Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors
Abstract
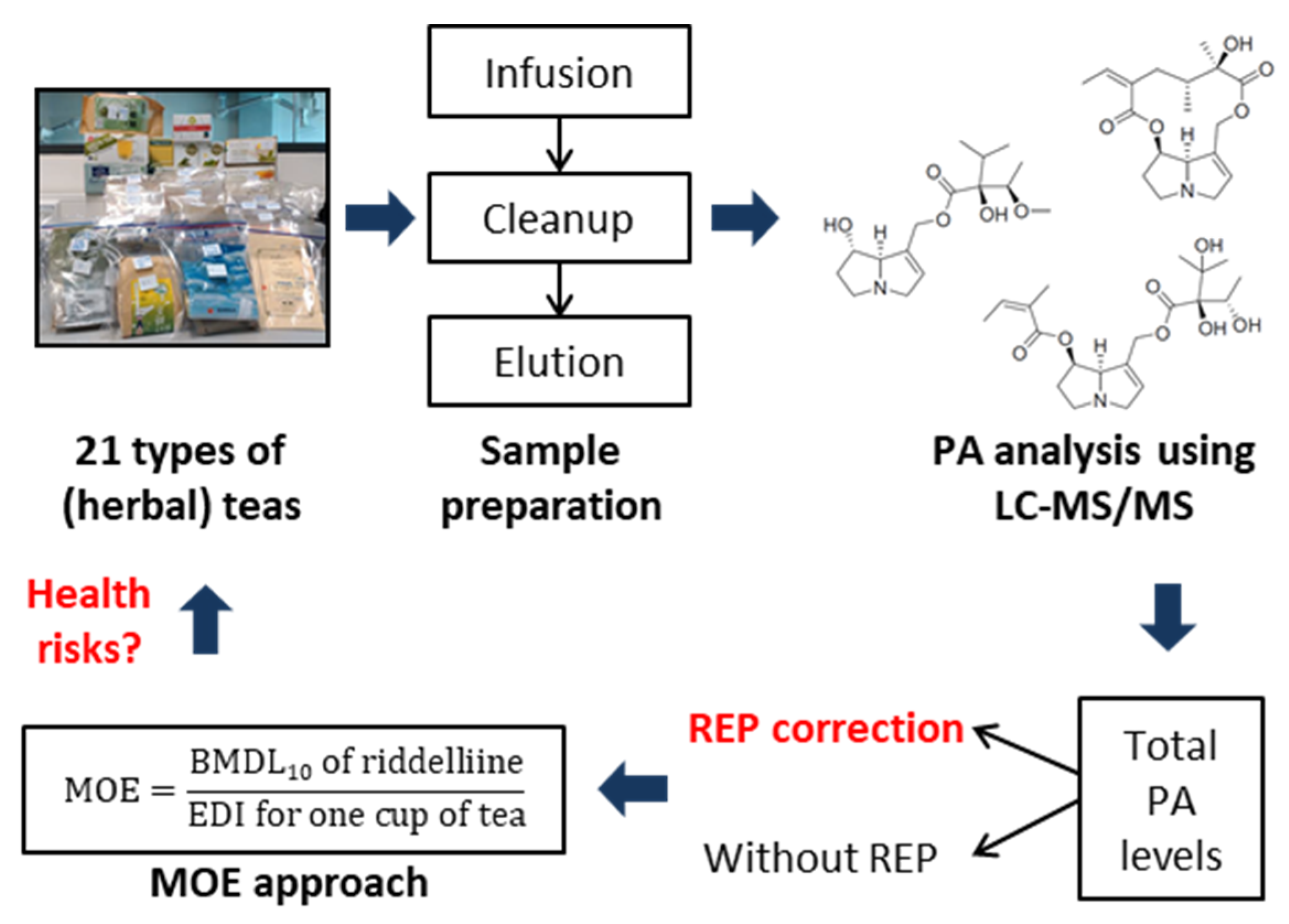
:1. Introduction
2. Materials and Methods
2.1. PA Extraction
2.2. Sample Cleanup
2.3. LC-MS/MS Analysis
2.4. Quality Assurance and Quality Control (QA/QC)
2.5. Estimated Daily Intake (EDI) and MOE Calculation
3. Results
3.1. PA Concentrations in (Herbal) Teas
3.2. Estimated Daily Intake of PAs
3.3. Risk Assessment for the (Herbal) Teas Based on Lifetime and Shorter Duration Exposure
3.4. Risk Assessment for the (Herbal) Teas Based on Shorter-Than-Lifetime Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Griffin, C.T.; Danaher, M.; Elliott, C.T.; Glenn Kennedy, D.; Furey, A. Detection of Pyrrolizidine Alkaloids in Commercial Honey Using Liquid Chromatography-Ion Trap Mass Spectrometry. Food Chem. 2013, 136, 1577–1583. [Google Scholar] [CrossRef]
- Bodi, D.; Ronczka, S.; Gottschalk, C.; Behr, N.; Skibba, A.; Wagner, M.; Lahrssen-Wiederholt, M.; Preiss-Weigert, A.; These, A. Determination of Pyrrolizidine Alkaloids in Tea, Herbal Drugs and Honey. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2014, 31, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- EFSA, E.F.S. Dietary Exposure Assessment to Pyrrolizidine Alkaloids in the European Population. EFSA J. 2016, 14, e04572. [Google Scholar] [CrossRef]
- Garcia-Alvarez, A.; Egan, B.; de Klein, S.; Dima, L.; Maggi, F.M.; Isoniemi, M.; Ribas-Barba, L.; Raats, M.M.; Meissner, E.M.; Badea, M.; et al. Usage of Plant Food Supplements across Six European Countries: Findings from the PlantLIBRA Consumer Survey. PLoS ONE 2014, 9, e92265. [Google Scholar] [CrossRef] [PubMed]
- Mohabbat, O.; Younos, M.S.; Merzad, A.A.; Srivastava, R.N.; Sediq, G.G.; Aram, G.N. An Outbreak of Hepatic Veno-Occlusive Disease in North-Western Afghanistan. Lancet Lond. Engl. 1976, 2, 269–271. [Google Scholar] [CrossRef]
- Tandon, B.N.; Tandon, H.D.; Tandon, R.K.; Narndranathan, M.; Joshi, Y.K. An Epidemic of Veno-Occlusive Disease of Liver in Central India. Lancet Lond. Engl. 1976, 2, 271–272. [Google Scholar] [CrossRef]
- Kumana, C.R.; Ng, M.; Lin, H.J.; Ko, W.; Wu, P.C.; Todd, D. Herbal Tea Induced Hepatic Veno-Occlusive Disease: Quantification of Toxic Alkaloid Exposure in Adults. Gut 1985, 26, 101–104. [Google Scholar] [CrossRef]
- Roulet, M.; Laurini, R.; Rivier, L.; Calame, A. Hepatic Veno-Occlusive Disease in Newborn Infant of a Woman Drinking Herbal Tea. J. Pediatr. 1988, 112, 433–436. [Google Scholar] [CrossRef]
- Prakash, A.S.; Pereira, T.N.; Reilly, P.E.; Seawright, A.A. Pyrrolizidine Alkaloids in Human Diet. Mutat. Res. 1999, 443, 53–67. [Google Scholar] [CrossRef]
- Sperl, W.; Stuppner, H.; Gassner, I.; Judmaier, W.; Dietze, O.; Vogel, W. Reversible Hepatic Veno-Occlusive Disease in an Infant after Consumption of Pyrrolizidine-Containing Herbal Tea. Eur. J. Pediatr. 1995, 154, 112–116. [Google Scholar] [CrossRef]
- Schulz, M.; Meins, J.; Diemert, S.; Zagermann-Muncke, P.; Goebel, R.; Schrenk, D.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Detection of Pyrrolizidine Alkaloids in German Licensed Herbal Medicinal Teas. Phytomed. Int. J. Phytother. Phytopharm. 2015, 22, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.-H.; Schrenk, D. Interim Relative Potency Factors for the Toxicological Risk Assessment of Pyrrolizidine Alkaloids in Food and Herbal Medicines. Toxicol. Lett. 2016, 263, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.J.; Sánchez, P.L.; These, A.; Preiss-Weigert, A.; Castellari, M. Occurrence of Pyrrolizidine Alkaloids in Food. EFSA Support. Publ. 2015, 12, 859E. [Google Scholar] [CrossRef]
- BfR (Bundesinstitut für Risikobewertung). Pyrrolizidine Alkaloids in Herbal Teas and Teas; No. 018/2013; BfR Opinion: Berlin, German, 5 July 2013. [Google Scholar]
- Chen, L.; Mulder, P.P.J.; Louisse, J.; Peijnenburg, A.; Wesseling, S.; Rietjens, I.M.C.M. Risk Assessment for Pyrrolizidine Alkaloids Detected in (Herbal) Teas and Plant Food Supplements. Regul. Toxicol. Pharmacol. RTP 2017, 86, 292–302. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Committee on a Request from EFSA Related to A Harmonised Approach for Risk Assessment of Substances Which Are Both Genotoxic and Carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- National Toxicology Program. Bioassay of Lasiocarpine for Possible Carcinogenicity. Natl. Cancer Inst. Carcinog. Techical Rep. Ser. 1978, 39, 1–66. [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Riddelliine (CAS No. 23246-96-0) in F344/N Rats and B6C3F1 Mice (Gavage Studies). Natl. Toxicol. Program Techical Rep. Ser. 2003, 508, 1–280. [Google Scholar]
- EFSA (CONTAM), E.P. on C. in the F. Scientific Opinion on the Risks to Animal and Public Health and the Environment Related to the Presence of Nickel in Feed. EFSA J. 2015, 13, 4074. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risks for Human Health Related to the Presence of Pyrrolizidine Alkaloids in Honey, Tea, Herbal Infusions and Food Supplements. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04908. [Google Scholar] [CrossRef]
- Chen, L.; Mulder, P.P.J.; Peijnenburg, A.; Rietjens, I.M.C.M. Risk Assessment of Intake of Pyrrolizidine Alkaloids from Herbal Teas and Medicines Following Realistic Exposure Scenarios. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 130, 142–153. [Google Scholar] [CrossRef]
- Chou, M.W.; Wang, Y.-P.; Yan, J.; Yang, Y.-C.; Beger, R.D.; Williams, L.D.; Doerge, D.R.; Fu, P.P. Riddelliine N-Oxide Is a Phytochemical and Mammalian Metabolite with Genotoxic Activity That Is Comparable to the Parent Pyrrolizidine Alkaloid Riddelliine. Toxicol. Lett. 2003, 145, 239–247. [Google Scholar] [CrossRef]
- EFSA. Guidance on Selected Default Values to Be Used by the EFSA Scientific Committee, Scientific Panels and Units in the Absence of Actual Measured Data. EFSA J. 2012, 10, 2579. [Google Scholar] [CrossRef]
- Bekanntmachung über die Zulassung und Registrierung von Arzneimitteln. Transfus. Med. Hemother. 1987, 14, 131–132. [CrossRef]
- Beatrix, W. Besluit van 19 januari 2001, houdende vaststelling van het Warenwetbesluit Kruidenpreparaten. Staatsblad Van Het Koninkr. Der Ned. 2001, 56, 12. [Google Scholar]
- JECFA (World Health Organization: Joint FAO/WHO Expert Committee on Food Additives). Summary and Conclusions; Issued 6 July 2015, JECFA/80/SC; JECFA: Geneva, Switzerland, 2015. [Google Scholar]
- COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment). COT Statement on Pyrrolizidine Alkaloids in Food; 2008/06; COT: London, UK, 2008. [Google Scholar]
- Chen, T.; Mei, N.; Fu, P.P. Genotoxicity of Pyrrolizidine Alkaloids. J. Appl. Toxicol. JAT 2010, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Edgar, J.A.; Molyneux, R.J.; Colegate, S.M. Pyrrolizidine Alkaloids: Potential Role in the Etiology of Cancers, Pulmonary Hypertension, Congenital Anomalies, and Liver Disease. Chem. Res. Toxicol. 2015, 28, 4–20. [Google Scholar] [CrossRef]
- Fu, P.P. Pyrrolizidine Alkaloids: Metabolic Activation Pathways Leading to Liver Tumor Initiation. Chem. Res. Toxicol. 2017, 30, 81–93. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, J.; Xia, Q.; Fu, P.P.; Lin, G. The Long Persistence of Pyrrolizidine Alkaloid-Derived DNA Adducts in Vivo: Kinetic Study Following Single and Multiple Exposures in Male ICR Mice. Arch. Toxicol. 2017, 91, 949–965. [Google Scholar] [CrossRef]
- Yang, M.; Ruan, J.; Gao, H.; Li, N.; Ma, J.; Xue, J.; Ye, Y.; Fu, P.P.-C.; Wang, J.; Lin, G. First Evidence of Pyrrolizidine Alkaloid N-Oxide-Induced Hepatic Sinusoidal Obstruction Syndrome in Humans. Arch. Toxicol. 2017, 91, 3913–3925. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Sun, Y.; Guo, X.; Huang, W.; Peng, Y.; Zheng, J. Comparative Study of Hepatotoxicity of Pyrrolizidine Alkaloids Retrorsine and Monocrotaline. Chem. Res. Toxicol. 2017, 30, 532–539. [Google Scholar] [CrossRef]
- EFSA. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 2097. [Google Scholar] [CrossRef]
- Kempf, M.; Reinhard, A.; Beuerle, T. Pyrrolizidine Alkaloids (PAs) in Honey and Pollen-Legal Regulation of PA Levels in Food and Animal Feed Required. Mol. Nutr. Food Res. 2010, 54, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, P.; Seraglio, S.K.T.; Schulz, M.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Pyrrolizidine Alkaloids and Beehive Products: A Review. Food Chem. 2021, 342, 128384. [Google Scholar] [CrossRef]
- BfR (Bundesinstitut für Risikobewertung). Analytik und Toxizität von Pyrrolizidinalkaloiden Sowie Eine Einschätzung des Gesundheitlichen Risikos Durch Deren Vorkommen in Honig; Stellungnahme Nr. 038/2011 des BfR vom 11; BfR Opinion: Berlin, German, 2011; ergänzt am 21 Januar 2013. [Google Scholar]
- BfR (Bundesinstitut für Risikobewertung). Pyrrolizidinalkaloide Gehalte in Lebensmitteln Sollen Nach Wie vor so Weit Wie Möglich Gesenkt Werden; Stellungnahme Nr. 030/2016 des BfR vom 28; BfR Opinion: Berlin, German, 2016. [Google Scholar]
- Mädge, I.; Cramer, L.; Rahaus, I.; Jerz, G.; Winterhalter, P.; Beuerle, T. Pyrrolizidine Alkaloids in Herbal Teas for Infants, Pregnant or Lactating Women. Food Chem. 2015, 187, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Habs, M.; Binder, K.; Krauss, S.; Müller, K.; Ernst, B.; Valentini, L.; Koller, M. A Balanced Risk-Benefit Analysis to Determine Human Risks Associated with Pyrrolizidine Alkaloids (PA)—The Case of Tea and Herbal Infusions. Nutrients 2017, 9, 717. [Google Scholar] [CrossRef] [PubMed]
- Mulder, P.P.J.; López, P.; Castellari, M.; Bodi, D.; Ronczka, S.; Preiss-Weigert, A.; These, A. Occurrence of Pyrrolizidine Alkaloids in Animal- and Plant-Derived Food: Results of a Survey across Europe. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2018, 35, 118–133. [Google Scholar] [CrossRef]
- Dusemund, B.; Nowak, N.; Sommerfeld, C.; Lindtner, O.; Schäfer, B.; Lampen, A. Risk Assessment of Pyrrolizidine Alkaloids in Food of Plant and Animal Origin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 63–72. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Review Article: Herbal and Dietary Supplement Hepatotoxicity. Aliment. Pharmacol. Ther. 2013, 37, 3–17. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, C.-Y.; Li, D.-P.; Chen, H.-B.; Ma, J.; Gao, H.; Ye, Y.; Wang, J.-Y.; Fu, P.P.; Lin, G. Tu-San-Qi (Gynura Japonica): The Culprit behind Pyrrolizidine Alkaloid-Induced Liver Injury in China. Acta Pharmacol. Sin. 2021, 42, 1212–1222. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Duebecke, A.; Mulder, P.P.J.; Cuneah, O.; Barel, S. Pyrrolizidine and Tropane Alkaloids in Teas and the Herbal Teas Peppermint, Rooibos and Chamomile in the Israeli Market. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2015, 32, 2058–2067. [Google Scholar] [CrossRef]
- Kaltner, F.; Stiglbauer, B.; Rychlik, M.; Gareis, M.; Gottschalk, C. Development of a Sensitive Analytical Method for Determining 44 Pyrrolizidine Alkaloids in Teas and Herbal Teas via LC-ESI-MS/MS. Anal. Bioanal. Chem. 2019, 411, 7233–7249. [Google Scholar] [CrossRef] [PubMed]


| Type of (Herbal) Teas | Ingredients | Origin from PA-Producing Plants | Nation of Origin | Year of Sampling |
|---|---|---|---|---|
| Asteraceae | Asteraceae | Yes | CN | 2021 |
| Boraga | Boraga | Yes | ES | 2020 |
| Camomile | Chamazulene, bisabolol, apigenin, luteolin | Not | NL | 2020 |
| Citroen melisse | Lemon balm (melissa officinalis), rooibos, lemon flavouring | Not | NL | 2020 |
| Earl grey | Black tea, bergamot and lemon flavouring | Not | NL | 2020 |
| Eupatorium | Eupatorium Sp. | Yes | CN | 2021 |
| Forest fruit tea | Mint, strawberry, cherry, blueberry, cranberry, guava | Not | NL | 2020 |
| Fresh peppermint | Peppermint | Not | NL | 2020 |
| Green tea lemon | Green tea, natural flavouring, lemon flavouring (2%) | Not | NL | 2020 |
| Gynura segetum | Gynura segetum | Yes | CN | 2021 |
| Heliotrope | Heliotrope Sp. | Yes | CN | 2021 |
| Lemon balm & liquorice | Lemon balm leaves (51.5%), rooibos, camomile, liquorice root (6%), strawberry flavouring, orange blossom, sweet blackberry leaves | Not | UK | 2020 |
| Lemon balm (melissa) | Rooibos, orange flavouring, lemon balm (melissa officinalis) | Not | NL | 2020 |
| Lemon verbena | Lemon verbena, lemongrass | Not | NL | 2020 |
| Lungwort | Lungwort (pulmonaria officinalis) | Yes | NL | 2020 |
| Mix herb (1) | Verbena, lemon grass, rosemary, stevia, green tea, jasmine | Not | CN | 2021 |
| Mix herb (2) | Lemon balm, hops, lemongrass, raspberry | Not | CN | 2021 |
| Mix herb (3) | Mint leaves, lemongrass, lemon balm, flowers, roots | Not | CN | 2021 |
| Rooibos | Rooibos | Not | NL | 2020 |
| Sage & lemon myrtle | Lemon balm leaves, camomile, nettle, sweet blackberry leaves, sage leaves (6%), lime flavouring, lemon myrtle (2%), angelica root, red clover | Not | UK | 2020 |
| Tephroseris | Tephroseris sp. | Yes | CN | 2021 |
| Type of Tea | Number of PAs above the LOD | Mean (Range) Concentration of Total PAs (μg/kg d.m.) | Daily Intake of Total PAs (μg/Day) | Top Three PAs a and Their Concentration (μg/kg d.m.) | ||||
|---|---|---|---|---|---|---|---|---|
| no REP | REP | no REP | REP | no REP | REP | no REP | REP | |
| Asteraceae | 4 | 4 | 36.7 (16.5–53.2) | 29.6 (14.3–47.1) | 0.0734 | 0.0592 | Senkirkine (26.6); lycopsamine N-oxide (4.3); neosenkirkine (3.0) | Senkirkine (26.6); neosenkirkine (2.97); lycopsamine N-oxide (0.04) |
| Borage | 13 | 13 | 167,846.6 (68,935.8–465,953.6) | 1440.6 (698.4–5782.1) | 336 | 2.88 | Supinine N-oxide (65452.4); lycopsamine N-oxide (47295.8); supinine (3174.9) | Supinine N-oxide (654.5); lycopsamine N-oxide (473.0); supinine (31.7) |
| Chamomile | 13 | 13 | 772.8 (393.0–1053.2) | 703.3 (382.0–997.2) | 1.55 | 1.41 | Retrorsine N-oxide (520.6); senecionine N-oxide (74.3); echimidine N-oxide (67.4) | Retrorsine N-oxide (520.6); senecionine N-oxide (74.3); echimidine N-oxide (67.4) |
| Citroen melisse | 0 | 0 | n.d. | n.a. | n.a. | n.a. | n.d. | n.a. |
| Earl grey | 3 | 3 | 189.9 (59.7–353.1) | 47.0 (17.5–87.2) | 0.38 | 0.094 | Echinatine N-oxide (124.9); lycopsamine N-oxide (34.3); echinatine (30.7) | Echinatine N-oxide (37.5); echinatine (9.2); lycopsamine N-oxide (0.3) |
| Eupatorium | 8 | 7 | 154.0 (86.9–257.4) | 140.7 (80.9–239.5) | 0.308 | 0.281 | Senkirkine (109.7); neosenkirkine (25.0); echinatine (6.5) | Senkirkine (109.7); neosenkirkine (25.0); echinatine (2.0) |
| Forest fruit tea | 2 | 2 | 21.3 (7.4–35.2) | 6.4 (2.1–10.7) | 0.0426 | 0.0128 | Echinatine N-oxide (15.3); echinatine (6.0) | Echinatine N-oxide (4.6); echinatine (1.8) |
| Fresh peppermint | 0 | 0 | n.d. | n.a. | n.a. | n.a. | n.d. | n.a. |
| Green tea | 5 | 5 | 72.8 (38.2–298.2) | 72.8 (38.2–298.2) | 0.146 | 0.146 | Integerrimine N-oxide (42.2); integerrimine (35.9); retrorsine N-oxide (18.5) | Integerrimine N-oxide (42.2); integerrimine (35.9); retrorsine N-oxide (18.5) |
| Gynura segetum | 2 | 2 | 13.4 (n.d.–26.8) | 13.4 (n.d.–26.8) | 0.0268 | 0.0268 | Senkirkine (10.2); neosenkirkine (3.2) | Senkirkine (10.2); neosenkirkine (3.2) |
| Heliotropium | 7 | 7 | 29.4 (8.5–73.9) | 20.4 (7.8–56.2) | 0.0588 | 0.0408 | Senkirkine (15.2); lycopsamine N-oxide (4.5); neosenkirkine (3.8) | Senkirkine (15.2); neosenkirkine (3.8); echinatine N-oxide (0.6) |
| Lemon balm (melissa) | 17 | 17 | 845.1 (169.5–1258.3) | 831.2 (163.5–1216.3) | 1.69 | 1.66 | Senecionine N-oxide (539.0); integerrimine N-oxide (107.6); senecionine (85.0) | Senecionine N-oxide (539.0); integerrimine N-oxide (107.6); senecionine (85.0) |
| Lemon balm & liquorice | 15 | 13 | 52.0 (17.5–163.2) | 16.9 (6.5–54.6) | 0.104 | 0.0338 | Atropine (18.8); scopolamine (18.5); europine N-oxide (10.9) | Lasiocarpine N-oxide (4.9); europine N-oxide (3.3); heliotrine N-oxide (3.3) |
| Lemon verbena | 4 | 4 | 647.1 (218.9–987.2) | 210.2 (69.3– 353.1) | 1.29 | 0.42 | Echinatine N-oxide (579.0); echinatine (45.1); heliosupine N-oxide (17.8) | Echinatine N-oxide (173.7); echinatine (13.5); heliosupine N-oxide (17.8) |
| Lungwort | 8 | 8 | 1769.7 (996.5–2753.2) | 37.8 (18.6–54.8) | 3.54 | 0.0756 | Intermedine N-oxide (589.9); lycopsamine N-oxide (421.8); intermedine (300.8) | 7-acetyllycopsamine/intermedine N-oxide (12.7); senkirkine (6.2); intermedine N-oxide (5.9) |
| Mix herb (1) | 5 | 5 | 84.1 (14.5–253.6) | 78.8 (14.5–214.2) | 0.168 | 0.158 | Monocrotaline (38.6); senkirkine (23.2); neosenkirkine (10.8) | Monocrotaline (38.6); senkirkine (23.2); neosenkirkine (10.8) |
| Mix herb (2) | 4 | 3 | 16.1 (5.4–39.0) | 11.4 (5.4–23.0) | 0.0322 | 0.0228 | Atropine (76.4); senecionine (9.5); heliotrine N-oxide (3.6) | Senecionine (9.5); heliotrine N-oxide (1.1); heliotrine (1.0) |
| Mix herb (3) | 6 | 4 | 15.0 (0.4–63.6) | 1.3 (0.4–3.2) | 0.03 | 0.0026 | Lycopsamine N-oxide (3.8); intermedine N-oxide (3.5); rinderine N-oxide (2.7) | Rinderine N-oxide (0.8); echinatine N-oxide (0.4); intermedine N-oxide (0.04) |
| Rooibos | 12 | 12 | 218.4 (116.8–754.3) | 218.4 (116.8– 754.3) | 0.437 | 0.437 | Senecionine N-oxide (62.3); integerrimine N-oxide (44.6); retrorsine N-oxide (32.2) | Senecionine N-oxide (62.3); integerrimine N-oxide (44.6); retrorsine N-oxide (32.2) |
| Sage & lemon myrtle | 14 | 12 | 114.4 (17.6–554.2) | 29.2 (4.2–114.8) | 0.229 | 0.0584 | Echinatine N-oxide (44.1); echinatine (27.0); intermedine (6.8) | Echinatine N-oxide (13.2); echinatine (8.1); Rinderine (1.6) |
| Tephroseris | 16 | 14 | 286,682.2 (185,233.6–366,599.4) | 286,648.3 (185,201.6– 366,544.4) | 573 | 573 | Senkirkine (191241.4); neosenkirkine (50250.6); petasitenine (37318.2) | Senkirkine (191241.4); neosenkirkine (50250.6); petasitenine (37318.2) |
| Type of Tea | EDI (μg/kg bw/day) | |
|---|---|---|
| without REP Correction | Corrected by REP Factors | |
| Asteraceae | 1.05 × 10−3 | 8.46 × 10−4 |
| Borage | 4.80 | 4.12 × 10−2 |
| Chamomile | 2.21 × 10−2 | 2.01 × 10−2 |
| Citroen melisse | n.a. | n.a. |
| Earl grey | 5.43 × 10−3 | 1.34 × 10−3 |
| Eupatorium | 4.40 × 10−3 | 4.02 × 10−3 |
| Forest fruit tea | 6.09 × 10−4 | 1.83 × 10−4 |
| Fresh peppermint | n.a. | n.a. |
| Green tea | 2.08 × 10−3 | 2.08 × 10−3 |
| Gynura segetum | 3.83 × 10−4 | 3.83 × 10−4 |
| Heliotropium | 8.40 × 10−4 | 5.83 × 10−4 |
| Lemon balm (melissa) | 2.41 × 10−2 | 2.37 × 10−2 |
| Lemon balm & liquorice | 1.49 × 10−3 | 4.83 × 10−4 |
| Lemon verbena | 1.85 × 10−2 | 6.01 × 10−3 |
| Lungwort | 5.06 × 10−2 | 1.08 × 10−3 |
| Mix herb (1) | 2.40 × 10−3 | 2.25 × 10−3 |
| Mix herb (2) | 4.60 × 10−4 | 3.26 × 10−4 |
| Mix herb (3) | 4.29 × 10−4 | 3.71 × 10−5 |
| Rooibos | 6.24 × 10−3 | 6.24 × 10−3 |
| Sage & lemon myrtle | 3.27 × 10−3 | 8.34 × 10−4 |
| Tephroseris | 8.19 | 8.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, Q.; Yi, Z.; Chen, Y.; Xiao, W.; Su, D.; Shi, W. Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods 2022, 11, 2946. https://doi.org/10.3390/foods11192946
Chen L, Zhang Q, Yi Z, Chen Y, Xiao W, Su D, Shi W. Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods. 2022; 11(19):2946. https://doi.org/10.3390/foods11192946
Chicago/Turabian StyleChen, Lu, Qian Zhang, Ziwei Yi, Yu Chen, Weihan Xiao, Dan Su, and Wenbiao Shi. 2022. "Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors" Foods 11, no. 19: 2946. https://doi.org/10.3390/foods11192946
APA StyleChen, L., Zhang, Q., Yi, Z., Chen, Y., Xiao, W., Su, D., & Shi, W. (2022). Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods, 11(19), 2946. https://doi.org/10.3390/foods11192946







