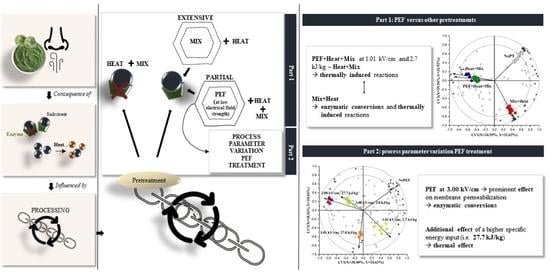
The Volatile Profile of Brussels Sprouts (Brassica oleracea Var. gemmifera) as Affected by Pulsed Electric Fields in Comparison to Other Pretreatments, Selected to Steer (Bio)Chemical Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Implementing PEF versus Other Pretreatments to Compare the Volatile Profiles
2.1.1. Raw Material
2.1.2. No Pretreatment (NoPT) (Control)
2.1.3. Mixing Followed by Heating (Mix + Heat)
2.1.4. Heating Followed by Mixing (Heat + Mix)
2.1.5. Pulsed Electric Fields (PEF) Followed by Heating and Mixing (PEF + Heat + Mix)
2.2. Impact of Parameter Variation in the PEF Treatment
2.2.1. Raw Material
2.2.2. PEF + Incubation
2.3. Analysis of the Volatile Profile
2.3.1. Sample Preparation
2.3.2. HS-SPME-GC-MS Fingerprinting
2.4. Multivariate Data Analysis
2.5. Statistical Analysis
3. Results and Discussion
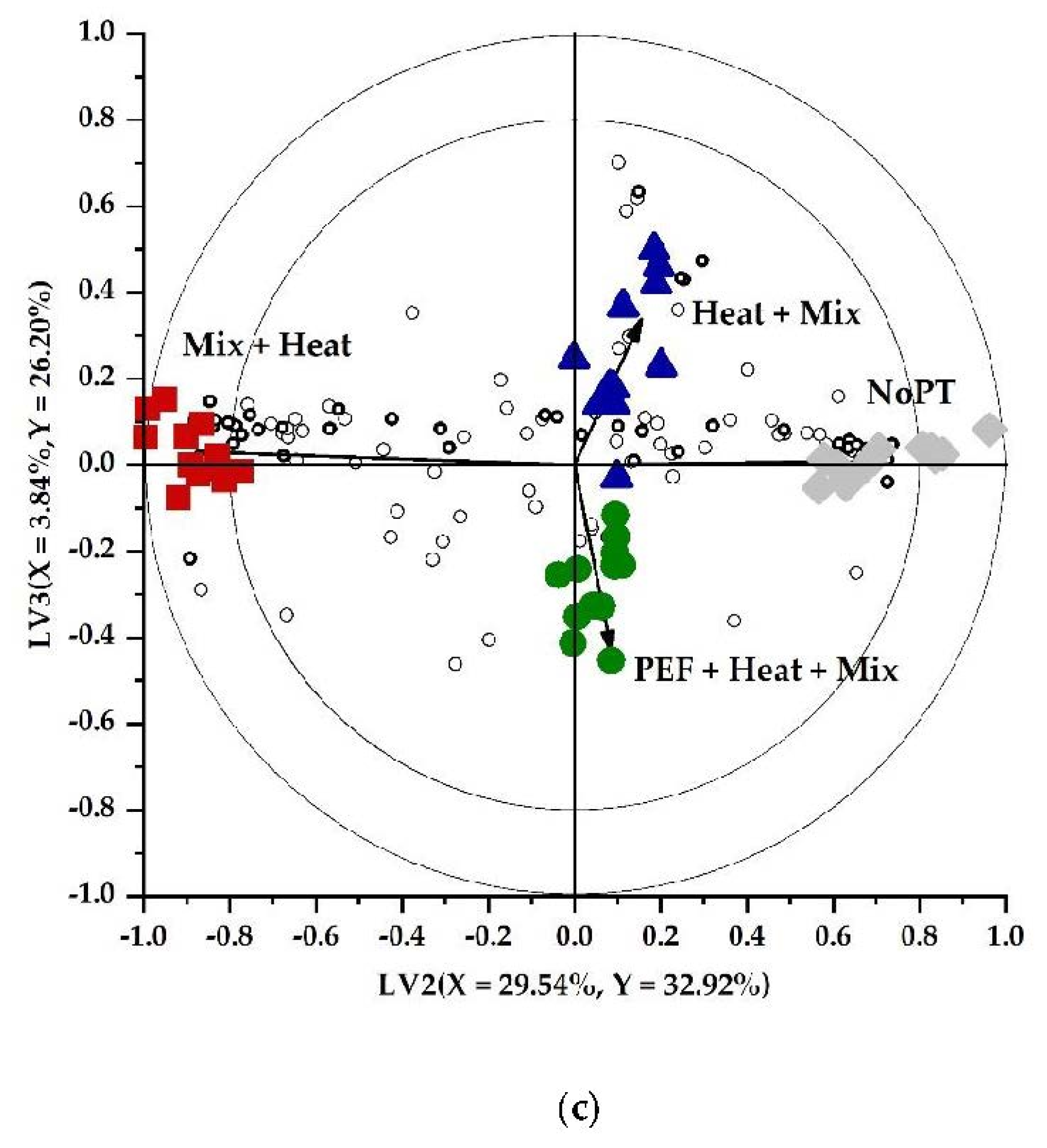
3.1. Impacts of PEF and Other Pretreatments on the Volatile Profile of Brussels Sprouts
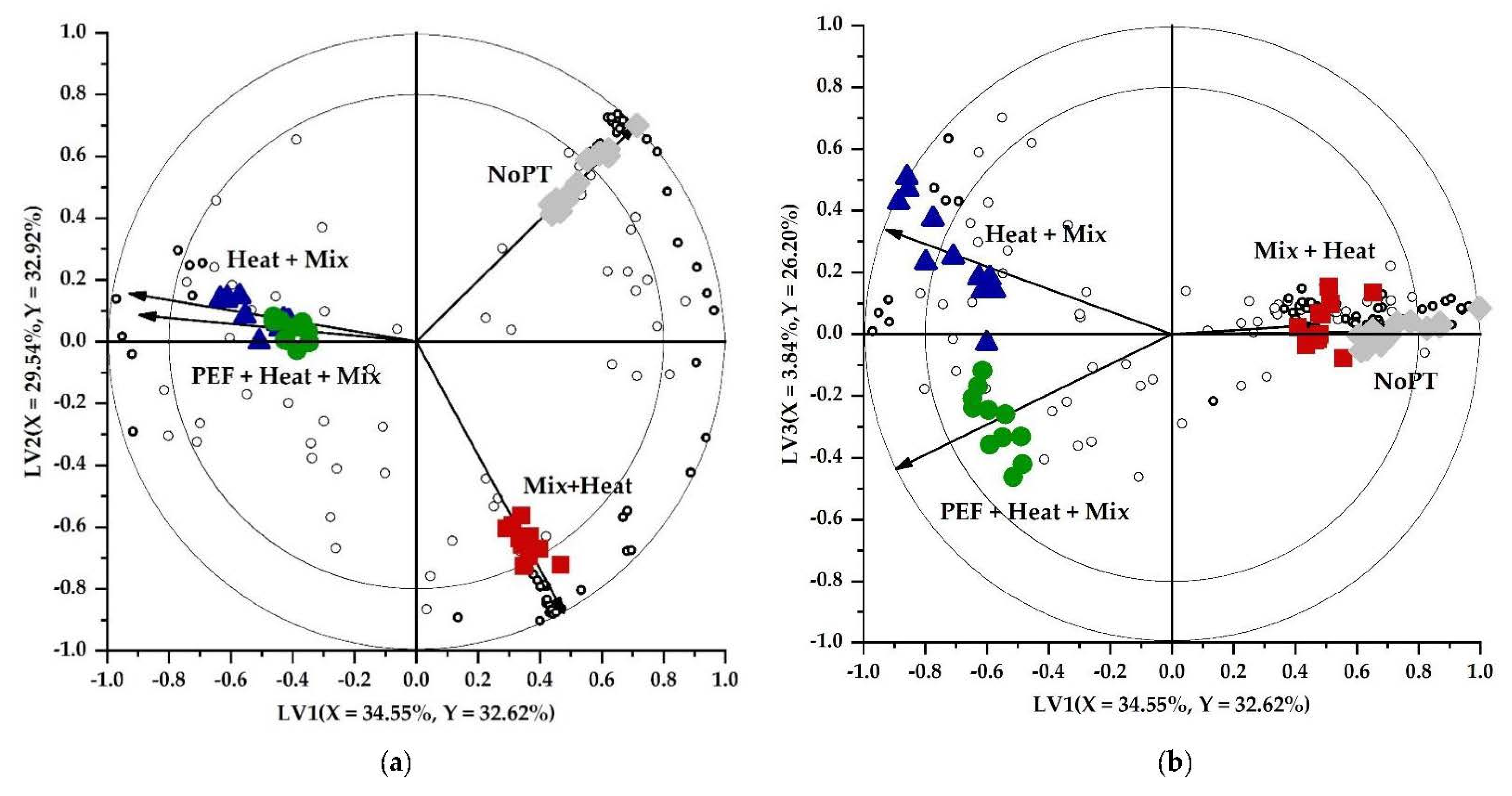
3.1.1. Qualitative and Quantitative Classification of the Volatile Profiles
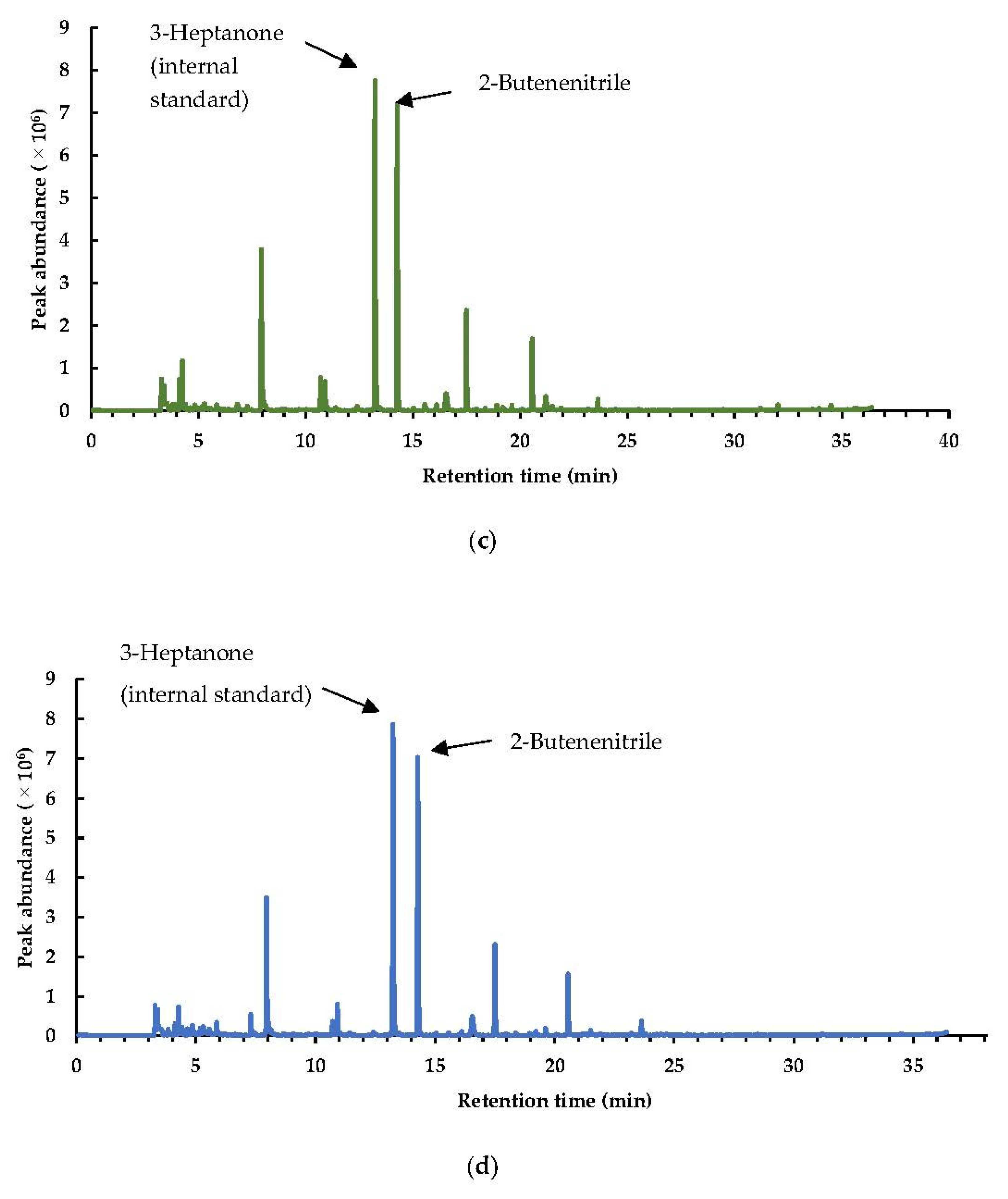
3.1.2. Interpretation of the Markers in the Headspace after Different Pretreatments on Brussels Sprouts
Sulfurous Compounds, Nitriles, and Isothiocyanates
Alcohols, Aldehydes, Ketones, and Furanic Compounds
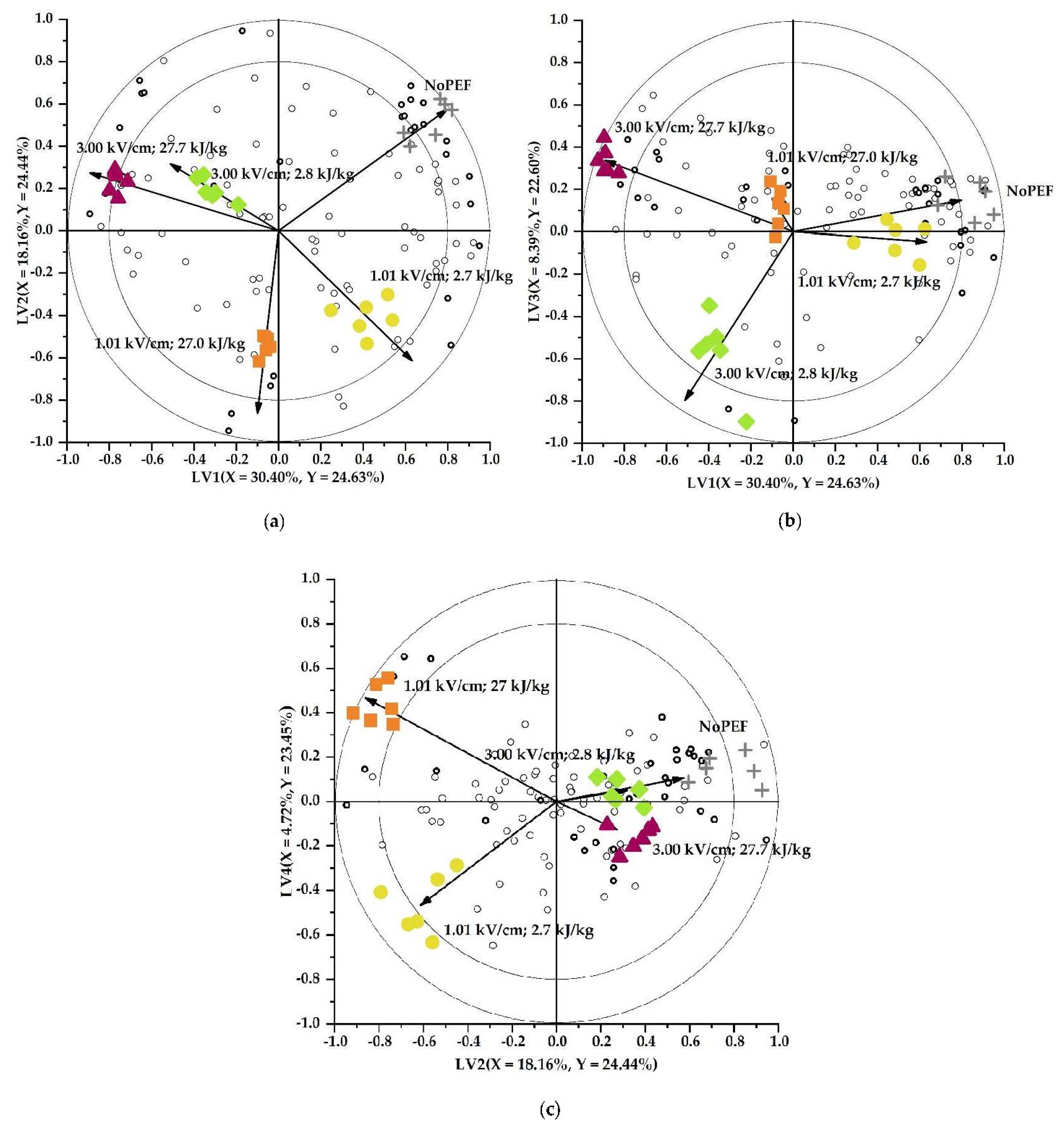
3.2. Impact of PEF Conditions on the Volatile Profile of Brussels Sprouts
3.2.1. Qualitative and Quantitative Classification of the Volatile Profiles
3.2.2. Linking Markers to (Bio)Chemical Reaction Pathways Possibly Occurring during and after PEF Treatment
Nitriles
Aldehydes and Alcohols
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) allyl isothiocyanate; (b) 4-isothiocyanato-1-butene. Statistically significant differences are designated by different letters ( < 0.05, = 12).
) Heat + Mix). (a) allyl isothiocyanate; (b) 4-isothiocyanato-1-butene. Statistically significant differences are designated by different letters ( < 0.05, = 12).
 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) allyl isothiocyanate; (b) 4-isothiocyanato-1-butene. Statistically significant differences are designated by different letters ( < 0.05, = 12).
) Heat + Mix). (a) allyl isothiocyanate; (b) 4-isothiocyanato-1-butene. Statistically significant differences are designated by different letters ( < 0.05, = 12).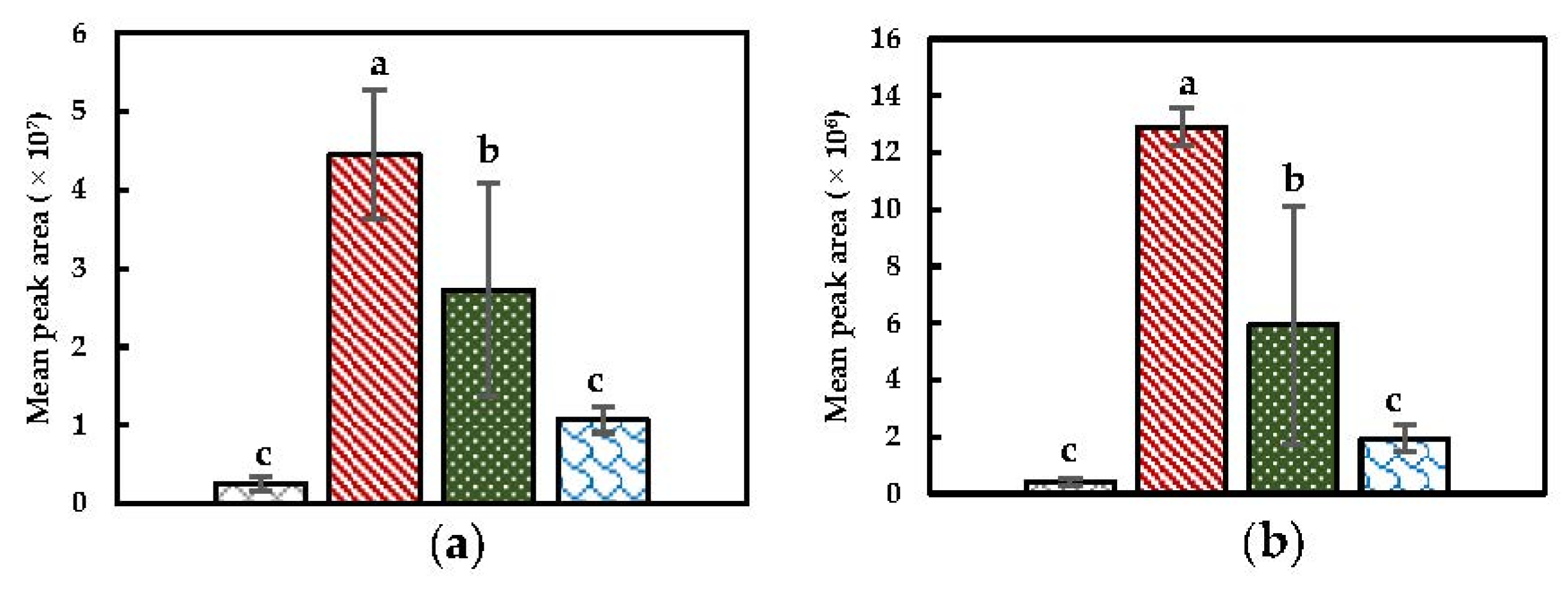
References
- Wieczorek, M.N.; Dunkel, A.; Szwengiel, A.; Czaczyk, K.; Drożdżyńska, A.; Wojtasiak, R.Z.; Jeleń, H.H. The relation between phytochemical composition and sensory traits of selected Brassica vegetables. LWT 2021, 156, 113028. [Google Scholar] [CrossRef]
- Engel, E.; Baty, C.; le Corre, D.; Souchon, I.; Martin, N. Flavor-Active Compounds Potentially Implicated in Cooked Cauliflower Acceptance. J. Agric. Food Chem. 2002, 50, 6459–6467. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (Brassica oleracea var. italica) to Improve the Nutritional Value and Consumer Acceptance. Mater. Veg. 2014, 69, 228–234. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Jeleń, H.H. Determination of the odor threshold concentrations and partition coefficients of isothiocyanates from Brassica vegetables in aqueous solution. LWT 2020, 131, 109793. [Google Scholar] [CrossRef]
- Bell, L.; Oloyede, O.O.; Lignou, S.; Wagstaff, C.; Methven, L. Taste and Flavor Perceptions of Glucosinolates, Isothiocyanates, and Related Compounds. Mol. Nutr. Food Res. 2018, 62, e1700990. [Google Scholar] [CrossRef]
- Olivera, D.F.; Viña, S.Z.; Marani, C.M.; Ferreyra, R.M.; Mugridge, A.; Chaves, A.R.; Mascheroni, R.H. Effect of blanching on the quality of Brussels sprouts (Brassica oleracea L. gemmifera DC) after frozen storage. J. Food Eng. 2008, 84, 148–155. [Google Scholar] [CrossRef]
- Kebede, B.T.; Grauwet, T.; Magpusao, J.; Palmers, S.; Michiels, C.; Hendrickx, M.E.; Van Loey, A. Chemical changes of thermally sterilized broccoli puree during shelf-life: Investigation of the volatile fraction by fingerprinting-kinetics. Food Res. Int. 2015, 67, 264–271. [Google Scholar] [CrossRef]
- Oloyede, O.O.; Wagstaff, C.; Methven, L. Influence of Cabbage (Brassica oleracea) Accession and Growing Conditions on Myrosinase Activity, Glucosinolates and Their Hydrolysis Products. Foods 2021, 10, 2903. [Google Scholar] [CrossRef]
- Tian, Q.; Rosselot, R.A.; Schwartz, S. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal. Biochem. 2005, 343, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, J.; Van Der Kruk, G.; Van Holst, G.; Schoofs, M.; Broer, J.; De Nijs, J. Quantitative inheritance of the progoitrin and sinigrin content in Brussels sprouts. Euphytica 1999, 108, 41–52. [Google Scholar] [CrossRef]
- Van Eylen, D.; Oey, I.; Hendrickx, M.; Van Loey, A. Behavior of mustard seed (Sinapis alba L.) myrosinase during temperature/pressure treatments: A case study on enzyme activity and stability. Eur. Food Res. Technol. 2007, 226, 545–553. [Google Scholar] [CrossRef]
- Koutidou, M.; Grauwet, T.; Van Loey, A.; Acharya, P. Potential of different mechanical and thermal treatments to control off-flavour generation in broccoli puree. Food Chem. 2017, 217, 531–541. [Google Scholar] [CrossRef]
- Frank, D.; Piyasiri, U.; Archer, N.; Jenifer, J.; Appelqvist, I. Influence of saliva on individual in-mouth aroma release from raw cabbage (Brassica oleracea var. capitata f. rubra L.) and links to perception. Heliyon 2018, 4, e01045. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Cheng, H.; Pu, Y.; Ren, S.; Hu, L.; Chen, J.; Ye, X.; Liu, D. Characterization of volatile compounds in pickled and dried mustard (Brassica juncea, Coss.) using optimal HS-SPME-GC-MS. CyTA-J. Food 2018, 16, 331–339. [Google Scholar] [CrossRef]
- Aires, A. Brassica Composition and Food Processing. In Processing and Impact on Active Components in Food; Preedy, V., Ed.; Elsevier Inc.: Amsterdam, the Netherlands, 2015; pp. 17–25. [Google Scholar]
- Kang, Y.; Liu, W.; Guan, C.; Guan, M.; He, X. Evolution and functional diversity of lipoxygenase (LOX) genes in allotetraploid rapeseed (Brassica napus L.). Int. J. Biol. Macromol. 2021, 188, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Engelberth, M. Variability in the Capacity to Produce Damage-Induced Aldehyde Green Leaf Volatiles among Different Plant Species Provides Novel Insights into Biosynthetic Diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Bones, A.; Rossiter, J. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Ortner, E.; Granvogl, M. Thermally Induced Generation of Desirable Aroma-Active Compounds from the Glucosinolate Sinigrin. J. Agric. Food Chem. 2017, 66, 2485–2490. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Walczak, M.; Skrzypczak-Zielinska, M.; Jeleń, H.H. Bitter taste of Brassica vegetables: The role of genetic factors, receptors, isothiocyanates, glucosinolates, and flavor context. Crit. Rev. Food Sci. Nutr. 2018, 58, 3130–3140. [Google Scholar] [CrossRef]
- Pecháček, R.; Velíšek, A.J.; Hrabcová, H. Decomposition Products of Allyl Isothiocyanate in Aqueous Solutions. J. Agric. Food Chem. 1997, 45, 4584–4588. [Google Scholar] [CrossRef]
- Bricker, G.V.; Riedl, K.; Ralston, R.A.; Tober, K.L.; Oberyszyn, T.; Schwartz, S.J. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol. Nutr. Food Res. 2014, 58, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Xiao, X.; Wei, L.; Li, L.; Li, G.; Liu, F.; Xie, J.; Yu, J.; Zhong, Y. Development and comprehensive HS-SPME/GC–MS analysis optimization, comparison, and evaluation of different cabbage cultivars (Brassica oleracea L. var. capitata L.) volatile components. Food Chem. 2020, 340, 128166. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Deng, L.; Zhu, X.-M.; Fan, Y.; Hu, J.-N.; Li, J.; Deng, Z.-Y. Novel Approach to Evaluate the Oxidation State of Vegetable Oils Using Characteristic Oxidation Indicators. J. Agric. Food Chem. 2014, 62, 12545–12552. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Laustsen, A.; Olsen, C.E.; Poll, L.; Sørensen, H. Chemical and sensory quality of broccoli (Brassica oleracea L. var italica). J. Food Qual. 1997, 20, 441–459. [Google Scholar] [CrossRef]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The ‘mustard oil bomb’: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2008, 8, 69–86. [Google Scholar] [CrossRef]
- Duncan, A.J.; Milne, J.A. Effects of oral administration of brassica secondary metabolites, allyl cyanide, allyl isothiocyanate and dimethyl disulphide, on the voluntary food intake and metabolism of sheep. Br. J. Nutr. 1993, 70, 631–645. [Google Scholar] [CrossRef]
- Hanschen, F.S.; Kühn, C.; Nickel, M.; Rohn, S.; Dekker, M. Leaching and degradation kinetics of glucosinolates during boiling of Brassica oleracea vegetables and the formation of their breakdown products. Food Chem. 2018, 263, 240–250. [Google Scholar] [CrossRef]
- Mao, X.; Zhao, X.; Huyan, Z.; Liu, T.; Yu, X. Relationship of Glucosinolate Thermal Degradation and Roasted Rapeseed Oil Volatile Odor. J. Agric. Food Chem. 2019, 67, 11187–11197. [Google Scholar] [CrossRef]
- Tulio, A.Z.; Yamanaka, H.; Ueda, Y.; Imahori, Y. Formation of Methanethiol and Dimethyl Disulfide in Crushed Tissues of Broccoli Florets and Their Inhibition by Freeze−Thawing. J. Agric. Food Chem. 2002, 50, 1502–1507. [Google Scholar] [CrossRef]
- Aganovic, K.; Grauwet, T.; Siemer, C.; Toepfl, S.; Heinz, V.; Hendrickx, M.; Van Loey, A. Headspace fingerprinting and sensory evaluation to discriminate between traditional and alternative pasteurization of watermelon juice. Eur. Food Res. Technol. 2015, 242, 787–803. [Google Scholar] [CrossRef]
- Shirakawa, M.; Hara-Nishimura, I. Specialized Vacuoles of Myrosin Cells: Chemical Defense Strategy in Brassicales Plants. Plant. Cell Physiol. 2018, 59, 1309–1316. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Gu, Z. Effect of freezing methods on sulforaphane formation in broccoli sprouts. RSC Adv. 2015, 5, 32290–32297. [Google Scholar] [CrossRef]
- Hanschen, F.; Brüggemann, N.; Brodehl, A.; Mewis, I.; Schreiner, M.; Rohn, S.; Kroh, L.W. Characterization of Products from the Reaction of Glucosinolate-Derived Isothiocyanates with Cysteine and Lysine Derivatives Formed in Either Model Systems or Broccoli Sprouts. J. Agric. Food Chem. 2012, 60, 7735–7745. [Google Scholar] [CrossRef]
- Hennig, K.; de Vos, R.; Maliepaard, C.; Dekker, M.; Verkerk, R.; Bonnema, G. A metabolomics approach to identify factors influencing glucosinolate thermal degradation rates in Brassica vegetables. Food Chem. 2014, 155, 287–297. [Google Scholar] [CrossRef]
- Dekker, M.; Hennig, K.; Verkerk, R. Differences in Thermal Stability of Glucosinolates in Five Brassica Vegetables. Czech. J. Food Sci. 2009, 27, S85–S88. [Google Scholar] [CrossRef]
- Koutidou, M.; Grauwet, T.; Acharya, P. Effect of different combined mechanical and thermal treatments on the volatile fingerprint of a mixed tomato–carrot system. J. Food Eng. 2016, 168, 137–147. [Google Scholar] [CrossRef]
- Luan, C.; Zhang, M.; Fan, K.; Devahastin, S. Effective pretreatment technologies for fresh foods aimed for use in central kitchen processing. J. Sci. Food Agric. 2020, 101, 347–363. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Santos, M.V.; Zaritzky, N. Processing of pre-cooked frozen Brussels sprouts: Heat transfer modelling as related to enzyme inactivation and quality stability. Food Bioprod. Process. 2019, 118, 114–129. [Google Scholar] [CrossRef]
- Viña, S.; Olivera, D.F.; Marani, C.M.; Ferreyra, R.M.; Mugridge, A.; Chaves, A.R.; Mascheroni, R.H. Quality of Brussels sprouts (Brassica oleracea L. gemmifera DC) as affected by blanching method. J. Food Eng. 2007, 80, 218–225. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Suarez, M.; Plaza, L.; Hossain, M.B.; Brunton, N.; Lyng, J.G.; Rai, D.K. Optimization of pulsed electric field pre-treatments to enhance health-promoting glucosinolates in broccoli flowers and stalk. J. Sci. Food Agric. 2014, 95, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, E.; Saldaña, G.; Raso, J. Pulsed Electric Field Treatment for Fruit and Vegetable Processing. In Handbook of Electroporation; Springer: Cham, Switzerland, 2017; pp. 2495–2515. [Google Scholar] [CrossRef]
- Kanduser, M.; Miklavcic, D. Electroporation in Biological Cell and Tissue: An Overview. In Electrotechnologies for Extraction from Food Plants and Biomaterials; Vorobiev, E., Lebovka, N., Eds.; Springer: New York, NY, USA, 2008; pp. 1–37. [Google Scholar]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent Advances on Application of Ultrasound and Pulsed Electric Field Technologies in the Extraction of Bioactives from Agro-Industrial By-products. Food Bioprocess. Technol. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martinez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Enhancing phenolic content in carrots by pulsed electric fields during post-treatment time: Effects on cell viability and quality attributes. Innov. Food Sci. Emerg. Technol. 2019, 59, 102252. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. Applications of Pulsed Electric Fields Technology for the Food Industry. In Pulsed Electric Fields Technology for the Food Industry; Springer: Boston, MA, USA, 2006; pp. 197–221. [Google Scholar] [CrossRef]
- Blahovec, J.; Vorobiev, E.; Lebovka, N. Pulsed Electric Fields Pretreatments for the Cooking of Foods. Food Eng. Rev. 2017, 9, 226–236. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving Mass Transfer to Soften Tissues by Pulsed Electric Fields: Fundamentals and Applications. Annu. Rev. Food Sci. Technol. 2012, 3, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Moens, L.G.; De Laet, E.; Van Wambeke, J.; Van Loey, A.M.; Hendrickx, M.E. Pulsed electric field and mild thermal processing affect the cooking behaviour of carrot tissues (Daucus carota) and the degree of methylesterification of carrot pectin. Innov. Food Sci. Emerg. Technol. 2020, 66, 102483. [Google Scholar] [CrossRef]
- Mañas, P.; Vercet, A. Effect of Pulsed Electric Fields on Enzymes and Food Constituents. In Pulsed Electric Fields Technology for the Food Industry; Raso, J., Heinz, V., Eds.; Springer: New York, NY, USA, 2006; pp. 131–152. [Google Scholar]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Knorr, D.; Froehling, A.; Jaeger, H.; Reineke, K.; Schlueter, O.; Schoessler, K. Emerging Technologies in Food Processing. Annu. Rev. Food Sci. Technol. 2011, 2, 203–235. [Google Scholar] [CrossRef] [PubMed]
- Mannozzi, C.; Rompoonpol, K.; Fauster, T.; Tylewicz, U.; Romani, S.; Rosa, M.D.; Jaeger, H. Influence of Pulsed Electric Field and Ohmic Heating Pretreatments on Enzyme and Antioxidant Activity of Fruit and Vegetable Juices. Foods 2019, 8, 247. [Google Scholar] [CrossRef]
- López-Gámez, G.; Elez-Martínez, P.; Martín-Belloso, O.; Soliva-Fortuny, R. Pulsed electric fields affect endogenous enzyme activities, respiration and biosynthesis of phenolic compounds in carrots. Postharvest Biol. Technol. 2020, 168, 111284. [Google Scholar] [CrossRef]
- Nandakumar, R.; Eyres, G.T.; Burritt, D.J.; Kebede, B.; Leus, M.; Oey, I. Impact of Pulsed Electric Fields on the Volatile Compounds Produced in Whole Onions (Allium cepa and Allium fistulosum). Foods 2018, 7, 183. [Google Scholar] [CrossRef]
- Adebooye, O.C.; Vijayalakshmi, R.; Singh, V. Peroxidase activity, chlorophylls and antioxidant profile of two leaf vegetables (Solanum nigrum L. and Amaranthus cruentus L.) under six pretreatment methods before cooking. Int. J. Food Sci. Technol. 2008, 43, 173–178. [Google Scholar] [CrossRef]
- McLellan, K. The effect of heat on cabbage and Brussels sprout peroxidase enzymes. Food Chem. 1981, 7, 257–266. [Google Scholar] [CrossRef]
- Qi, D.; Zhou, Y.; Wang, J.; Fei, T.; Wu, D.; Lu, J. Determination of Volatiles in Flue-Cured Tobacco by Gas Chromatography–Mass Spectrometry (GC–MS) with Chemometrics. Anal. Lett. 2021, 55, 1398–1411. [Google Scholar] [CrossRef]
- Ren, X.; Wang, L.; Xu, B.; Wei, B.; Liu, Y.; Zhou, C.; Ma, H.; Wang, Z. Influence of microwave pretreatment on the flavor attributes and oxidative stability of cold-pressed rapeseed oil. Dry. Technol. 2018, 37, 397–408. [Google Scholar] [CrossRef]
- Buvé, C.; Neckebroeck, B.; Haenen, A.; Kebede, B.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Combining untargeted, targeted and sensory data to investigate the impact of storage on food volatiles: A case study on strawberry juice. Food Res. Int. 2018, 113, 382–391. [Google Scholar] [CrossRef]
- Grauwet, T.; Vervoort, L.; Colle, I.; Van Loey, A.; Hendrickx, M. From fingerprinting to kinetics in evaluating food quality changes. Trends Biotechnol. 2014, 32, 125–131. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Zheng, C.; Yang, Y.; Wang, X.; Hui, J.; Zhou, Q. Key Odorant Differences in Fragrant Brassica napus and Brassica juncea Oils Revealed by Gas Chromatography–Olfactometry, Odor Activity Values, and Aroma Recombination. J. Agric. Food Chem. 2020, 68, 14950–14960. [Google Scholar] [CrossRef]
- Oh, J.; Cho, I.H. The aroma profile and aroma-active compounds of Brassica oleracea (kale) tea. Food Sci. Biotechnol. 2021, 30, 1205–1211. [Google Scholar] [CrossRef]
- Marcinkowska, M.; Frank, S.; Steinhaus, M.; Jeleń, H.H. Key Odorants of Raw and Cooked Green Kohlrabi (Brassica oleracea var. gongylodes L.). J. Agric. Food Chem. 2021, 69, 12270–12277. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, X.; Yao, Y.-Z.; Wang, B.; Wei, C.-Q.; Zhang, M.; Huang, F. Characterization of the Aroma-Active Compounds in Commercial Fragrant Rapeseed Oils via Monolithic Material Sorptive Extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef]
- Petersen, K.D.; Kleeberg, K.K.; Jahreis, G.; Busch-Stockfisch, M.; Fritsche, J. Comparison of analytical and sensory lipid oxidation parameters in conventional and high-oleic rapeseed oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1193–1203. [Google Scholar] [CrossRef]
- Kebede, B.; Grauwet, T.; Tabilo-Munizaga, G.; Palmers, S.; Vervoort, L.; Hendrickx, M.E.; Van Loey, A. Headspace components that discriminate between thermal and high pressure high temperature treated green vegetables: Identification and linkage to possible process-induced chemical changes. Food Chem. 2013, 141, 1603–1613. [Google Scholar] [CrossRef]
- Wieczorek, M.N.; Majcher, M.; Jeleń, H. Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli. Foods 2020, 9, 398. [Google Scholar] [CrossRef]
- Raseetha, S.; Oey, I.; Burritt, D.; Hamid, N. Monitoring colour, volatiles in the headspace and enzyme activity to assess the quality of broccoli florets (Brassica oleracea L. italica cv. Bellstar and Legacy) during postharvest storage. Int. J. Food Sci. Technol. 2013, 49, 280–287. [Google Scholar] [CrossRef]
- Rössner, J.; Kubec, R.; Velíšek, J.; Davídek, J. Formation of aldehydes from S -alk(en)ylcysteines and their sulfoxides. Eur. Food Res. Technol. 2002, 215, 124–130. [Google Scholar] [CrossRef]
- Grebenteuch, S.; Kroh, L.W.; Drusch, S.; Rohn, S. Formation of Secondary and Tertiary Volatile Compounds Resulting from the Lipid Oxidation of Rapeseed Oil. Foods 2021, 10, 2417. [Google Scholar] [CrossRef] [PubMed]
- Grebenteuch, S.; Kanzler, C.; Klaußnitzer, S.; Kroh, L.; Rohn, S. The Formation of Methyl Ketones during Lipid Oxidation at Elevated Temperatures. Molecules 2021, 26, 1104. [Google Scholar] [CrossRef]
- Limacher, A.; Kerler, J.; Davidek, T.; Schmalzried, F.; Blank, I. Formation of Furan and Methylfuran by Maillard-Type Reactions in Model Systems and Food. J. Agric. Food Chem. 2008, 56, 3639–3647. [Google Scholar] [CrossRef]
- Pliquett, U. Joule heating during solid tissue electroporation. Med. Biol. Eng. Comput. 2003, 41, 215–219. [Google Scholar] [CrossRef]
- Akpolat, H.; Barringer, S.A. The Effect of pH and Temperature on Cabbage Volatiles During Storage. J. Food Sci. 2015, 80, S1878–S1884. [Google Scholar] [CrossRef]
 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) Dimethyl disulfide; (b) dimethyl trisulfide; (c) methyl (methylthio)methyl disulfide; (d) 4-(methylthio) butanenitrile; (e) benzyl nitrile; (f) hexanenitrile; (g) 2-butenenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
) Heat + Mix). (a) Dimethyl disulfide; (b) dimethyl trisulfide; (c) methyl (methylthio)methyl disulfide; (d) 4-(methylthio) butanenitrile; (e) benzyl nitrile; (f) hexanenitrile; (g) 2-butenenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) Dimethyl disulfide; (b) dimethyl trisulfide; (c) methyl (methylthio)methyl disulfide; (d) 4-(methylthio) butanenitrile; (e) benzyl nitrile; (f) hexanenitrile; (g) 2-butenenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
) Heat + Mix). (a) Dimethyl disulfide; (b) dimethyl trisulfide; (c) methyl (methylthio)methyl disulfide; (d) 4-(methylthio) butanenitrile; (e) benzyl nitrile; (f) hexanenitrile; (g) 2-butenenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 12).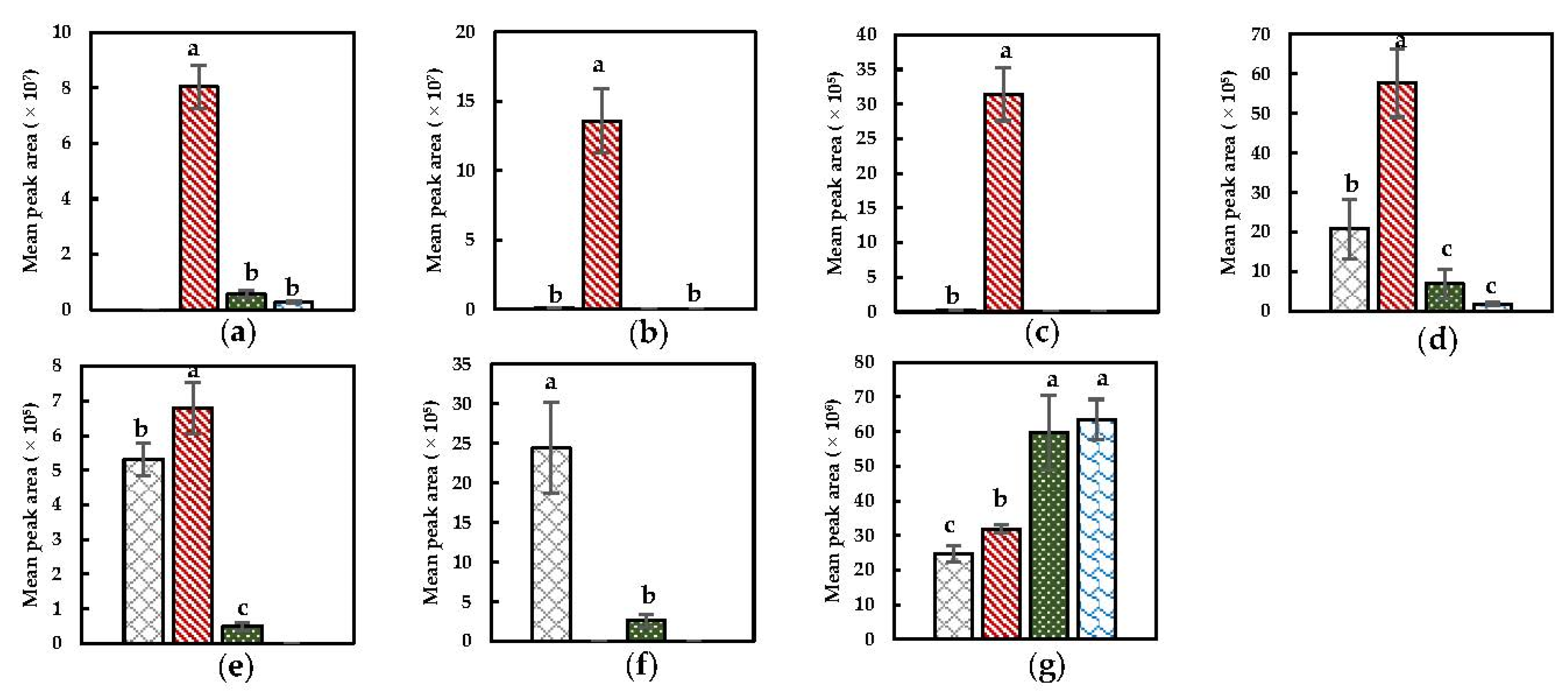
 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) 1-hexanol; (b) 3-hexen-1-ol; (c) (E)-3-hexen-1-ol; (d) 2-hexen-1-ol; (e) 1-penten-3-ol; (f) pentanal; (g) hexanal; (h) (E)-2-hexenal; (i) (E)-2-pentenal; (j) 1-penten-3-one; (k) (E,E)-2,4-heptadienal; (l) 2-ethyl furan. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
) Heat + Mix). (a) 1-hexanol; (b) 3-hexen-1-ol; (c) (E)-3-hexen-1-ol; (d) 2-hexen-1-ol; (e) 1-penten-3-ol; (f) pentanal; (g) hexanal; (h) (E)-2-hexenal; (i) (E)-2-pentenal; (j) 1-penten-3-one; (k) (E,E)-2,4-heptadienal; (l) 2-ethyl furan. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
 ) NoPT, (
) NoPT, ( ) Mix + Heat, (
) Mix + Heat, ( ) PEF + Heat + Mix, and (
) PEF + Heat + Mix, and ( ) Heat + Mix). (a) 1-hexanol; (b) 3-hexen-1-ol; (c) (E)-3-hexen-1-ol; (d) 2-hexen-1-ol; (e) 1-penten-3-ol; (f) pentanal; (g) hexanal; (h) (E)-2-hexenal; (i) (E)-2-pentenal; (j) 1-penten-3-one; (k) (E,E)-2,4-heptadienal; (l) 2-ethyl furan. Statistically significant differences are designated by different letters (p < 0.05, n = 12).
) Heat + Mix). (a) 1-hexanol; (b) 3-hexen-1-ol; (c) (E)-3-hexen-1-ol; (d) 2-hexen-1-ol; (e) 1-penten-3-ol; (f) pentanal; (g) hexanal; (h) (E)-2-hexenal; (i) (E)-2-pentenal; (j) 1-penten-3-one; (k) (E,E)-2,4-heptadienal; (l) 2-ethyl furan. Statistically significant differences are designated by different letters (p < 0.05, n = 12).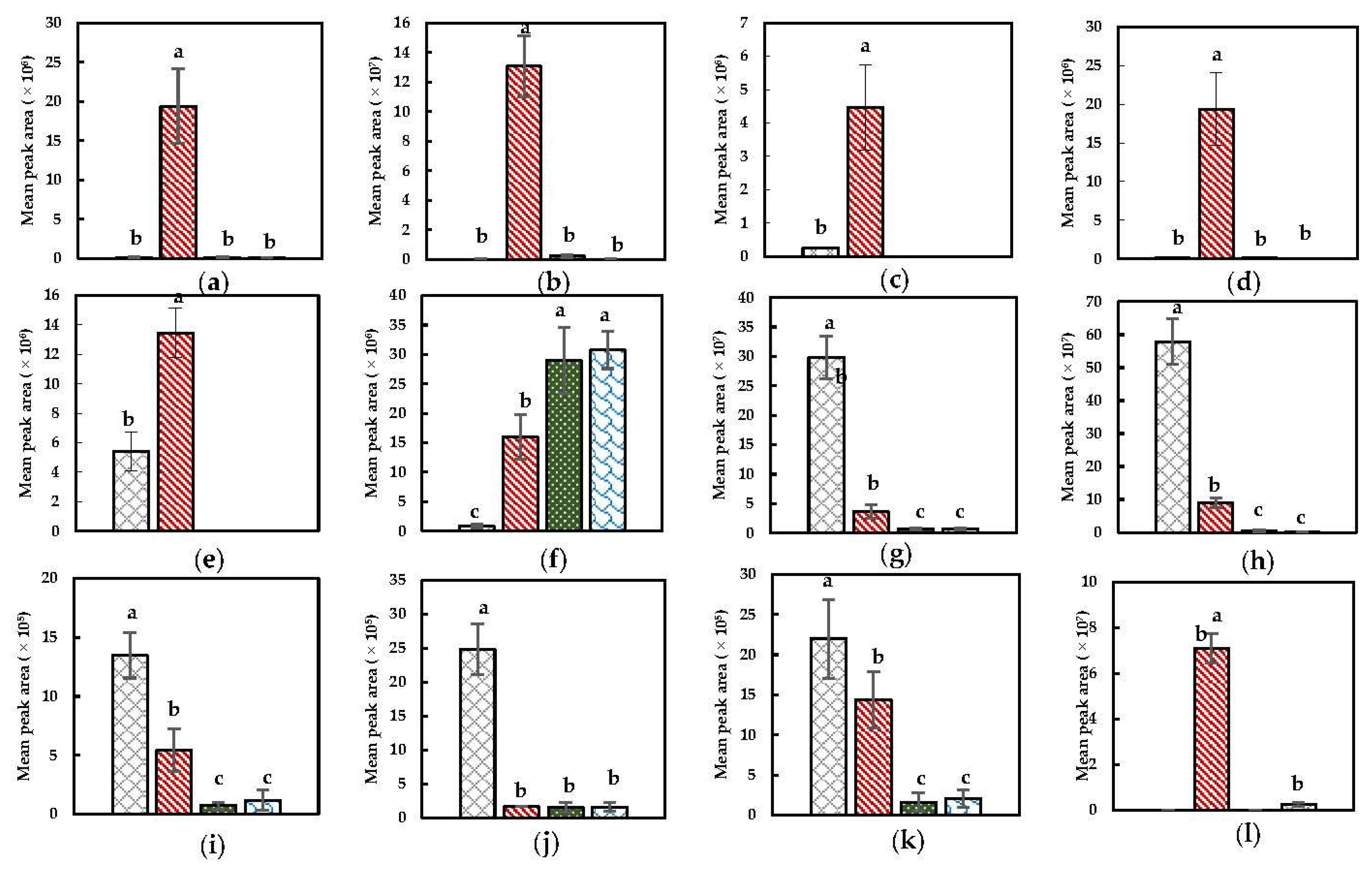
 ) NoPEF (
) NoPEF ( ), 1.01 kV/cm and 2.7 kJ/kg, (
), 1.01 kV/cm and 2.7 kJ/kg, ( ) 1.01 kV/cm and 27 kJ/kg, (
) 1.01 kV/cm and 27 kJ/kg, ( ) 3.00 kV/cm and 2.8 kJ/kg, and (
) 3.00 kV/cm and 2.8 kJ/kg, and ( ) 3.00 kV/cm and 27.7 kJ/kg). (a) Benzyl nitrile; (b) 2-butenenitrile; (c) 3-methyl butenenitrile; (d) 4-(methylthio) butanenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
) 3.00 kV/cm and 27.7 kJ/kg). (a) Benzyl nitrile; (b) 2-butenenitrile; (c) 3-methyl butenenitrile; (d) 4-(methylthio) butanenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
 ) NoPEF (
) NoPEF ( ), 1.01 kV/cm and 2.7 kJ/kg, (
), 1.01 kV/cm and 2.7 kJ/kg, ( ) 1.01 kV/cm and 27 kJ/kg, (
) 1.01 kV/cm and 27 kJ/kg, ( ) 3.00 kV/cm and 2.8 kJ/kg, and (
) 3.00 kV/cm and 2.8 kJ/kg, and ( ) 3.00 kV/cm and 27.7 kJ/kg). (a) Benzyl nitrile; (b) 2-butenenitrile; (c) 3-methyl butenenitrile; (d) 4-(methylthio) butanenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
) 3.00 kV/cm and 27.7 kJ/kg). (a) Benzyl nitrile; (b) 2-butenenitrile; (c) 3-methyl butenenitrile; (d) 4-(methylthio) butanenitrile. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
 ) NoPEF (
) NoPEF ( ), 1.01 kV/cm and 2.7 kJ/kg, (
), 1.01 kV/cm and 2.7 kJ/kg, ( ) 1.01 kV/cm and 27 kJ/kg, (
) 1.01 kV/cm and 27 kJ/kg, ( ) 3.00 kV/cm and 2.8 kJ/kg, and (
) 3.00 kV/cm and 2.8 kJ/kg, and ( ) 3.00 kV/cm and 27.7 kJ/kg). (a) Hexanal; (b) (E)-2-hexenal; (c) (Z)-2-hexenal; (d) 3-hexenal; (e) (Z)-3-hexen-1-ol; (f) (E,Z)-2,6-nonadienal; (g) (E,E)-2,4-heptadienal. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
) 3.00 kV/cm and 27.7 kJ/kg). (a) Hexanal; (b) (E)-2-hexenal; (c) (Z)-2-hexenal; (d) 3-hexenal; (e) (Z)-3-hexen-1-ol; (f) (E,Z)-2,6-nonadienal; (g) (E,E)-2,4-heptadienal. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
 ) NoPEF (
) NoPEF ( ), 1.01 kV/cm and 2.7 kJ/kg, (
), 1.01 kV/cm and 2.7 kJ/kg, ( ) 1.01 kV/cm and 27 kJ/kg, (
) 1.01 kV/cm and 27 kJ/kg, ( ) 3.00 kV/cm and 2.8 kJ/kg, and (
) 3.00 kV/cm and 2.8 kJ/kg, and ( ) 3.00 kV/cm and 27.7 kJ/kg). (a) Hexanal; (b) (E)-2-hexenal; (c) (Z)-2-hexenal; (d) 3-hexenal; (e) (Z)-3-hexen-1-ol; (f) (E,Z)-2,6-nonadienal; (g) (E,E)-2,4-heptadienal. Statistically significant differences are designated by different letters (p < 0.05, n = 6).
) 3.00 kV/cm and 27.7 kJ/kg). (a) Hexanal; (b) (E)-2-hexenal; (c) (Z)-2-hexenal; (d) 3-hexenal; (e) (Z)-3-hexen-1-ol; (f) (E,Z)-2,6-nonadienal; (g) (E,E)-2,4-heptadienal. Statistically significant differences are designated by different letters (p < 0.05, n = 6).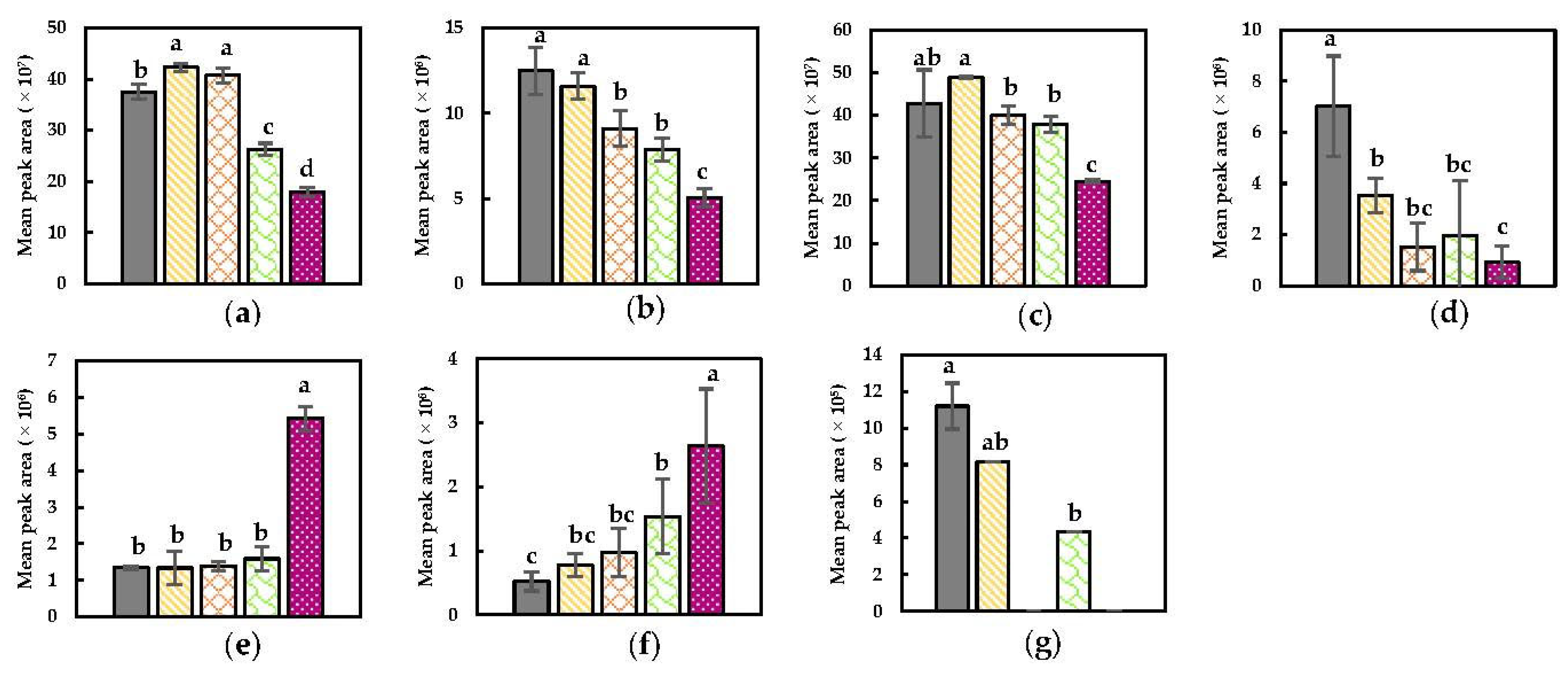
| Medium–Sized Treatment Chamber | Small–Sized Treatment Chamber | |||
|---|---|---|---|---|
| Dimensions of the electrodes (width × height × thickness) | 20.0 × 20.5 × 0.5 cm | 10.0 × 10.0 × 0.5 cm | ||
| Interelectrode distance | 29.7 cm | 10.0 cm | ||
| Volume of the treatment chamber | 12.2 L | 1.0 L | ||
| Amount of sample inserted into the treatment chamber | 650 g | 188 g | ||
| Amount of standardized tap water | 4350 mL | 300 mL | ||
| Electrical field strength at 30 kV | 1.01 kV/cm | 3.00 kV/cm | ||
| Pulse width | 225 ± 19 µs a | 233 ± 6 µs a | ||
| Energy input per pulse | 450 J/pulse | 450 J/pulse | ||
| Specific energy input per pulse | 90 J/kg·pulse | 922 J/kg·pulse | ||
| (Total) specific energy input | 2.7 kJ/kg (30 pulses) | 27 kJ/kg (300 pulses) | 2.8 kJ/kg (3 pulses) | 27.7 kJ/kg (30 pulses) |
| NoPT | ||||
|---|---|---|---|---|
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.991 | Hexanal | Aldehyde | 1096 | Fatty, green, grassy, apple, rancid, tallow c,d,e,f,g |
| 0.987 | (E)-2-Hexenal | Aldehyde | 1232 | Green, banana, fatty, apple, tallow c,f,g |
| 0.982 | Unidentified | - | 1480 | |
| 0.980 | 1,3-Pentadiene b | Alkene | 597 | |
| 0.979 | (E,E)-2,4-Hexadienal | Aldehyde | 1418 | Green, fruity, citrus, waxy g |
| 0.977 | Unidentified | - | 1150 | |
| 0.959 | (E,E)-2,4-Hexadienal | Aldehyde | 1423 | Green, fruity, citrus, waxy g |
| 0.953 | (E,Z)-2,6-Nonadienal | Aldehyde | 1599 | |
| 0.952 | Unidentified | - | 1422 | |
| 0.952 | 1-Penten-3-one | Ketone | 1028 | |
| 0.950 | Acetone | Ketone | 819 | |
| 0.937 | (E)-2-Nonenal | Aldehyde | 1549 | Rancid, cucumber, green, tallowy, fatty, oily g |
| 0.920 | (E)-2-Pentenal | Aldehyde | 1142 | Strawberry, fruity, tomato, pungent, apple g |
| 0.874 | 8-Hydroxy-2,2-dimethyl-dec-5-en-3-one b | Ketone | 1977 | |
| 0.874 | Cis-1,2-dimethyl cyclopropane b | Cycloalkane | 519 | |
| 0.873 | 2-Pentene | Alkene | 523 | |
| 0.863 | Unidentified | - | 1155 | |
| 0.833 | 5-Ethyl-2(5H)-furanone | Furanic compound | 1784 | |
| 0.828 | Hexanenitrile | Nitrile | 1315 | |
| 0.815 | Methyl thiocyanate | Thiocyanate | 1292 | |
| −0.856 | Pentanal | Aldehyde | 984 | Pungent e |
| Mix + Heat | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.988 | Unidentified | - | 1727 | |
| 0.987 | 4-Ethyl benzaldehyde | Aldehyde | 1731 | |
| 0.985 | 2-Ethyl furan | Furanic compound | 957 | Burnt, smoky, sweet c |
| 0.985 | Dimethyl disulfide | Sulfurous compound | 1088 | Onion, sulfurous, cabbage, putrid, cauliflower, ripened cheese c,d,e,h,i,j |
| 0.981 | Dimethyl trisulfide | Sulfurous compound | 1404 | Onion, sulfurous, alliaceous, fish, cabbage, spoiled, cooked, cauliflower c,d,e,f,h,j |
| 0.978 | 3-Hexen-1-ol | Alcohol | 1401 | Green, grassy j |
| 0.974 | 3-(Methylthio) nonanal b | Aldehyde | 1634 | |
| 0.971 | 2-Hexen-1-ol | Alcohol | 1639 | |
| 0.963 | 2,2,6-Trimethyl-1-cyclohexene-1-carboxaldehyde | Aldehyde | 1639 | |
| 0.955 | 1-Hexanol | Alcohol | 1367 | Resin, flower, green d |
| 0.948 | Butanal | Aldehyde | 881 | Pungent e |
| 0.939 | Cis-2-(2-pentenyl)furan | Furanic compound | 1318 | |
| 0.937 | Methyl (methylthio)methyl disulfide | Sulfurous compound | 1685 | |
| 0.925 | 4-(Methylthio) butanenitrile | Nitrile | 1813 | |
| 0.923 | 1-Penten-3-ol | Alcohol | 1167 | Buttery, pungent g |
| 0.898 | 1-Chloro pentane | Haloalkane | 944 | |
| 0.890 | (E)-3-Hexen-1-ol | Alcohol | 1379 | Green, grassy j |
| 0.889 | 5-Methyl-isoxazolidin-3-one b | Ketone | 1822 | |
| 0.867 | 2-Methyl-3-methylene-cyclopentanecarboxaldehyde b | Aldehyde | 1479 | |
| 0.846 | 2,4-Pentadienenitrile b | Nitrile | 1345 | |
| 0.841 | 4-Isothiocyanato-1-butene | Isothiocyanate | 1474 | |
| 0.821 | Unidentified | - | 946 | |
| 0.821 | 2-Ethyl thiophene | Sulfurous compound | 1186 | |
| 0.811 | 1-(Bicyclo [3.2.1]oct-2-en-4-yl)-4-phenyl-1,2,4-triazolidine-3,5-dione b | Ketone | 1781 | |
| PEF + Heat + Mix | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.877 | Pentane | Alkane | 496 | |
| 0.823 | 2-Butenenitrile | Nitrile | 1192 | Pungent d |
| −0.806 | Methyl thiocyanate | Thiocyanate | 1292 | |
| −0.863 | (E,E)-2,4-Heptadienal | Aldehyde | 1506 | Fatty, green, nut c,d,f,j |
| −0.867 | Cyano-3,4-epithiobutane b | Nitrile/sulfurous compound | 1973 | |
| −0.878 | (Z)-2-Penten-1-ol | Alcohol | 1334 | |
| −0.893 | Isothiocyanato cyclopropane b | Isothiocyanate/cycloalkane | 1861 | |
| −0.903 | Benzyl nitrile | Nitrile | 1955 | Pickled, pungent f |
| Heat + Mix | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.925 | Pentane | Alkane | 496 | |
| 0.922 | 2,4-Dimethyl hexane b | Alkane | 801 | |
| 0.911 | 2,2,4,6,6-Pentamethyl heptane | Alkane | 956 | |
| 0.909 | 2-Butenenitrile | Nitrile | 1192 | Pungent d |
| 0.885 | 3-Methyl-2-butenenitrile b | Nitrile | 1289 | |
| 0.866 | Ethyl cyclohexane | Cycloalkane | 888 | |
| 0.827 | Cis-1,3-dimethyl cyclohexane | Cycloalkane | 839 | |
| 0.819 | Pentanal | Aldehyde | 984 | Pungent e |
| −0.811 | Cyano-3,4-epithiobutane b | Nitrile/sulfurous compound | 1973 | |
| −0.822 | (E,E)-2,4-Heptadienal | Aldehyde | 1506 | Fatty, green, nut c,d,f,j |
| −0.847 | Isothiocyanato cyclopropane b | Isothiocyanate/cycloalkane | 1861 | |
| −0.853 | (Z)-2-Penten-1-ol | Alcohol | 1334 | |
| −0.890 | Benzyl nitrile | Nitrile | 1955 | Pickled, pungent f |
| NoPEF | ||||
|---|---|---|---|---|
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.955 | Ethyl cyclohexane | Cycloalkane | 886 | |
| 0.948 | Cis-1,3-dimethyl cyclohexane | Cycloalkane | 815 | |
| 0.908 | 2,2,4,6,6-Pentamethyl heptane | Alkane | 954 | |
| 0.897 | 1,3-Pentadiene b | Alkene | 596 | |
| 0.874 | 3-Ethyl-1,5-octadiene | Alkene | 1029 | |
| 0.872 | Vinyl crotonate b | Ester | 1045 | |
| 0.859 | Trans-1,2-dimethyl cyclohexane b | Cycloalkane | 838 | |
| 0.835 | 3-Hexenal | Aldehyde | 1155 | Green, leafy, fruity, apple-like c |
| 0.834 | 5,5-Dimethyl-1,3-hexadiene b | Alkene | 898 | |
| 0.828 | 2,2,4-Trimethyl pentane | Alkane | 709 | |
| 0.826 | (E,E)-2,4-Heptadienal | Aldehyde | 1479 | Fatty, green, nut c,d,e,f |
| 0.818 | 2,4-Dimethyl hexane | Alkane | 799 | |
| 0.803 | 3-Ethyl-1,5-octadiene | Alkene | 1013 | |
| 1.01 kV/cm and 2.7 kJ/kg | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| −0.806 | 4,4-Dimethyl-3-oxopentanenitrile b | Nitrile | 1247 | |
| −0.820 | Tetrahydrofuran | Furanic compound | 861 | |
| −0.906 | Benzyl nitrile | Nitrile | 1954 | Pickled, pungent f |
| 1.01 kV/cm and 27.0 kJ/kg | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.946 | 2,4-Dimethyl-1-heptene | Alkene | 881 | |
| 0.936 | 4-Methyl heptane | Alkane | 720 | |
| 0.869 | 2-Methyl-3-methylene cyclopentanecarboxaldehyde b | Aldehyde | 1479 | |
| 0.856 | 2-Methyl-3-methylene cyclopentanecarboxaldehyde b | Aldehyde | 1432 | |
| 0.815 | 1-Nonen-4-ol b | Alcohol | 1662 | |
| −0.873 | Hexanenitrile | Nitrile | 1315 | |
| 3.00 kV/cm and 2.8 kJ/kg | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.897 | Ethyl acetate | Ester | 893 | Fruity, brandy-like, pineapple c |
| 0.812 | 4-(Methylthio) butanenitrile | Nitrile | 1812 | |
| 3.00 kV/cm and 27.7 kJ/kg | ||||
| VID | Identity | Chemical Class | RI | Odor Description |
| 0.969 | 2-Butenenitrile | Nitrile | 1192 | Pungent d |
| 0.953 | (Z)-3-Hexen-1-ol | Alcohol | 1399 | Green, grass c,g |
| 0.898 | 3-Methyl butanenitrile | Nitrile | 1139 | |
| 0.886 | 4,4-Dimethyl-3-oxopentanenitrile b | Nitrile | 1247 | |
| 0.876 | (E,Z)-2,6-Nonadienal | Aldehyde | 1598 | |
| 0.837 | Benzyl nitrile | Nitrile | 1954 | Pickled, pungent f |
| 0.829 | Tetrahydrofuran | Furanic compound | 861 | |
| 0.825 | Unidentified | - | 1289 | |
| −0.888 | (Z)-2-Hexenal | Aldehyde | 1230 | |
| −0.891 | Hexanal | Aldehyde | 1095 | Fatty, green, grassy, apple, rancid, tallow c,d,f,h,i |
| −0.909 | (E)-2-Hexenal | Aldehyde | 1212 | Green, banana, fatty, apple, tallow c,f,i |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delbaere, S.M.; Bernaerts, T.; Vangrunderbeek, M.; Vancoillie, F.; Hendrickx, M.E.; Grauwet, T.; Van Loey, A.M. The Volatile Profile of Brussels Sprouts (Brassica oleracea Var. gemmifera) as Affected by Pulsed Electric Fields in Comparison to Other Pretreatments, Selected to Steer (Bio)Chemical Reactions. Foods 2022, 11, 2892. https://doi.org/10.3390/foods11182892
Delbaere SM, Bernaerts T, Vangrunderbeek M, Vancoillie F, Hendrickx ME, Grauwet T, Van Loey AM. The Volatile Profile of Brussels Sprouts (Brassica oleracea Var. gemmifera) as Affected by Pulsed Electric Fields in Comparison to Other Pretreatments, Selected to Steer (Bio)Chemical Reactions. Foods. 2022; 11(18):2892. https://doi.org/10.3390/foods11182892
Chicago/Turabian StyleDelbaere, Sophie M., Tom Bernaerts, Mirte Vangrunderbeek, Flore Vancoillie, Marc E. Hendrickx, Tara Grauwet, and Ann M. Van Loey. 2022. "The Volatile Profile of Brussels Sprouts (Brassica oleracea Var. gemmifera) as Affected by Pulsed Electric Fields in Comparison to Other Pretreatments, Selected to Steer (Bio)Chemical Reactions" Foods 11, no. 18: 2892. https://doi.org/10.3390/foods11182892
APA StyleDelbaere, S. M., Bernaerts, T., Vangrunderbeek, M., Vancoillie, F., Hendrickx, M. E., Grauwet, T., & Van Loey, A. M. (2022). The Volatile Profile of Brussels Sprouts (Brassica oleracea Var. gemmifera) as Affected by Pulsed Electric Fields in Comparison to Other Pretreatments, Selected to Steer (Bio)Chemical Reactions. Foods, 11(18), 2892. https://doi.org/10.3390/foods11182892