogenes EGD-e: Role in adherence and differential expression pattern. Appl. Environ. Microbiol. 2007, 73, 6125–6133. [Google Scholar] [CrossRef]
- Longhi, C.; Scoarughi, G.L.; Poggiali, F.; Cellini, A.; Carpentieri, A.; Seganti, L.; Pucci, P.; Amoresano, A.; Cocconcelli, P.S.; Artini, M.; et al. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microb. Pathog. 2008, 45, 45–52. [Google Scholar] [CrossRef]
- Josse, J.; Laurent, F.; Diot, A. Staphylococcal adhesion and host cell invasion: Fibronectin-binding and other mechanisms. Front. Microbiol. 2017, 8, 2433. [Google Scholar] [CrossRef]
- Kıran, F.; Akoğlu, A.; Çakır, İ. Control of Listeria monocytogenes biofilm on industrial surfaces by cell-free extracts of Lactobacillus plantarum. J. Food Process. Preserv. 2021, 45, e15042. [Google Scholar] [CrossRef]
- Gómez, N.C.; Ramiro, J.M.; Quecan, B.X.; de Melo Franco, B.D. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157: H7 biofilms formation. Front. Microbiool. 2016, 7, 863. [Google Scholar] [CrossRef]
- Huang, J.; Lacroix, C.; Daba, H.; Simard, R.E. Growth of Listeria monocytogenes in milk and its control by pediocin 5 produced by Pediococcus acidilactici UL5. Int. Dairy J. 1994, 4, 429–443. [Google Scholar] [CrossRef]
- Abbasiliasi, S.; Tan, J.S.; Bashokouh, F.; Ibrahim, T.A.T.; Mustafa, S.; Vakhshiteh, F.; Sivasamboo, S.; Ariff, A.B. In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Nielsen, J.W.; Dickson, J.S.; Crouse, J.D. Use of a bacteriocin produced by Pediococcus acidilactici to inhibit Listeria monocytogenes associated with fresh meat. Appl. Environ. Microbiol. 1990, 56, 2142–2145. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Beuchat, L. Growth inhibition of Listeria monocytogenes by a bacteriocin of Pediococcus acidilactici M during fermentation of kimchi. Food Microbiol. 1994, 11, 301–307. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimaraes, J.T.; Yilmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Mardani, K.; Tajik, H. Characterization and application of postbiotics of Lactobacillus spp. on Listeria monocytogenes in vitro and in food models. LWT-Food Sci. Technol. 2019, 111, 457–464. [Google Scholar] [CrossRef]
- Boyer, M.; Wisniewski-Dye, F. Cell–cell signalling in bacteria: Not simply a matter of quorum. FEMS Microbiol. Ecol. 2009, 70, 1–19. [Google Scholar] [CrossRef]
- Li, R.; Du, W.; Yang, J.; Liu, Z.; Yousef, A.E. Control of Listeria monocytogenes biofilm by paenibacterin, a natural antimicrobial lipopeptide. Food Control 2018, 84, 529–535. [Google Scholar] [CrossRef]
- Bezar, I.F.; Mashruwala, A.A.; Boyd, J.M.; Stock, A.M. Drug-like fragments inhibit agr-mediated virulence expression in Staphylococcus aureus. Sci. Rep. 2019, 9, 6786. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.K.; Jaiswal, S.K.; Sharma, V.K. Prediction of biofilm inhibiting peptides: An In Silico approach. Front. Microbiol. 2016, 7, 949. [Google Scholar] [CrossRef] [PubMed]
- Segev-Zarko, L.; Saar-Dover, R.; Brumfeld, V.; Mangoni, M.L.; Shai, Y. Mechanisms of biofilm inhibition and degradation by antimicrobial peptides. Biochem. J. 2015, 468, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals (Basel) 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Han, X.; Du, W.; Kou, Z.; Jiang, F. Trp-containing antibacterial peptides impair quorum sensing and biofilm development in multidrug-resistant Pseudomonas aeruginosa and exhibit synergistic effects with antibiotics. Front. Microbiol. 2021, 12, 611009. [Google Scholar] [CrossRef]
- Sánchez, B.; Urdaci, M.C. Extracellular proteins from Lactobacillus plantarum BMCM12 prevent adhesion of enteropathogens to mucin. Curr. Microbiol. 2012, 64, 592–596. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Xayarath, B.; Marquis, H.; Port, G.C.; Freitag, N.E. Listeria monocytogenes CtaP is a multifunctional cysteine transport-associated protein required for bacterial pathogenesis. Mol. Microbiol. 2009, 74, 956–973. [Google Scholar] [CrossRef]
- Shao, X.; Fang, K.; Medina, D.; Wan, J.; Lee, J.L.; Hong, S.H. The probiotic, Leuconostoc mesenteroides, inhibits Listeria monocytogenes biofilm formation. J. Food Saf. 2020, 40, e12750. [Google Scholar] [CrossRef]
- Mathipa, M.G.; Thantsha, M.S.; Bhunia, A.K. Lactobacillus casei expressing internalins A and B reduces Listeria monocytogenes interaction with Caco-2 cells in vitro. Microb. Biotechnol. 2019, 12, 715–729. [Google Scholar] [CrossRef]
- Hymes, J.P.; Klaenhammer, T.R. Stuck in the Middle: Fibronectin-binding proteins in Gram-positive bacteria. Front. Microbiol. 2016, 7, 1504. [Google Scholar] [CrossRef] [PubMed]
- Osanai, A.; Li, S.J.; Asano, K.; Sashinami, H.; Hu, D.L.; Nakane, A. Fibronectin-binding protein, FbpA, is the adhesin responsible for pathogenesis of Listeria monocytogenes infection. Microbiol. Immunol. 2013, 57, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Marquis, H.; Boor, K.J. σB contributes to Listeria monocytogenes invasion by controlling expression of inlA and inlB. Microbiology (Reading) 2005, 151, 3215–3222. [Google Scholar] [CrossRef] [PubMed]
- Travier, L.; Guadagnini, S.; Gouin, E.; Dufour, A.; Chenal-Francisque, V.; Cossart, P.; Olivo-Marin, J.C.; Ghigo, J.M.; Disson, O.; Lecuit, M. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog. 2013, 9, e1003131. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).