Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Material
2.3. Microwave-Assisted Extraction (MAE)
2.4. Spray Drying
2.5. Characterization of the Microcapsules
2.5.1. Process Yield
2.5.2. Moisture Content
2.5.3. Solubility
2.5.4. Hygroscopicity
2.5.5. Encapsulation and Loading Capacity
2.5.6. Bioavailability
2.5.7. Antioxidant Capacity
FRAP (Ferric Reducing Antioxidant Power) Assay
DPPH Radical Scavenging Assay
2.5.8. Scanning Electron Microscopy (SEM)
2.5.9. UPLC-MS/MS Analysis of Polyphenols
2.6. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Process Yield
3.2. Moisture Content
3.3. Solubility
3.4. Hygroscopicity
3.5. Encapsulation and Loading Capacity
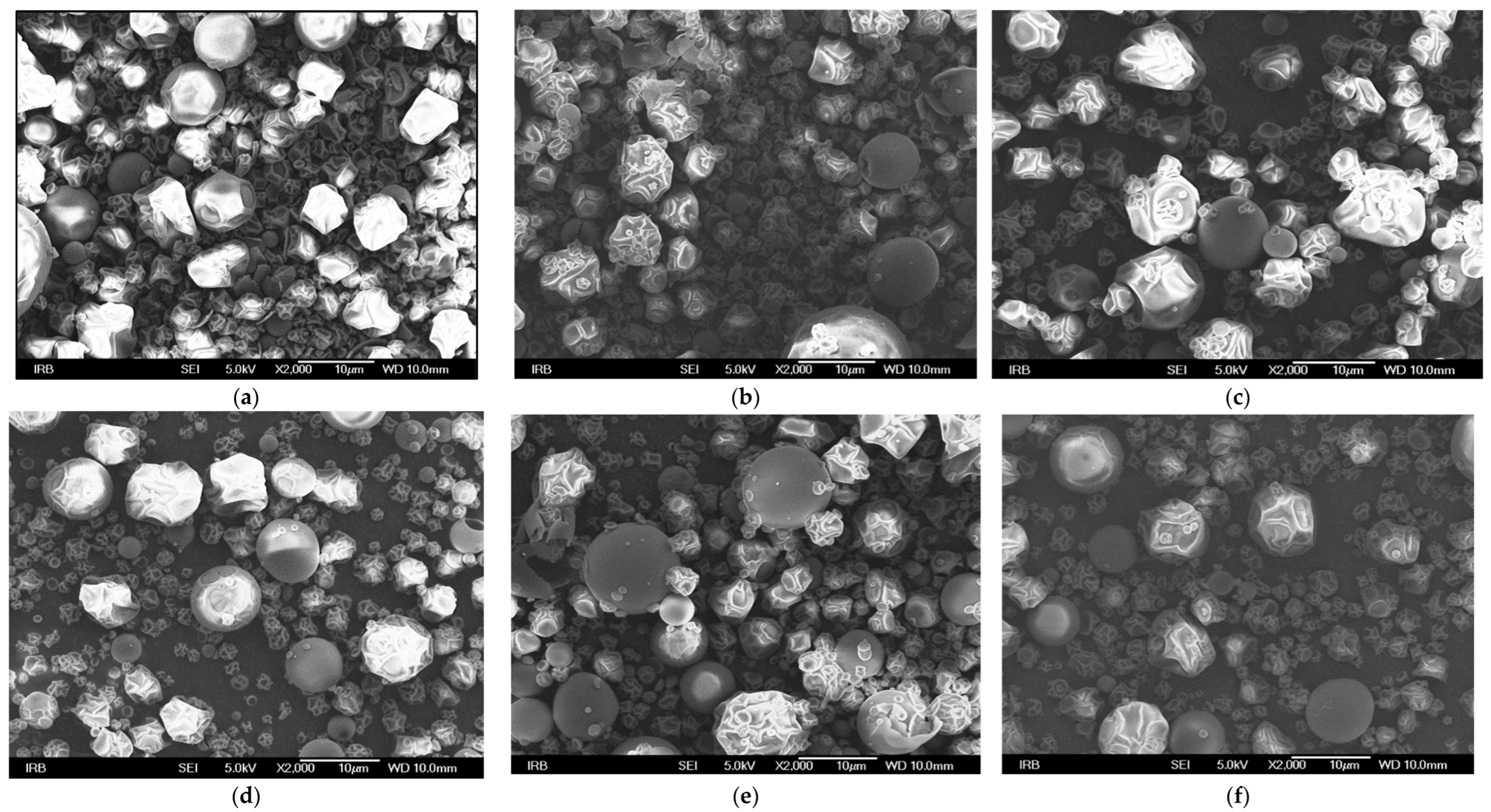
3.6. SEM Analysis
3.7. Bioavailability of Polyphenols
3.8. Antioxidant Capacity
3.9. UPLC-MS/MS Identification and Quantification of Polyphenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of Plant Extracts upon Human Health: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Bornkessel, S.; Bröring, S.; Omta, S.W.F.; van Trijp, H. What Determines Ingredient Awareness of Consumers? A Study on Ten Functional Food Ingredients. Food Qual. Prefer. 2014, 32, 330–339. [Google Scholar] [CrossRef]
- Grauso, L.; Emrick, S.; Bonanomi, G.; Lanzotti, V. Metabolomics of the Alimurgic Plants Taraxacum Officinale, Papaver Rhoeas and Urtica dioica by Combined NMR and GC–MS Analysis. Phytochem. Anal. 2019, 30, 535–546. [Google Scholar] [CrossRef]
- Di Virgilio, N.; Papazoglou, E.G.; Jankauskiene, Z.; di Lonardo, S.; Praczyk, M.; Wielgusz, K. The Potential of Stinging Nettle (Urtica dioica L.) as a Crop with Multiple Uses. Ind. Crops Prod. 2015, 68, 42–49. [Google Scholar] [CrossRef]
- Kukrić, Z.Z.; Topalić-Trivunović, L.N.; Kukavica, B.M.; Matoš, S.B.; Pavičić, S.S.; Boroja, M.M.; Savić, A.V. Characterization of Antioxidant and Antimicrobial Activities of Nettle Leaves (Urtica dioica L.). Acta Per. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Garofulić, I.E.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Yalcin, B. Phenolic Compounds Analysis of Root, Stalk, and Leaves of Nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef] [PubMed]
- Upton, R. Stinging Nettles Leaf (Urtica dioica L.): Extraordinary Vegetable Medicine. J. Herb. Med. 2013, 3, 9–38. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polysaccharides as Carriers of Polyphenols: Comparison of Freeze-Drying and Spray-Drying as Encapsulation Techniques. Molecules 2022, 27, 5069. [Google Scholar] [CrossRef] [PubMed]
- Igual, M.; García-Herrera, P.; Cámara, R.M.; Martínez-Monzó, J.; García-Segovia, P.; Cámara, M. Bioactive Compounds in Rosehip (Rosa Canina) Powder with Encapsulating Agents. Molecules 2022, 27, 4737. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray Drying for the Production of Nutraceutical Ingredients-A Review. Food Bioproc. Technol. 2012, 5, 3–14. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Morassi, A.G.; Vanzo, A.A.; Park, K.J.; Hubinger, M.D. Influence of Spray Drying Conditions on Physicochemical Properties of Chicken Meat Powder. Dry Technol. 2009, 27, 1248–1257. [Google Scholar] [CrossRef]
- Peng, Z.; Li, J.; Guan, Y.; Zhao, G. Effect of Carriers on Physicochemical Properties, Antioxidant Activities and Biological Components of Spray-Dried Purple Sweet Potato Flours. Food Sci. Technol. 2013, 51, 348–355. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Vulić, J.; Šeregelj, V.; Kalušević, A.; Lević, S.; Nedović, V.; Šaponjac, V.T.; Čanadanović-Brunet, J.; Ćetković, G. Bioavailability and Bioactivity of Encapsulated Phenolics and Carotenoids Isolated from Red Pepper Waste. Molecules 2019, 24, 2837. [Google Scholar] [CrossRef]
- Kalajahi, M.S.E.; Ghandiha, S. Optimization of Spray Drying Parameters for Encapsulation of Nettle (Urtica dioica L.) Extract. LWT 2022, 158, 113149. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Malin, V.; Repajić, M.; Zorić, Z.; Pedisić, S.; Sterniša, M.; Možina, S.S.; Dragović-Uzelac, V. Phenolic Profile, Antioxidant Capacity and Antimicrobial Activity of Nettle Leaves Extracts Obtained by Advanced Extraction Techniques. Molecules 2021, 26, 6153. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, X.; Fu, N.; Tang, X.; Sun, Y.; Lin, L. Maltodextrin: A Consummate Carrier for Spray-Drying of Xylooligosaccharides. Food Res. Inter. 2018, 106, 383–393. [Google Scholar] [CrossRef]
- Anderson, R.A.; Anderson, R.A.; Conway, H.F.; Pfiefer, V.F.; Griffin, E.L. Roll and Extrusion-Cooking of Grain Sorghum Grits. Cereal Sci. Today 1969, 14, 373–381. [Google Scholar]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chavez, J.; Saenz, C. Encapsulation of Polyphenols and Anthocyanins from Pomegranate (Punica granatum) by Spray Drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, J.; Hu, Q.; Gao, N.; Wang, S.; Sun, Y.; Yang, X. Microencapsulation of Brucea Javanica Oil: Characterization, Stability and Optimization of Spray Drying Conditions. J. Drug Deliv. Sci. Technol. 2016, 36, 46–54. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by Spray Drying of Bioactive Compounds from Cactus Pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Mcdougall, G.; Dobson, P.; Shpiro, F.; Smith, P.; Stewart, D.; Fyffe, S. Assessing Bioavailability of Soft Fruit Polyphenols In Vitro. Int. Symp. Hum. Health Eff. Fruits Veg. 2005, 744, 135–148. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P.V. Change of Phenolics, Carotenoids, and Antioxidant Capacity Following Simulated Gastrointestinal Digestion and Dialysis of Selected Edible Green Leaves. Food Chem. 2018, 245, 371–379. [Google Scholar] [CrossRef]
- Dobroslavić, E.; Elez Garofulić, I.; Šeparović, J.; Zorić, Z.; Pedisić, S.; Dragović-Uzelac, V. Pressurized Liquid Extraction as a Novel Technique for the Isolation of Laurus nobilis L. Leaf Polyphenols. Molecules 2022, 27, 5099. [Google Scholar] [CrossRef]
- Elez Garofulić, I.; Zorić, Z.; Pedisić, S.; Brnčić, M.; Dragović-Uzelac, V. UPLC-MS2 Profiling of Blackthorn Flower Polyphenols Isolated by Ultrasound-Assisted Extraction. J. Food Sci. 2018, 83, 2782–2789. [Google Scholar] [CrossRef]
- Bhandari, B.R.; Datta, N.; Howes, T. Problems Associated with Spray Drying of Sugar-Rich Foods. Dry Technol. 1997, 15, 671–684. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Effect of Spray Drying and Storage on the Stability of Bayberry Polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef]
- Vidović, S.S.; Vladić, J.Z.; Vaštag, Ž.G.; Zeković, Z.P.; Popović, L.M. Maltodextrin as a Carrier of Health Benefit Compounds in Satureja Montana Dry Powder Extract Obtained by Spray Drying Technique. Powder Technol. 2014, 258, 209–215. [Google Scholar] [CrossRef]
- Şahin-Nadeem, H.; Dinçer, C.; Torun, M.; Topuz, A.; Özdemir, F. Influence of Inlet Air Temperature and Carrier Material on the Production of Instant Soluble Sage (Salvia fruticosa Miller) by Spray Drying. Food Sci. Technol. 2013, 52, 31–38. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.D.R.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef] [PubMed]
- Şahin-Nadeem, H.; Torun, M.; Özdemir, F. Spray Drying of the Mountain Tea (Sideritis stricta) Water Extract by Using Different Hydrocolloid Carriers. Food Sci. Technol. 2011, 44, 1626–1635. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of Spray Drying Conditions on the Physical Properties of Cagaita (Eugenia dysenterica DC.) Fruit Extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Sablania, V.; Bosco, S.J.D. Optimization of Spray Drying Parameters for Murraya koenigii (Linn) Leaves Extract Using Response Surface Methodology. Powder Technol. 2018, 335, 35–41. [Google Scholar] [CrossRef]
- Tran, T.T.A.; Nguyen, H.V.H. Effects of Spray-Drying Temperatures and Carriers on Physical and Antioxidant Properties of Lemongrass Leaf Extract Powder. Beverages 2018, 4, 84. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of Spray Drying Conditions and Feed Composition on the Physical Properties of Black Mulberry Juice Powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Bastías-Montes, J.M.; Choque-Chávez, M.C.; Alarcón-Enos, J.; Quevedo-León, R.; Muñoz-Fariña, O.; Vidal-San-martín, C. Effect of Spray Drying at 150, 160, and 170 °C on the Physical and Chemical Properties of Maqui Extract (Aristotelia Chilensis (Molina) Stuntz). Chil. J. Agric. Res. 2019, 79, 144–152. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Gunjal, S.D.; Shirolkar, S.V. An Overview of Process Parameters and Spray Drying Agents Involved in Spray Drying of Herbal Extracts. Paid. J. 2020, 13, 102–118. [Google Scholar]
- Giovagnoli-Vicuña, C.; Briones-Labarca, V.; Romero, M.S.; Giordano, A.; Pizarro, S. Effect of Extraction Methods and In Vitro Bio-Accessibility of Microencapsulated Lemon Extract. Molecules 2022, 27, 4166. [Google Scholar] [CrossRef] [PubMed]
- Susantikarn, P.; Donlao, N. Optimization of Green Tea Extracts Spray Drying as Affected by Temperature and Maltodextrin Content. Int. Food Res. J. 2016, 23, 1327–1331. [Google Scholar]
- Phisut, N. Spray Drying Technique of Fruit Juice Powder: Some Factors Influencing the Properties of Product. Int. Food Res. J. 2012, 19, 1297–1306. [Google Scholar]
- Fernandes, R.V.D.B.; Borges, S.V.; Botrel, D.A. Gum Arabic/Starch/Maltodextrin/Inulin as Wall Materials on the Microencapsulation of Rosemary Essential Oil. Carbohydr. Polym. 2014, 101, 524–532. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia Ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef]
- Zokti, J.A.; Baharin, B.S.; Mohammed, A.S.; Abas, F. Green Tea Leaves Extract: Microencapsulation, Physicochemical and Storage Stability Study. Molecules 2016, 21, 940. [Google Scholar] [CrossRef]
- Ali, B.H.; Ziada, A.; Blunden, G. Biological Effects of Gum Arabic: A Review of Some Recent Research. Food Chem. Toxicol. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, G.R.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Abud-Archila, M. Spray-Drying of Cactus Pear Juice (Opuntia streptacantha): Effect on the Physicochemical Properties of Powder and Reconstituted Product. Dry Technol. 2005, 23, 955–973. [Google Scholar] [CrossRef]
- Tonon, R.V.; Freitas, S.S.; Hubinger, M.D. Spray Drying of Açai (Euterpe oleraceae Mart.) Juice: Effect of Inlet Air Temperature and Type of Carrier Agent. J. Food Process. Preserv. 2011, 35, 691–700. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, S.; Mahanta, C.L. Effect of Maltodextrin Concentration and Inlet Temperature during Spray Drying on Physicochemical and Antioxidant Properties of Amla (Emblica officinalis) Juice Powder. Food Bioprod. Process. 2014, 92, 252–258. [Google Scholar] [CrossRef]
- Zorić, Z.; Pelaić, Z.; Pedisić, S.; Elez Garofulić, I.; Bursać Kovačević, D.; Dragović–Uzelac, V. Effect of Storage Conditions on Phenolic Content and Antioxidant Capacity of Spray Dried Sour Cherry Powder. Food Sci. Technol. 2017, 79, 251–259. [Google Scholar] [CrossRef]
- Watson, M.A.; Lea, J.M.; Bett-Garber, K.L. Spray Drying of Pomegranate Juice Using Maltodextrin/Cyclodextrin Blends as the Wall Material. Food Sci. Nutr. 2017, 5, 820–826. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.H.; Yusof, Y.A.; Aziz, M.G.; Nazli, N. Mohd.; Chin, N.L.; Muhammad, S.K.S. Effects of Spray Drying Conditions of Microencapsulation of Amaranthus Gangeticus Extract on Drying Behaviour. Agric. Agric. Sci. Proc. 2014, 2, 33–42. [Google Scholar] [CrossRef]
- Shahidi, F.; Peng, H. Bioaccessibility and Bioavailability of Phenolic Compounds. J. Food Bioact. 2018, 4, 11–68. [Google Scholar] [CrossRef]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.E. Effect of in Vitro Gastrointestinal Digestion on Encapsulated and Nonencapsulated Phenolic Compounds of Carob (Ceratonia siliqua L.) Pulp Extracts and Their Antioxidant Capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef]
- Tuan, M.P.; Hoang, T.V.A.; Le, T.H.C.; Vu, N.Q.C.; Dang, T.B.O.; Dang, B.K.; Pham, D.T.M.; Mai, H.T.; Nguyen, T.T.S.; Dam, S.M.; et al. Extraction and Encapsulation of Polyphenols of Guava Leaves. Annals. Food Sci. Technol. 2016, 17, 34–40. [Google Scholar]
- Jovanović, M.; Drinić, Z.; Bigović, D.; Zdunić, G.; Mudrić, J.; Šavikin, K. Effect of Carrier Type on the Spray-Dried Willowherb (Epilobium angustifolium L.) Leaves Extract, Powder Properties and Bioactive Compounds Encapsulation. Lek. Sirovine 2021, 41, 41–45. [Google Scholar] [CrossRef]
- Bonetti, G.; Tedeschi, P.; Meca, G.; Bertelli, D.; Mañes, J.; Brandolini, V.; Maietti, A. In Vitro Bioaccessibility, Transepithelial Transport and Antioxidant Activity of Urtica dioica L. Phenolic Compounds in Nettle Based Food Products. Food Funct. 2016, 7, 4222–4230. [Google Scholar] [CrossRef]
- Bhusari, S.N.; Kumar, P. Antioxidant activities of spray dried tamarind pulp powder as affected by carrier type and their addition rate. In Proceedings of the International Conference on Food, Biological and Medical Sciences (FBMS-2014), Bangkok, Thailand, 28–29 January 2014 . [Google Scholar] [CrossRef]
- Fernandes, M.R.V.; Kabeya, L.M.; Souza, C.R.F.; Massarioli, A.P.; Alencar, S.M.; Oliveira, W.P. Antioxidant Activity of Spray-Dried Extracts of Psidium Guajava Leaves. J. Food Res. 2018, 7, 141. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Ramaswamy, H.S. Effect of Microencapsulation on Antioxidant and Antifungal Properties of Aqueous Extract of Pomegranate Peel. J. Food Sci. Technol. 2020, 57, 723–733. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.V.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica Spp.: Phenolic Composition, Safety, Antioxidant and Anti-Inflammatory Activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC Analysis of Phenolic Compounds in Leaves, Stalks, and Textile Fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]


| Sample | Carrier | Sample: Carrier Ratio | Temperature (°C) | Process Yield (%) | Moisture Content (%) | Solubility (%) | Hygroscopicity (g 100 g−1) | Encapsulation Capacity (%) | Loading Capacity (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MD | 1:1 | 120 | 74.92 ± 0.29 | 3.8 ± 0.62 | 82.21 ± 0.46 | 23.03 ± 0.11 | 97.61 ± 0.04 | 15.32 ± 0.09 |
| 2 | MD:GA (1:1) | 73.52 ± 0.03 | 7.29 ± 0.67 | 82.01 ± 0.79 | 25.93 ± 0.49 | 97.07 ± 0.16 | 14.29 ± 0.32 | ||
| 3 | MD:GA (3:1) | 73.12 ± 0.35 | 5.18 ± 0.49 | 87.46 ± 0.14 | 29.96 ± 0.25 | 97.37 ± 0.09 | 16.27 ± 0.16 | ||
| 4 | β-CD | 72.96 ± 0.82 | 4.55 ± 0.75 | 76.91 ± 1.34 | 24.26 ± 0.10 | 97.37 ± 0.26 | 17.33 ± 1.30 | ||
| 5 | β-CD:GA (1:1) | 70.87 ± 0.36 | 5.8 ± 0.37 | 90.63 ± 0.60 | 30.21 ± 1.21 | 97.59 ± 0.04 | 16.35 ± 0.22 | ||
| 6 | β-CD:GA (3:1) | 70.35 ± 0.99 | 5.49 ± 0.85 | 75.18 ± 0.72 | 27.66 ± 0.00 | 97.64 ± 0.09 | 17.67 ± 0.15 | ||
| 7 | MD | 1:2 | 75.22 ± 0.68 | 5.36 ± 0.01 | 83.75 ± 0.54 | 13.35 ± 0.65 | 98.51 ± 0.04 | 9.81 ± 0.25 | |
| 8 | MD:GA (1:1) | 73.83 ± 1.23 | 6.88 ± 0.35 | 85.18 ± 0.51 | 15.04 ± 0.27 | 98.66 ± 0.06 | 9.32 ± 0.12 | ||
| 9 | MD:GA (3:1) | 77.61 ± 0.09 | 3.27 ± 0.64 | 80.95 ± 0.12 | 21.77 ± 0.42 | 97.76 ± 0.04 | 10.59 ± 0.04 | ||
| 10 | β-CD | 74.76 ± 0.45 | 4.87 ± 0.21 | 88.86 ± 0.24 | 14.11 ± 0.32 | 97.46 ± 0.23 | 10.56 ± 0.14 | ||
| 11 | β-CD:GA (1:1) | 75.93 ± 0.42 | 4.12 ± 0.28 | 88.35 ± 0.68 | 19.49 ± 0.74 | 97.76 ± 0.11 | 11.37 ± 0.07 | ||
| 12 | β-CD:GA (3:1) | 73.49 ± 0.13 | 4.81 ± 0.53 | 78.98 ± 0.29 | 20.29 ± 0.52 | 97.51 ± 0.11 | 10.98 ± 0.48 | ||
| 13 | MD | 1:3 | 77.17 ± 0.53 | 4.11 ± 0.46 | 90.41 ± 0.24 | 14.57 ± 0.75 | 98.40 ± 0.04 | 6.69 ± 0.07 | |
| 14 | MD:GA (1:1) | 76.25 ± 0.59 | 3.74 ± 0.06 | 89.27 ± 0.57 | 18.08 ± 0.21 | 98.65 ± 0.09 | 7.24 ± 0.04 | ||
| 15 | MD:GA (3:1) | 82.42 ± 0.86 | 4.9 ± 0.6 | 91.32 ± 0.06 | 22.71 ± 0.84 | 97.72 ± 0.20 | 7.55 ± 0.08 | ||
| 16 | β-CD | 74.90 ± 0.44 | 4.49 ± 0.89 | 86.10 ± 0.75 | 14.60 ± 0.65 | 95.42 ± 0.13 | 11.02 ± 0.09 | ||
| 17 | β-CD:GA (1:1) | 81.47 ± 0.76 | 3.97 ± 0.52 | 91.43 ± 0.44 | 19.27 ± 0.54 | 97.84 ± 0.03 | 9.47 ± 0.04 | ||
| 18 | β-CD:GA (3:1) | 77.00 ± 0.45 | 3.48 ± 0.75 | 89.25 ± 0.79 | 18.19 ± 0.64 | 96.82 ± 0.05 | 9.60 ± 0.08 | ||
| 19 | MD | 1:1 | 160 | 80.35 ± 0.61 | 3.55 ± 0.83 | 85.01 ± 0.75 | 27.59 ± 0.36 | 98.14 ± 0.04 | 19.07 ± 0.26 |
| 20 | MD:GA (1:1) | 73.61 ± 0.10 | 4.9 ± 0.33 | 90.19 ± 0.03 | 32.92 ± 0.47 | 97.03 ± 0.07 | 14.31 ± 0.25 | ||
| 21 | MD:GA (3:1) | 75.06 ± 0.66 | 4.88 ± 0.64 | 87.28 ± 0.84 | 32.11 ± 0.62 | 97.26 ± 0.07 | 17.10 ± 0.06 | ||
| 22 | β-CD | 68.98 ± 0.42 | 4.9 ± 0.89 | 57.09 ± 0.15 | 25.55 ± 0.62 | 96.61 ± 0.05 | 12.88 ± 0.08 | ||
| 23 | β-CD:GA (1:1) | 64.63 ± 0.71 | 6.92 ± 0.6 | 88.24 ± 0.78 | 31.41 ± 0.23 | 97.11 ± 0.02 | 15.29 ± 0.19 | ||
| 24 | β-CD:GA (3:1) | 70.38 ± 0.23 | 4 ± 0.62 | 78.20 ± 0.92 | 29.36 ± 0.84 | 96.55 ± 0.03 | 14.50 ± 0.20 | ||
| 25 | MD | 1:2 | 77.56 ± 0.40 | 2.31 ± 0.49 | 89.59 ± 0.09 | 19.98 ± 0.41 | 97.51 ± 0.03 | 9.30 ± 0.04 | |
| 26 | MD:GA (1:1) | 75.92 ± 0.12 | 2.66 ± 0.64 | 87.88 ± 0.86 | 24.21 ± 0.91 | 97.68 ± 0.09 | 9.93 ± 0.13 | ||
| 27 | MD:GA (3:1) | 80.16 ± 0.12 | 6.43 ± 0.3 | 87.63 ± 0.17 | 21.98 ± 0.40 | 97.31 ± 0.04 | 10.07 ± 0.07 | ||
| 28 | β-CD | 74.41 ± 0.08 | 3.52 ± 0.84 | 62.13 ± 0.96 | 15.88 ± 0.35 | 96.92 ± 0.13 | 11.56 ± 0.05 | ||
| 29 | β-CD:GA (1:1) | 78.52 ± 0.84 | 2.48 ± 0.77 | 88.64 ± 0.22 | 21.85 ± 0.28 | 97.72 ± 0.05 | 12.61 ± 0.02 | ||
| 30 | β-CD:GA (3:1) | 73.85 ± 0.32 | 4.45 ± 0.11 | 80.82 ± 0.41 | 21.79 ± 0.87 | 97.23 ± 0.02 | 12.96 ± 0.03 | ||
| 31 | MD | 1:3 | 87.23 ± 0.41 | 2.98 ± 0.76 | 87.77 ± 0.85 | 13.93 ± 0.13 | 97.91 ± 0.09 | 8.69 ± 0.20 | |
| 32 | MD:GA (1:1) | 84.54 ± 0.93 | 3.61 ± 0.09 | 91.11 ± 0.80 | 15.92 ± 0.49 | 98.16 ± 0.15 | 7.61 ± 0.08 | ||
| 33 | MD:GA (3:1) | 78.59 ± 0.86 | 4.08 ± 0.55 | 83.63 ± 0.72 | 20.13 ± 0.65 | 97.90 ± 0.12 | 9.66 ± 0.18 | ||
| 34 | β-CD | 76.01 ± 0.80 | 2.65 ± 0.71 | 86.53 ± 0.04 | 16.37 ± 0.43 | 95.68 ± 0.07 | 13.20 ± 0.02 | ||
| 35 | β-CD:GA (1:1) | 81.86 ± 0.71 | 3.79 ± 0.66 | 89.51 ± 0.47 | 22.57 ± 0.37 | 97.47 ± 0.11 | 10.42 ± 0.08 | ||
| 36 | β-CD:GA (3:1) | 80.28 ± 0.70 | 3.6 ± 0.1 | 90.69 ± 0.73 | 18.25 ± 0.44 | 98.67 ± 0.07 | 20.28 ± 0.17 | ||
| 37 | MD | 1:1 | 200 | 76.32 ± 1.05 | 4.05 ± 0.61 | 90.24 ± 0.62 | 20.82 ± 0.56 | 96.68 ± 0.10 | 13.11 ± 0.05 |
| 38 | MD:GA (1:1) | 74.51 ± 0.80 | 4.82 ± 0.45 | 91.37 ± 0.14 | 22.75 ± 0.55 | 95.52 ± 0.20 | 11.68 ± 0.07 | ||
| 39 | MD:GA (3:1) | 72.41 ± 0.04 | 3.87 ± 0.19 | 88.96 ± 0.55 | 28.11 ± 0.47 | 96.56 ± 0.00 | 14.59 ± 0.05 | ||
| 40 | β-CD | 69.50 ± 0.55 | 3.27 ± 0.03 | 60.24 ± 0.53 | 24.23 ± 0.72 | 96.81 ± 0.14 | 16.10 ± 0.33 | ||
| 41 | β-CD:GA (1:1) | 72.57 ± 0.18 | 2.69 ± 0.35 | 90.36 ± 0.17 | 29.76 ± 0.85 | 96.96 ± 0.14 | 17.13 ± 0.53 | ||
| 42 | β-CD:GA (3:1) | 68.96 ± 1.03 | 1.64 ± 0.55 | 75.80 ± 1.01 | 26.01 ± 0.61 | 96.54 ± 0.18 | 15.41 ± 0.37 | ||
| 43 | MD | 1:2 | 78.25 ± 0.68 | 3.92 ± 0.6 | 86.89 ± 0.06 | 15.77 ± 0.67 | 96.68 ± 0.00 | 9.20 ± 0.12 | |
| 44 | MD:GA (1:1) | 76.60 ± 0.00 | 3.43 ± 0.27 | 89.37 ± 0.73 | 17.47 ± 0.53 | 97.32 ± 0.03 | 11.20 ± 0.10 | ||
| 45 | MD:GA (3:1) | 79.42 ± 0.71 | 4.13 ± 0.4 | 90.72 ± 0.59 | 21.31 ± 0.61 | 97.48 ± 0.03 | 10.19 ± 0.03 | ||
| 46 | β-CD | 67.96 ± 1.41 | 1.4 ± 0.04 | 64.80 ± 0.77 | 19.08 ± 0.16 | 96.03 ± 0.08 | 11.31 ± 0.08 | ||
| 47 | β-CD:GA (1:1) | 76.18 ± 0.94 | 3.87 ± 0.53 | 90.48 ± 0.85 | 26.40 ± 0.26 | 96.31 ± 0.06 | 11.49 ± 0.08 | ||
| 48 | β-CD:GA (3:1) | 74.78 ± 0.41 | 3.5 ± 0.76 | 81.52 ± 0.92 | 24.01 ± 1.18 | 96.00 ± 0.01 | 11.20 ± 0.07 | ||
| 49 | MD | 1:3 | 83.42 ± 0.07 | 3.11 ± 0.3 | 83.07 ± 0.30 | 13.64 ± 0.55 | 96.13 ± 0.03 | 7.16 ± 0.09 | |
| 50 | MD:GA (1:1) | 83.29 ± 0.48 | 3.38 ± 0.11 | 91.52 ± 0.75 | 17.10 ± 0.25 | 97.47 ± 0.09 | 7.75 ± 0.03 | ||
| 51 | MD:GA (3:1) | 81.62 ± 0.56 | 2.63 ± 0.72 | 92.83 ± 0.67 | 19.90 ± 1.30 | 97.45 ± 0.16 | 7.95 ± 0.03 | ||
| 52 | β-CD | 78.54 ± 0.81 | 2.66 ± 0.22 | 87.24 ± 0.84 | 15.63 ± 0.38 | 95.57 ± 0.10 | 13.70 ± 0.02 | ||
| 53 | β-CD:GA (1:1) | 75.44 ± 0.31 | 4.32 ± 0.15 | 90.05 ± 1.15 | 23.17 ± 0.90 | 97.37 ± 0.03 | 9.14 ± 0.24 | ||
| 54 | β-CD:GA (3:1) | 70.07 ± 0.28 | 2.51 ± 0.78 | 91.22 ± 0.92 | 18.01 ± 0.45 | 96.16 ± 0.09 | 9.24 ± 0.01 | ||
| MEAN | 75.88 | 4.02 | 89.94 | 21.62 | 97.20 | 12.01 |
| N | Process Yield (%) | Moisture Content (%) | Solubility (%) | Hygroscopicity (g 100 g−1) | Encapsulation Capacity (%) | Loading Capacity (%) | |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | p = 0.23 | p < 0.01 | p = 0.12 | p = 0.26 | p < 0.01 | p = 0.36 | |
| 120 | 36 | 75.32 ± 0.51 a | 4.78 ± 0.19 c | 85.46 ± 0.84 a | 20.70 ± 0.89 a | 97.62 ± 0.13 c | 11.75 ± 0.58 a |
| 160 | 36 | 76.77 ± 0.91 a | 3.98 ± 0.22 b | 84.00 ± 1.57 a | 22.88 ± 0.97 a | 97.38 ± 0.11 b | 12.74 ± 0.58 a |
| 200 | 36 | 75.55 ± 0.78 a | 3.29 ± 0.15 a | 85.37 ± 1.54 a | 21.29 ± 0.76 a | 96.61 ± 0.11 a | 11.53 ± 0.49 a |
| Carrier | p < 0.01 | p = 0.28 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |
| MD | 18 | 78.94 ± 0.94 b | 3.69 ± 0.21 a | 86.55 ± 0.73 ab | 18.08 ± 1.16 a | 97.51 ± 0.19 b | 10.93 ± 0.93 ab |
| MD:GA (1:1) | 18 | 76.90 ± 0.96 ab | 4.52 ± 0.37 a | 88.66 ± 0.74 c | 21.05 ± 1.35 ab | 97.51 ± 0.23 b | 10.37 ± 0.62 a |
| MD:GA (3:1) | 18 | 77.82 ± 0.83 b | 4.38 ± 0.27 a | 87.87 ± 0.86 b | 24.22 ± 1.05 b | 97.41 ± 0.09 b | 11.55 ± 0.80 ab |
| β-CD | 18 | 73.11 ± 0.83 a | 3.59 ± 0.30 a | 74.43 ± 3.04 a | 18.86 ± 1.05 a | 96.43 ± 0.21 a | 13.07 ± 0.53 ab |
| β-CD:GA (1:1) | 18 | 75.27 ± 1.24 ab | 4.22 ± 0.33 a | 89.74 ± 0.28 c | 24.90 ± 1.07 b | 97.35 ± 0.11 b | 12.08 ± 0.68 ab |
| β-CD:GA (3:1) | 18 | 73.24 ± 0.86 a | 3.72 ± 0.29 a | 82.41 ± 1.45 ab | 22.62 ± 1.00 ab | 97.01 ± 0.13 ab | 13.54 ± 0.86 b |
| Ratio sample:carrier | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.16 | p < 0.01 | |
| 1:1 | 36 | 72.39 ± 0.57 a | 4.53 ± 0.13 b | 82.08 ± 1.66 a | 27.32 ± 0.57 b | 97.02 ± 0.10 a | 15.47 ± 0.31 c |
| 1:2 | 36 | 75.80 ± 0.46 b | 3.97 ± 0.24 ab | 83.70 ± 1.35 a | 19.65 ± 0.61 a | 97.32 ± 0.12 a | 10.76 ± 0.18 b |
| 1:3 | 36 | 79.45 ± 0.70 c | 3.56 ± 0.24 a | 89.05 ± 0.46 b | 17.89 ± 0.50 a | 97.27 ± 0.17 a | 9.80 ± 0.54 a |
| Sample | Carrier | Sample: Carrier Ratio | Temperature (°C) | FRAP (mmol TE 100g−1 dm) | DPPH (mmol TE 100g−1 dm) |
|---|---|---|---|---|---|
| 31 | MD | 1:3 | 160 | 6.13 ± 0.18 a | 9.10 ± 0.08 a |
| 32 | MD:GA (1:1) | 8.57 ± 0.40 b | 12.42 ± 0.16 b | ||
| 33 | MD:GA (3:1) | 8.30 ± 0.62 b | 12.11 ± 0.03 b | ||
| 34 | β-CD | 9.59 ± 0.16 bc | 12.74 ± 0.12 b | ||
| 35 | β-CD:GA (1:1) | 10.55 ± 0.20 c | 12.13 ± 0.34 b | ||
| 36 | β-CD:GA (3:1) | 11.04 ± 0.27 cd | 15.57 ± 0.01 c |
| Mass Spectrometric Data | Concentration (mg 100 g−1) | |||
|---|---|---|---|---|
| Compound | Rt (min) | Precursor Ion (m/z) | Fragment Ion (m/z) | Encapsulated Sample 36 |
| FLAVONOLS | ||||
| Quercetin-acetyl-rutinoside | 11.317 | 653 | 303 | 1.45 ± 0.08 |
| Isorhamnetin 3-O-rutinoside | 1.384 | 625 | 317 | 0.36 ± 0.05 |
| Quercetin 3-O-rutinoside | 11.16 | 611 | 303 | 1.00 ± 0.05 |
| Quercetin-pentosyl-hexoside | 11.498 | 597 | 303 | 0.11 ± 0.03 |
| Kaempferol 3-O-rutinoside | 2.173 | 595 | 287 | 0.29 ± 0.03 |
| Kaempferol-pentosyl-hexoside | 11.344 | 581 | 287 | 0.28 ± 0.02 |
| Quercetin-acetyl-hexoside | 11.511 | 507 | 303 | 0.21 ± 0.04 |
| Kaempferol 3-O-glucoside * | 2.193 | 449 | 287 | 0.09 ± 0.03 |
| Quercetin 3-O-rhamnoside | 12.013 | 449 | 303 | 0.65 ± 0.10 |
| Quercetin-pentoside | 9.236 | 435 | 303 | 0.26 ± 0.07 |
| Kaempferol-rhamnoside | 10.551 | 433 | 287 | 0.63 ± 0.04 |
| Kaempferol-pentoside | 8.054 | 419 | 287 | 0.12 ± 0.07 |
| Quercetin | 7.732 | 301 | 151 | 0.01 ± 0.00 |
| Isorhamentin | 6.265 | 315 | 300 | 0.01 ± 0.00 |
| Myricetin * | 1.201 | 319 | 273 | 5.79 ± 0.12 |
| Kaempferol | 11.58 | 285 | 285 | 9.44 ± 0.04 |
| FLAVAN-3-OLS | ||||
| Epigallocatechin gallate * | 9.711 | 459 | 289, 139 | 0.31 ± 0.05 |
| Epicatechin gallate * | 10.872 | 443 | 291 | 0.09 ± 0.03 |
| Epicatechin | 12.067 | 291 | 139 | 57.66 ± 0.04 |
| Catechin * | 11.127 | 291 | 165 | 0.29 ± 0.07 |
| FLAVONES | ||||
| Apigenin 7-O-glucoside | 1.863 | 433 | 271 | 2.00 ± 0.11 |
| Apigenin * | 7.025 | 271 | 153 | 3.25 ± 0.05 |
| Luteolin * | 1.266 | 287 | 153 | 0.83 ± 0.07 |
| ISOFLAVONES | ||||
| Genistein | 7.65 | 269 | 133 | 2.76 ± 0.11 |
| FLAVANONES | ||||
| Naringenin * | 1.091 | 271 | 151 | 0.11 ± 0.05 |
| COUMARINS | ||||
| Umbelliferone | 0.803 | 161 | 133 | 0.99 ± 0.09 |
| Esculetin * | 1.417 | 177 | 133 | 13.59 ± 0.11 |
| Scopoletin * | 0.947 | 191 | 176 | 0.80 ± 0.05 |
| BENZOIC ACIDS | ||||
| Protocatechuic acid * | 0.807 | 153 | 109 | 14.41 ± 0.43 |
| Gallic acid * | 11.292 | 169 | 125 | 2.86 ± 0.12 |
| Syringic acid * | 10.037 | 197 | 182 | 0.25 ± 0.04 |
| Gentisic acid | 1.148 | 153 | 109 | 14.78 ± 0.63 |
| p-hydroxybenzoic acid | 11.313 | 137 | 93 | 6.70 ± 0.21 |
| CINNAMIC ACIDS | ||||
| Chlorogenic acid * | 0.909 | 353 | 191 | 1.73 ± 0.05 |
| Sinapic acid * | 4.213 | 223 | 193 | 0.25 ± 0.04 |
| Ferulic acid * | 6.544 | 193 | 178 | 8.82 ± 0.15 |
| Caffeic acid * | 1.414 | 179 | 135 | 194.82 ± 2.92 |
| p-coumaric acid * | 3.624 | 163 | 119 | 2.30 ± 0.10 |
| Cinnamic acid * | 4.465 | 147 | 103 | 828.47 ± 1.09 |
| OTHER ACIDS | ||||
| Quinic acid * | 0.786 | 191 | 85 | 109.61 ± 0.24 |
| TOTAL POLYPHENOLS | 1288.39 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegledi, E.; Garofulić, I.E.; Zorić, Z.; Roje, M.; Dragović-Uzelac, V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods 2022, 11, 2852. https://doi.org/10.3390/foods11182852
Cegledi E, Garofulić IE, Zorić Z, Roje M, Dragović-Uzelac V. Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods. 2022; 11(18):2852. https://doi.org/10.3390/foods11182852
Chicago/Turabian StyleCegledi, Ena, Ivona Elez Garofulić, Zoran Zorić, Marin Roje, and Verica Dragović-Uzelac. 2022. "Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability" Foods 11, no. 18: 2852. https://doi.org/10.3390/foods11182852
APA StyleCegledi, E., Garofulić, I. E., Zorić, Z., Roje, M., & Dragović-Uzelac, V. (2022). Effect of Spray Drying Encapsulation on Nettle Leaf Extract Powder Properties, Polyphenols and Their Bioavailability. Foods, 11(18), 2852. https://doi.org/10.3390/foods11182852










