Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of RT–Loaded Zein–CMS Nanoparticles
2.3. Determination of Particle Size, Polydispersity Index (PDI), ζ–Potential, and Turbidity
2.4. Characterization of the Complex
2.4.1. Fluorescence Spectrum Analysis
2.4.2. Fourier–Transform Infrared (FTIR) Spectroscopy
2.4.3. X–ray Diffraction (XRD)
2.4.4. Circular Dichroism (CD) Spectroscopy
2.4.5. Field Emission Scanning Electron Microscopy (FE–SEM) Analysis
2.5. Encapsulation Efficiency (EE) and Loading Capacity (LC) of Curcumin
2.6. Stability of Complex
2.6.1. Thermogravimetric Analysis (TGA)
2.6.2. pH Stability
2.7. Statistical Analysis
3. Results and Discussion
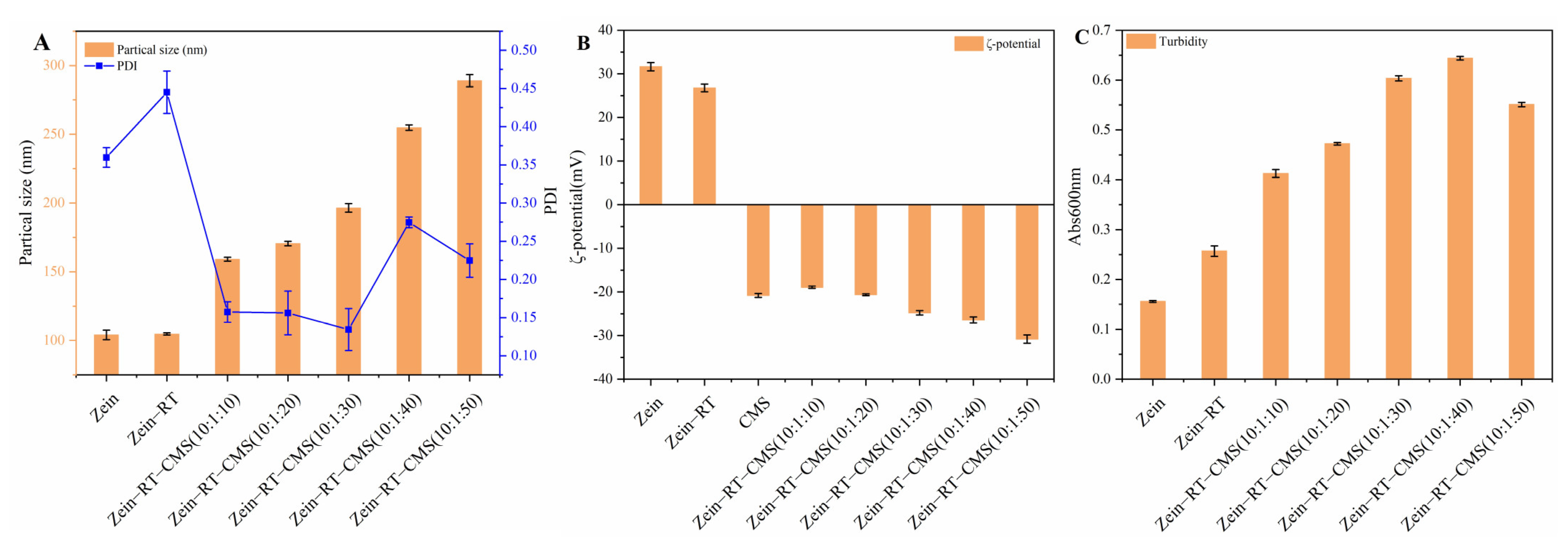
3.1. Particle Size, PDI, ζ–Potential, and Turbidity
3.2. Characterization of Nanoparticles
3.2.1. Fluorescence Spectrum Analysis
3.2.2. Fourier–Transform Infrared (FTIR) Spectroscopy
3.2.3. X–ray Diffraction (XRD)
3.2.4. Circular Dichroism (CD) Spectroscopy
3.2.5. Field Emission Scanning Electron Microscopy (FE–SEM) Analysis
3.3. Encapsulation Efficiency (EE) and Loading Capacity (LC) of RT
3.4. Stability of Composite Nanoparticles
3.4.1. TGA
3.4.2. pH Stability
4. Formation Mechanism of Composite Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Glossary
| RT | rutin |
| CMS | carboxymethyl starch |
| EE | encapsulation efficiency |
| LC | loading capacity |
| PDI | polydispersity index |
| FTIR | Fourier transform infrared |
| XRD | X–ray diffraction |
| CD | Circular dichroism |
| FE–SEM | Field Emission Scanning Electron Microscopy |
| DMSO | dimethyl sulfoxide |
References
- Ma, J.; Zheng, Y.M.; Tang, W.J.; Yan, W.X.; Nie, H.F.; Fang, J.; Liu, G. Dietary polyphenols in lipid metabolism: A role of gut microbiome. Anim. Nutr. 2020, 6, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Z.; Shen, T.; Lou, H.X. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 832–859. [Google Scholar] [CrossRef] [PubMed]
- Damin, F.; Meinhart, A.; Caldeirao, L.; Filho, M.; da Silva, L.; Constant, L.; Teixeira, J.; Wagner, R.; Godoy, H. Determination of rutin in fruits and vegetables in natura. J. Food Nutr. Res. 2019, 58, 328–338. [Google Scholar]
- Iscimen, E.M.; Berktas, S.; Cam, M.; Hayta, M. Optimization of ultrasound-assisted aqueous two-phase system (ATPS) of phenolics from apple pulp and peel. Prep. Biochem. Biotechnol. 2022, 1–8. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Kolackova, T.; Sumczynski, D.; Minarik, A.; Yalcin, E.; Orsavova, J. The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants 2022, 11, 889. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. an up-to-date review of rutin and its biological and pharmacological activities. EXCLI J. 2015, 14, 59–63. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Liu, B.G.; Zhao, J.; Thomas, D.S.; Hook, J.M. An investigation into the supramolecular structure, solubility, stability and antioxidant activity of rutin/cyclodextrin inclusion complex. Food Chem. 2013, 136, 186–192. [Google Scholar] [CrossRef]
- Hu, B.; Shen, Y.; Adamcik, J.; Fischer, P.; Schneider, M.; Loessner, M.J.; Mezzenga, R. Polyphenol-Binding Amyloid Fibrils Self-Assemble into Reversible Hydrogels with Antibacterial Activity. ACS Nano 2018, 12, 3385–3396. [Google Scholar] [CrossRef]
- Pavaloiu, R.D.; At, F.S.; Bubueanu, C.; Neagu, G.; Albulescu, A.; Hlevca, C.; Nechifor, G. Release of Polyphenols from Liposomes Loaded with Echinacea purpurea. Rev. Chim. 2018, 69, 2315–2317. [Google Scholar] [CrossRef]
- Aluani, D.; Kondeva-Burdina, M.; Tosheva, A.; Yoncheva, K.; Tzankova, V. Improvement of in vitro antioxidant activity of kaempferol by encapsulation in copolymer micelles. Pharmacia 2022, 69, 25–29. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Zhou, F.B.; Zeng, X.F.; Shen, P.H.; Yuan, D.; Zhong, M.; Zhao, Q.Z.; Zhao, M.M. pH-driven-assembled soy peptide nanoparticles as particulate emulsifier for oil-in-water Pickering emulsion and their potential for encapsulation of vitamin D-3. Food Chem. 2022, 383, 132489. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; McClements, D.J. Recent advances in colloidal delivery systems for nutraceuticals: A case study—Delivery by Design of curcumin. J. Colloid Interface Sci. 2019, 557, 506–518. [Google Scholar] [CrossRef]
- Chung, I.M.; Subramanian, U.; Thirupathi, P.; Venkidasamy, B.; Samynathan, R.; Gangadhar, B.H.; Rajakumar, G.; Thiruvengadam, M. Resveratrol Nanoparticles: A Promising Therapeutic Advancement over Native Resveratrol. Processes 2020, 8, 458. [Google Scholar] [CrossRef]
- Hu, K.; Huang, X.; Gao, Y.; Huang, X.; Xiao, H.; McClements, D.J. Core–shell biopolymer nanoparticle delivery systems: Synthesis and characterization of curcumin fortified zein–pectin nanoparticles. Food Chem. 2015, 182, 275–281. [Google Scholar] [CrossRef]
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412. [Google Scholar] [CrossRef]
- Chen, F.P.; Liu, L.L.; Tang, C.H. Spray-drying microencapsulation of curcumin nanocomplexes with soy protein isolate: Encapsulation, water dispersion, bioaccessibility and bioactivities of curcumin. Food Hydrocoll. 2020, 105, 105821. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Pho, T.V.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Oliveira, F.M.; Oliveira, R.M.; Buchweitz, L.T.G.; Pereira, J.R.; Hackbart, H.C.D.; Nalerio, E.S.; Borges, C.D.; Zambiazi, R.C. Encapsulation of olive leaf extract (Olea europaea L.) in gelatin/tragacanth gum by complex coacervation for application in sheep meat hamburger. Food Control 2022, 131, 108426. [Google Scholar] [CrossRef]
- Yue, Y.; Geng, S.; Shi, Y.; Liang, G.; Wang, J.; Liu, B. Interaction mechanism of flavonoids and zein in ethanol-water solution based on 3D-QSAR and spectrofluorimetry. Food Chem. 2019, 276, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Qin, Y.; Jiang, B.; Chen, J.J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, M.; Liu, F.; Peng, F.; Li, F.; Lou, X.; Jin, Y.; Wang, J.; Xu, H. Fabrication and characterization of zein-tea polyphenols-pectin ternary complex nanoparticles as an effective hyperoside delivery system: Formation mechanism, physicochemical stability, and in vitro release property. Food Chem. 2021, 364, 130335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Han, C.; Tian, Y.; Liu, T. Fabrication of curcumin-loaded zein nanoparticles stabilized by sodium caseinate/sodium alginate: Curcumin solubility, thermal properties, rheology, and stability. Process Biochem. 2020, 94, 30–38. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Enhancing the bioaccessibility of hydrophobic bioactive agents using mixed colloidal dispersions: Curcumin-loaded zein nanoparticles plus digestible lipid nanoparticles. Food Res. Int. 2016, 81, 74–82. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhou, C.; Zheng, P.; Zhao, M.; Liang, L. Improving Physicochemical Stability of Quercetin-Loaded Hollow Zein Particles with Chitosan/Pectin Complex Coating. Antioxidants 2021, 10, 1476. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Friciu, M.M.; Le, T.C.; Ispas-Szabo, P.; Mateescu, M.A. Carboxymethyl starch and lecithin complex as matrix for targeted drug delivery: I. Monolithic Mesalamine forms for colon delivery. Eur. J. Pharm. Biopharm. 2013, 85, 521–530. [Google Scholar] [CrossRef]
- Saboktakin, M.R.; Tabatabaie, R.M.; Maharramov, A.; Ramazanov, M.A. Synthesis and in vitro evaluation of carboxymethyl starch-chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011, 48, 381–385. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chi, C.D.; Huang, X.Y.; Zou, Q.; Li, X.X.; Chen, L. Starch-based nanocapsules fabricated through layer-by-layer assembly for oral delivery of protein to lower gastrointestinal tract. Carbohydr. Polym. 2017, 171, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of curcumin in zein/ caseinate/sodium alginate nanoparticles with improved physicochemical and controlled release properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.X.; Li, R.R.; Mao, L.K.; Liu, F.G.; Gao, Y.X. Structural characterization, formation mechanism and stability of curcumin in zein-lecithin composite nanoparticles fabricated by antisolvent co-precipitation. Food Chem. 2017, 237, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Fan, M. Interaction behaviors and structural characteristics of zein/NaTC nanoparticles. RSC Adv. 2019, 9, 5748–5755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.Y.; Yang, L.L.; Wang, S.Y.; Ying, X.G.; Ling, J.H.; Ouyang, X.K. Fabrication and characterization of zein-alginate oligosaccharide complex nanoparticles as delivery vehicles of curcumin. J. Mol. Liq. 2021, 342, 116937. [Google Scholar] [CrossRef]
- Rodriguez-Felix, F.; Del-Toro-Sanchez, C.L.; Tapia-Hernandez, J.A. A new design for obtaining of white zein micro- and nanoparticles powder: Antisolvent-dialysis method. Food Sci. Biotechnol. 2020, 29, 619–629. [Google Scholar] [CrossRef]
- Wang, X.; Peng, F.; Liu, F.; Xiao, Y.; Li, F.; Lei, H.; Wang, J.; Li, M.; Xu, H. Zein-pectin composite nanoparticles as an efficient hyperoside delivery system: Fabrication, characterization, and in vitro release property. LWT 2020, 133, 109869. [Google Scholar] [CrossRef]
- Dai, L.; Zhan, X.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Composite zein-propylene glycol alginate particles prepared using solvent evaporation: Characterization and application as Pickering emulsion stabilizers. Food Hydrocoll. 2018, 85, 281–290. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Xue, J.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Liu, C.; Zhu, J.; Fan, M.; Sun, X.; Wang, T.; Xu, Y.; Cao, Y. Fabrication of stable zein nanoparticles coated with soluble soybean polysaccharide for encapsulation of quercetin. Food Hydrocoll. 2019, 87, 342–351. [Google Scholar] [CrossRef]
- Cooper, C.L.; Dubin, P.L.; Kayitmazer, A.B.; Turksen, S. Polyelectrolyte–protein complexes. Curr. Opin. Colloid Interface Sci. 2005, 10, 52–78. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.E.; Chen, Z.X.; Wang, T.; Wang, L.; Zhong, Q.X. Biological macromolecule delivery system fabricated using zein and gum arabic to control the release rate of encapsulated tocopherol during in vitro digestion. Food Res. Int. 2018, 114, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Interaction and formation mechanism of binary complex between zein and propylene glycol alginate. Carbohydr. Polym. 2017, 157, 1638–1649. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.H.; Wang, Y.Q.; Yang, X.; Sun, C.X.; Mao, L.K.; Gao, Y.X. Zein-hyaluronic acid binary complex as a delivery vehicle of quercetagetin: Fabrication, structural characterization, physicochemical stability and in vitro release property. Food Chem. 2019, 276, 322–332. [Google Scholar] [CrossRef]
- Keppler, J.K.; Stuhldreier, M.C.; Temps, F.; Schwarz, K. Influence of mathematical models and correction factors on binding results of polyphenols and retinol with beta-lactoglobulin measured with fluorescence quenching. Food Biophys. 2014, 9, 158–168. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.R.; Hu, Q.B.; Luo, Y.C. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D.J. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef]
- Chen, S.; Sun, C.X.; Wang, Y.Q.; Han, Y.H.; Dai, L.; Abliz, A.; Gao, Y.X. Quercetagetin-Loaded Composite Nanoparticles Based on Zein and Hyaluronic Acid: Formation, Characterization, and Physicochemical Stability. J. Agric. Food Chem. 2018, 66, 7441–7450. [Google Scholar] [CrossRef]
- Rafe, A.; Razavi, S.M.A. Effect of Thermal Treatment on Chemical Structure of B-Lactoglobulin and Basil Seed Gum Mixture at Different States by ATR-FTIR Spectroscopy. Int. J. Food Prop. 2015, 18, 2652–2664. [Google Scholar] [CrossRef]
- Shinde, P.; Agraval, H.; Srivastav, A.K.; Yadav, U.C.S.; Kumar, U. Physico-chemical characterization of carvacrol loaded zein nanoparticles for enhanced anticancer activity and investigation of molecular interactions between them by molecular docking. Int. J. Pharm. 2020, 588, 119795. [Google Scholar] [CrossRef] [PubMed]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef] [PubMed]
- Tudorachi, N.; Lipsa, R.; Mustata, F.R. Thermal Degradation of Carboxymethyl Starch-g-Poly (lactic acid) Copolymer by TG-FTIR-MS Analysis. Ind. Eng. Chem. Res. 2012, 51, 15537–15545. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.; Xie, Q.-T.; Cheng, J.-S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2021, 340, 127893. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, M.; Rod, A.B. Engaging in Office Hours: A Study of Student-Faculty Interaction and Academic Performance. J. Political Sci. Educ. 2013, 9, 403–416. [Google Scholar] [CrossRef]
- Sun, C.; Dai, L.; Gao, Y. Formation and characterization of the binary complex between zein and propylene glycol alginate at neutral pH. Food Hydrocoll. 2017, 64, 36–47. [Google Scholar] [CrossRef]
- Boufi, S.; Bel Haaj, S.; Magnin, A.; Pignon, F.; Impéror-Clerc, M.; Mortha, G. Ultrasonic assisted production of starch nanoparticles: Structural characterization and mechanism of disintegration. Ultrason. Sonochem. 2018, 41, 327–336. [Google Scholar] [CrossRef]
- Ravi, G.S.; Charyulu, R.N.; Dubey, A.; Prabhu, P.; Hebbar, S.; Mathias, A.C. Nano-lipid Complex of Rutin: Development, Characterisation and In Vivo Investigation of Hepatoprotective, Antioxidant Activity and Bioavailability Study in Rats. Aaps Pharmscitech 2018, 19, 3631–3649. [Google Scholar] [CrossRef]
- Holder, C.F.; Schaak, R.E. Tutorial on Powder X-ray Diffraction for Characterizing Nanoscale Materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef]
- Wang, X.; Chu, X. Role of surfactant in the formation of zein/Tween-20 nanoparticles studied by fluorescence and circular dichroism. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 110–116. [Google Scholar] [CrossRef]
- Sun, C.X.; Dai, L.; Gao, Y.X. Binary Complex Based on Zein and Propylene Glycol Alginate for Delivery of Quercetagetin. Biomacromolecules 2016, 17, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Kittur, F.S.; Harish Prashanth, K.V.; Udaya Sankar, K.; Tharanathan, R.N. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2002, 49, 185–193. [Google Scholar] [CrossRef]
- Xu, A.Y.; Melton, L.D.; Jameson, G.B.; Williams, M.A.K.; McGillivray, D.J. Structural mechanism of complex assemblies: Characterisation of beta-lactoglobulin and pectin interactions. Soft Matter 2015, 11, 6790–6799. [Google Scholar] [CrossRef]
- Sun, C.; Xu, C.; Mao, L.; Wang, D.; Yang, J.; Gao, Y. Preparation, characterization and stability of curcumin-loaded zein-shellac composite colloidal particles. Food Chem. 2017, 228, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.G.; Ma, C.C.; McClements, D.J.; Gao, Y.X. A comparative study of covalent and non-covalent interactions between zein and polyphenols in ethanol-water solution. Food Hydrocoll. 2017, 63, 625–634. [Google Scholar] [CrossRef]
- Soradech, S.; Limatvapirat, S.; Luangtana-anan, M. Stability enhancement of shellac by formation of composite film: Effect of gelatin and plasticizers. J. Food Eng. 2013, 116, 572–580. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Zhou, Y.; Jiang, L.; Lyu, Q.; Liu, G.; Wang, X.; Chen, X.; Chen, L. Zein-whey protein isolate-carboxymethyl cellulose complex as carrier of apigenin via pH-driven method: Fabrication, characterization, stability, and in vitro release property. Food Chem. 2022, 387, 132926. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Sun, J.; Xue, C. Tremella polysaccharides-coated zein nanoparticles for enhancing stability and bioaccessibility of curcumin. Curr. Res. Food Sci. 2022, 5, 611–618. [Google Scholar] [CrossRef]
- Gonçalves da Rosa, C.; Zapelini de Melo, A.P.; Sganzerla, W.G.; Machado, M.H.; Nunes, M.R.; Vinicius de Oliveira Brisola Maciel, M.; Bertoldi, F.C.; Manique Barreto, P.L. Application in situ of zein nanocapsules loaded with Origanum vulgare Linneus and Thymus vulgaris as a preservative in bread. Food Hydrocoll. 2020, 99, 105339. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Wright, J.; Boffito, D.C.; Dubois, J.-L.; Patience, G.S. Cs, V, Cu Keggin-type catalysts partially oxidize 2-methyl-1,3-propanediol to methacrylic acid. Appl. Catal. A Gen. 2018, 554, 105–116. [Google Scholar] [CrossRef]
- Mahboub, M.J.D.; Lotfi, S.; Dubois, J.L.; Patience, G.S. Gas phase oxidation of 2-methyl-1,3-propanediol to methacrylic acid over heteropolyacid catalysts. Catal. Sci. Technol. 2016, 6, 6525–6535. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Meng, F.S.; Liu, F.J.; Lan, M.; Zou, T.T.; Li, L.H.; Cai, T.G.; Cai, Y. Preparation and evaluation of folate-modified albumin baicalin-loaded nanoparticles for the targeted treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2021, 65, 102603. [Google Scholar] [CrossRef]
- Hu, Y.; McClements, D.J.; Li, X.J.; Chen, L.; Long, J.; Jiao, A.Q.; Xie, F.; Wang, J.P.; Jin, Z.Y.; Qiu, C. Improved art bioactivity by encapsulation within cyclodextrin carboxylate. Food Chem. 2022, 384, 132429. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zhao, Z.L.; Xia, G.B.; Xue, F.; Chen, C.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020, 146, 179–192. [Google Scholar] [CrossRef]
- Cai, X.; Wang, Y.; Du, X.; Xing, X.; Zhu, G. Stability of pH-responsive Pickering emulsion stabilized by carboxymethyl starch/xanthan gum combinations. Food Hydrocoll. 2020, 109, 106093. [Google Scholar] [CrossRef]









| Zein | Zein–RT | Zein–RT–CMS (10:1:10) | Zein–RT–CMS (10:1:20) | Zein–RT–CMS (10:1:30) | Zein–RT–CMS (10:1:40) | Zein–RT–CMS (10:1:50) | |
|---|---|---|---|---|---|---|---|
| α–Helix (%) | 20.70 ± 0.21 e | 21.93 ± 0.12 c | 23.70 ± 0.25 a | 23.30 ± 0.26 b | 22.17 ± 0.10 c | 22.07 ± 0.15 c | 21.47 ± 0.06 d |
| β–sheet (%) | 28.37 ± 0.31 a | 26.67 ± 0.12 c | 25.33 ± 0.30 e | 25.80 ± 0.29 d | 27.20 ± 0.17 b | 27.33 ± 0.06 b | 28.17 ± 0.12 a |
| β–Turn (%) | 17.93 ± 0.06 a | 17.60 ± 0.06 e | 16.70 ± 0.12 f | 16.80 ± 0.06 e | 17.03 ± 0.00 d | 17.03 ± 0.06 d | 17.20 ± 0.00 c |
| Random coil (%) | 36.67 ± 0.15 d | 36.77 ± 0.21 d | 40.77 ± 0.30 a | 40.67 ± 0.26 ab | 40.40 ± 0.10 bc | 40.53 ± 0.10 abc | 40.33 ± 0.06 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Qiu, C.; Long, J.; Ji, H.; Zhao, J.; Zhou, X.; Jin, Z. Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles. Foods 2022, 11, 2827. https://doi.org/10.3390/foods11182827
Li C, Chen L, McClements DJ, Peng X, Qiu C, Long J, Ji H, Zhao J, Zhou X, Jin Z. Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles. Foods. 2022; 11(18):2827. https://doi.org/10.3390/foods11182827
Chicago/Turabian StyleLi, Cuicui, Long Chen, David Julian McClements, Xinwen Peng, Chao Qiu, Jie Long, Hangyan Ji, Jianwei Zhao, Xing Zhou, and Zhengyu Jin. 2022. "Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles" Foods 11, no. 18: 2827. https://doi.org/10.3390/foods11182827
APA StyleLi, C., Chen, L., McClements, D. J., Peng, X., Qiu, C., Long, J., Ji, H., Zhao, J., Zhou, X., & Jin, Z. (2022). Preparation and Characterization of Rutin–Loaded Zein–Carboxymethyl Starch Nanoparticles. Foods, 11(18), 2827. https://doi.org/10.3390/foods11182827











