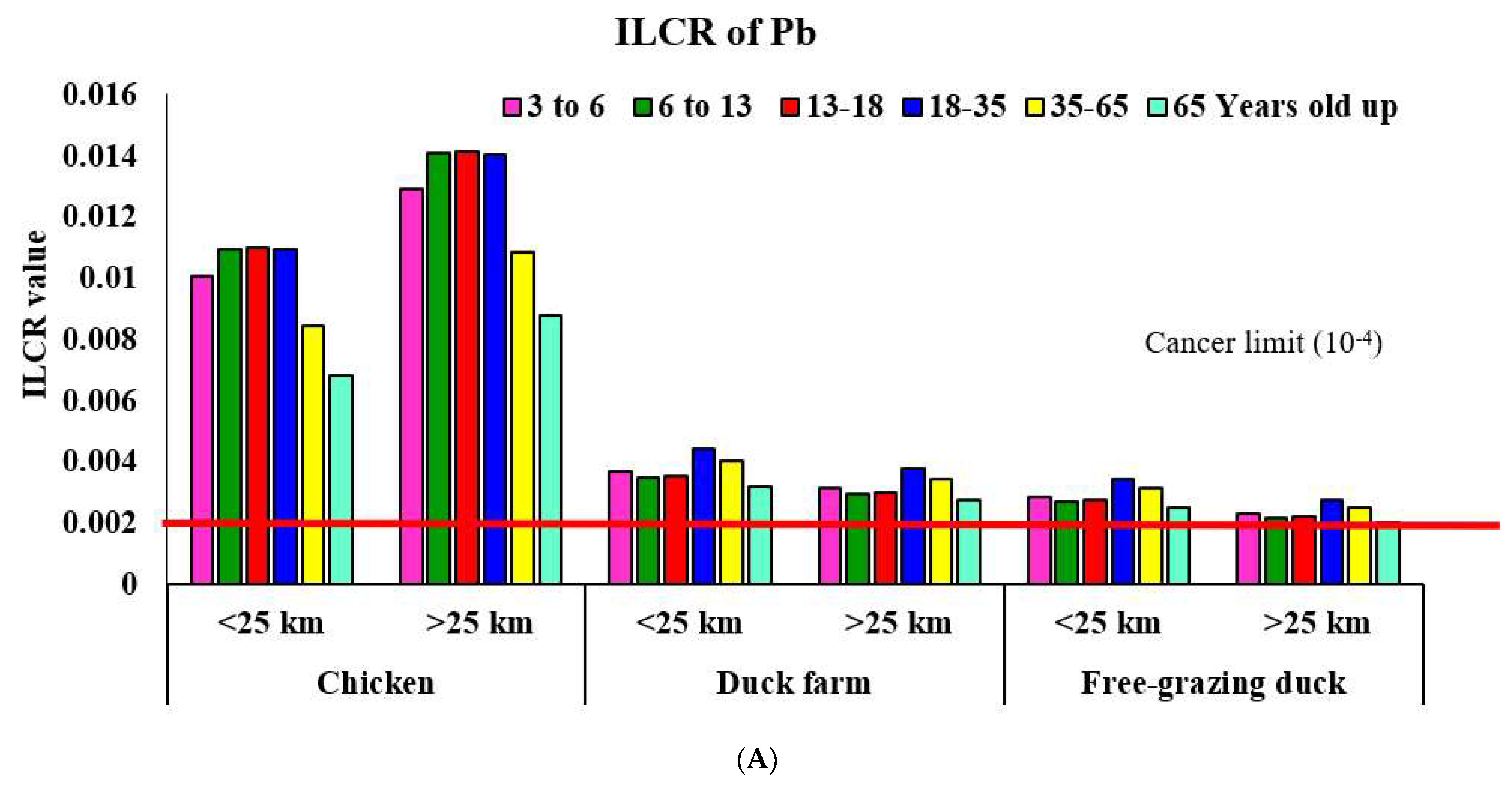
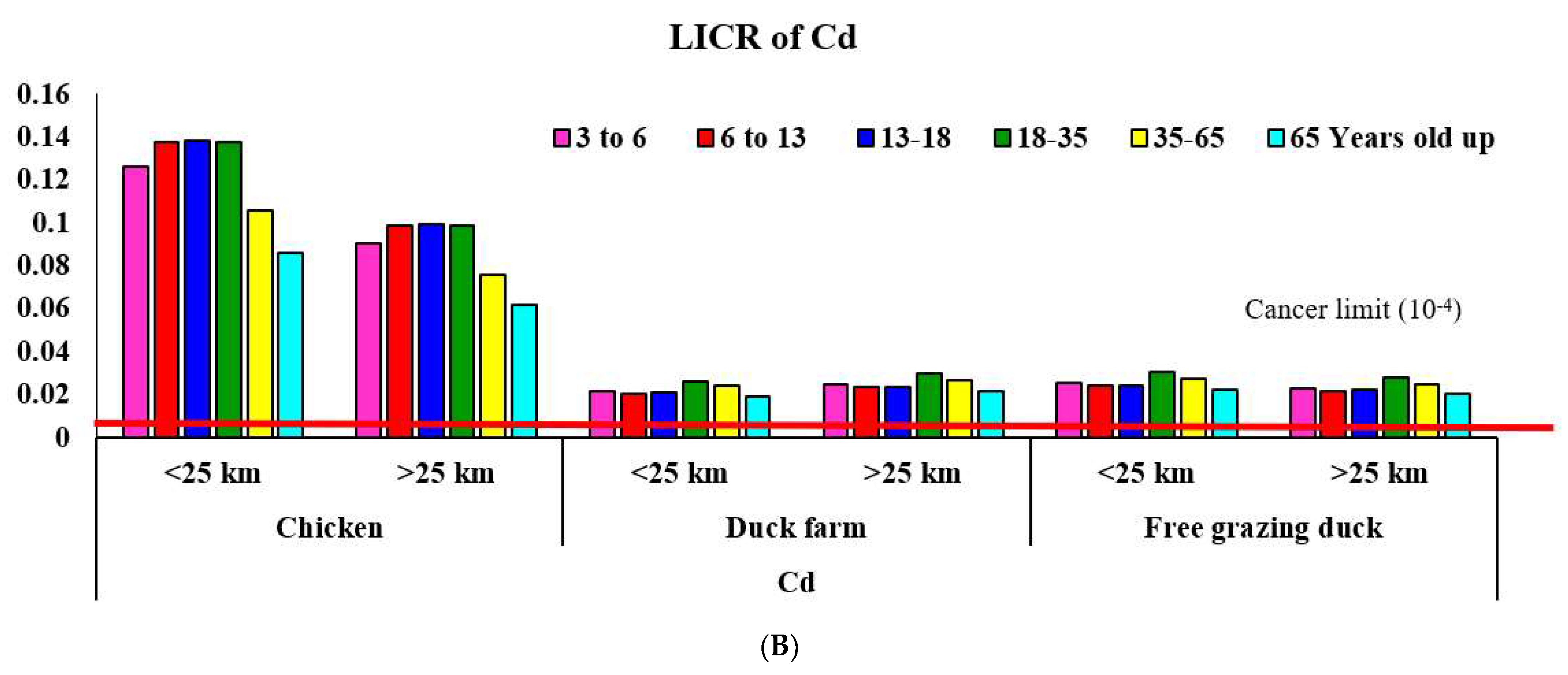
3.1.1. Area < 25 km from the Gold Mine
On farms located <25 km away from the gold mine, the average and standard variations in concentrations of Hg, Pb, and Mn in the eggs of free-grazing ducks were significantly higher than for farms located >25 km away (
p < 0.05). Moreover, the Hg concentrations in eggs from both farm ducks and free-grazing ducks were found to be 1.5–3 times higher than the standard limit set by the Ministry of Public Health of Thailand in 2020 (
Table 1). In seabirds, Hg contamination has been associated with reduced egg hatchability, possibly via altered egg-turning behavior by parents [
51]. Additionally, embryonic exposure to Hg may result in carry-over effects on later chick development [
52]. Williams et al. (2017) reported that, after Pb contamination in birds, the weight and length of bird eggs were significantly decreased, whereas lesions to the liver, kidney, spleen, and thymus were increased [
53]. In terms of Cd, the LD
50 of duck and chicken embryos was 8 μg, besides which they experienced a decrease in hatchability and hepatocyte damage [
54,
55]. The average concentrations of Cd in this study ranged between 8.94 and 13.13 μg kg
−1, which are also considered harmful levels to poultry in both areas. In blood, the average Hg concentration in free-grazing ducks was also significantly higher for farms located >25 km away from the mine (
p < 0.05;
Table 2).
People living and working in both artisanal and gold-mining areas are frequently exposed to Hg, which is used for gold extraction. It is estimated that about 15 million miners are affected globally [
56]. Additionally, exposure to other toxic metals such as arsenic (As), Pb, Cd, and Mn may occur through mining-related activities and could be ingested via air, sediment, water, or food contamination [
57,
58]. Mining activities such as excavation, crushing, and milling may result in the increased liberation of these toxic metals. Although the gold metal is collected at the end of the mining process, metals may end up in the tailing dumps at mining locations, thus presenting an exposure hazard for people living and working in these mining areas [
56]. Santos et al. (2020) found that surface-sediment samples collected in an area under the influence of gold mining were polluted (moderately to seriously) [
59]. Wilson et al. (2004) reported that Hg concentrations in blood increased during the breeding season in female birds from Northern Alaska, USA [
60]. Pb concentrations in the blood of female birds increased significantly (possibly via re-release of stored lead from bones) during incubation [
61]. The degree of contamination in the area depended on where the poultry lived, as well as species, age, sex, size, and time since the pyrite mine was opened. The trophic level influences the accumulation of metal in organs and tissue [
62,
63]. In our study, the age of farm ducks and free-grazing ducks in an area <25 km away was higher than in an area >25 km away, as shown in
Table S1, which was one of the factors correlated with heavy-metal contamination in poultry <25 km away.
Free-grazing ducks raised in fields are supplied by natural water sources, which may present a high risk of exposure to chemicals in contaminated environments [
64]. Similarly, Yabe et al. (2013) reported that free-range chickens raised near a lead–zinc mine in Zambia accumulated greater concentrations of Pb and Cd in the liver than confined broilers [
65]. Moreover, Grace and MacFarlane (2016) reported that the concentration of Pb in homegrown eggs in Australia was generally higher than in commercial eggs [
66]. In a previous study in Phichit Province, Northern Thailand, we found that Pb and Cd concentrations in the intestines of free-grazing ducks were significantly higher than in those of ducks from intensive farms, whereas Cd concentration in the livers of free-grazing ducks was also higher than in those on intensive duck farms [
67]. This study indicated that free-grazing ducks were a health risk and contamination risk due to their exposure to Hg, Pb, and Mn within 25-km areas, making it imperative to avoid grazing near gold-mine sites.
Table 1.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry egg (µgkg−1 dry weight).
Table 1.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry egg (µgkg−1 dry weight).
| Metals | Chicken | Duck Farm | Free-Grazing Duck | # Chicken Egg Limit
## Duck Egg Limit | *** Ministry of Health |
|---|
| <25 km | >25 km | <25 km | >25 km | <25 km | >25 km |
|---|
| Hg | Mean ± SD | 11.93 ± 5.08 | 17.74 ± 10.07 | 35.61 ± 16.85 | 43.90 ± 16.97 | 60.63 ± 9.42 * | 46.30 ± 3.28 * | - | 20 |
| Median | 9.82 | 14.40 | 35.57 | 44.03 | 61.57 | 45.37 |
| Min | 6.60 | 6.90 | 20.00 | 22.90 | 48.97 | 42.77 |
| Max | 19.53 | 33.10 | 51.20 | 60.90 | 71.80 | 50.93 |
| Pb | Mean ± SD | 44.38 ± 10.44 * | 57.03 ± 17.50 * | 85.78 ± 19.86 | 73.25 ± 18.41 | 66.96 ± 8.33 * | 53.52 ± 11.75 * | 100 | - |
| Median | 42.97 | 53.10 | 78.80 | 71.88 | 71.06 | 55.47 |
| Min | 32.07 | 29.85 | 66.87 | 52.20 | 53.18 | 34.70 |
| Max | 65.71 | 102.86 | 116.50 | 110.80 | 77.19 | 67.29 |
| Cd | Mean ± SD | 12.47 ± 15.01 | 8.94 ± 5.41 | 11.33 ± 7.71 | 12.84 ± 4.71 | 13.13 ± 13.23 | 11.92 ± 8.27 | - | - |
| Median | 6.32 | 7.99 | 11.19 | 13.99 | 6.92 | 7.45 |
| Min | 3.66 | 2.99 | 4.14 | 5.55 | 4.24 | 5.18 |
| Max | 65.28 | 25.46 | 25.28 | 20.31 | 45.11 | 27.44 |
| Mn | Mean ± SD | 2938.02 ± 741.92 | 3641.70 ± 1609.26 | 5178.25 ± 1425.92 | 4214.80 ± 1162.15 | 5021.75 ± 1320.39 * | 3413.13 ± 759.84 * | - | - |
| Median | 2704.64 | 3192.63 | 5474.80 | 4615.27 | 5060.37 | 3477.37 |
| Min | 1973.84 | 1794.86 | 3258.91 | 2108.09 | 3201.24 | 2259.31 |
| Max | 4759.35 | 8432.82 | 7139.69 | 5528.35 | 6920.93 | 4377.22 |
Table 2.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry blood (µL L−1).
Table 2.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry blood (µL L−1).
| Metals | Chicken | Duck Farm | Free-Grazing Duck |
|---|
| <25 km | >25 km | <25 km | >25 km | <25 km | >25 km |
|---|
| Hg | Mean ± SD | 0.96 ± 0.60 | 1.33 ± 0.81 | 2.19 ± 0.91 | 2.96 ± 1.17 | 3.07 ± 0.63 * | 2.48 ± 0.64 * |
| Median | 0.69 | 1.21 | 1.93 | 2.98 | 3.38 | 2.60 |
| Min | 0.33 | 0.29 | 1.37 | 1.27 | 2.30 | 1.58 |
| Max | 2.46 | 3.07 | 4.56 | 4.78 | 4.57 | 3.41 |
| Pb | Mean ± SD | 22.14 ± 14.85 | 20.41 ± 13.37 | 20.96 ± 6.22 | 26.62 ± 10.92 | 43.83 ± 20.27 | 33.08 ± 10.57 |
| Median | 18.70 | 15.93 | 20.53 | 23.83 | 29.30 | 23.27 |
| Min | 9.20 | 7.57 | 11.03 | 12.83 | 13.23 | 8.40 |
| Max | 77.53 | 55.97 | 32.23 | 44.73 | 74.10 | 46.60 |
| Cd | Mean ± SD | 2.92 ± 0.99 | 2.55 ± 0.73 | 5.45 ± 0.90 * | 4.50 ± 0.75 * | 5.25 ± 1.17 | 4.62 ± 0.85 |
| Median | 2.80 | 2.39 | 5.84 | 4.55 | 5.05 | 4.33 |
| Min | 1.65 | 1.50 | 4.19 | 3.35 | 4.31 | 3.47 |
| Max | 6.18 | 4.92 | 6.56 | 6.08 | 8.20 | 6.05 |
| Mn | Mean ± SD | 80.51 ± 27.48 | 72.14 ± 22.97 | 77.47 ± 20.55 | 76.31 ± 25.18 | 55.93 ± 19.66 | 45.84 ± 13.84 |
| Median | 77.14 | 72.53 | 81.54 | 78.01 | 55.86 | 45.87 |
| Min | 33.44 | 32.47 | 45.03 | 42.23 | 33.15 | 26.59 |
| Max | 149.18 | 129.10 | 118.31 | 146.56 | 88.26 | 68.37 |
Interestingly, this study revealed that there was a correlation between the Hg found in eggs in free-grazing ducks and blood at r
2 = 0.25 (
p < 0.05), as shown in
Table 3, which is consistent with the report of Heinz et al. (2010), which reported that the concentration of Hg in mallard blood was closely correlated with the concentration of Hg in their eggs (r
2 = 0.88;
p < 0.001) [
71]. Moreover, there was a correlation between the Pb found in eggs in both chickens and free-grazing ducks and blood at r
2 = 0.16 and r
2 = 0.33 (
p < 0.05), respectively, as shown in
Table 3, which is consistent with the report of Trampel et al. (2003), which found that Pb content of the egg yolks strongly correlated with blood Pb levels [
72]. Therefore, eggs and blood are considered good bioindicators for monitoring heavy-metal contamination, especially for Hg and Pb [
73,
74]. In poultry feed, we found no significant difference in heavy-metal concentrations between both areas, as shown in
Table 4. On the contrary, the average concentration of Cd in drinking water on chicken farms located in an area <25 km away (0.12 ± 0.05 μL L
−1) was significantly higher (
p < 0.05) than for those located >25 km away (0.06 ± 0.03 μL L
−1), as shown in
Table 5.
Farmers interviewed indicated that the water supplied to their animals on the intensive chicken farms located close to the mine (<25 km) came mainly from tap water (66.66%), and 33.33% came from canals and groundwater. For chicken farms located farther away from the mine (>25 km), 83.33% came from tap water and only 16.66% from canals (
Table S1). Torrance et al. (2021) reported that the geochemical data from surface water from streams around gold mining in Colombia were compared to a comprehensive data set of whole-rock analyses from drill-core and channel samples from the deposit, indicating that the deposit is significantly enriched in Pb and Cd compared to crustal averages [
75]. Therefore, gold mining may affect Cd contamination in water sources, particularly in the groundwater in this study. Dietary Cd exposure at ≥15 mg kg
−1 for 6 weeks induced hepatic damage, and increasing dietary Cd concentration increased the residues of Cd in the yolk in laying hens in China [
76]. Furthermore, there was a high correlation between the Pb (r
2 = 0.84) and Cd (r
2 = 0.42) found in drinking water and blood in free-grazing ducks in an area <25 km away at
p < 0.05, as shown in
Table 6. This is consistent with our previous study, which found a high correlation between Pb concentration in whole eggs and drinking water (r
2 = 0.806) at
p < 0.05 for the free-grazing duck farms in Central and Western Thailand [
77]. Free-grazing duck flocks raised in an area <25 km away from the gold mine mostly used 100% water (
Table S1). Thus, the canal water may be indicated as a primary source of Pb contamination in the blood of free-grazing ducks.
For the soil, there was a correlation of Pb between the soil on chicken farms in an area <25 km away and eggs at r
2 = 0.55 (
p < 0.05), as shown in
Table 7. This is consistent with a report by Waegeneers et al. (2009), which found that the Pb concentration in chicken eggs was significantly correlated to the Pb concentration in the soil in the outdoor run (r = 0.49,
p < 0.001) [
78]. Miller et al. (2004) reported significant Cd and Pb contamination of agricultural soils up to 200 km downstream of tin mines in Bolivia, with some concentrations exceeding the recommended guideline values for agricultural use in the Netherlands, Canada, and Germany. These metals flow into the soil, water (including rivers, irrigation canals, and drinking-water supplies), and crops on particular livestock and poultry farms [
79].
We also found a correlation in Mn concentrations recorded in soil and blood from chickens between the soil on chicken farms located <25 km away from the mine site (r
2 = 0.32,
p < 0.05;
Table 7). Hao et al. (2016) reported that the high concentration of Mn was likely due to residual chemicals in the soil after mining activity in China, which had a more significant impact on local water quality than terrace-field farming and poultry-breeding activities [
80]. The average concentration of Mn in drinking water on duck farms and free-grazing ducks in both areas was above the water standards for animal consumption by 5–11 times, as shown in
Table 5. The 10–100 mg kg
−1 dosages of Mn can increase apoptosis in young turkeys, increase global DNA methylation, and decrease the activity of antioxidant enzymes [
81,
82]. Interestingly, the Mn concentration in the feed from chicken and duck farms in this study was found in a range between 57 and 147 mg kg
−1, which might be a potential risk to poultry health in both areas. There was no correlation found between the feed and eggs in both areas. We found a correlation between Mn levels in the feed and blood of chickens raised on farms located <25 km away (r
2 = 0.24,
p < 0.05), as shown in
Table 8. This is consistent with the report of Zhao et al. (2019), who reported that the Mn concentrations in the plasma and heart of broilers increased linearly as dietary Mn concentration increased [
83]. Furthermore, we also found a significant correlation between Cd in the feed and blood of ducks farmed nearest to the gold mine (r
2 = 0.95,
p < 0.05;
Table 8). Thus, Cd and Mn concentrations found on the duck and chicken farms <25 km away might be related to the feed used, since the farmers used 50% commercial and 50% semi-commercial feed for duck farms and used 50% commercial and 50% commercial and semi-commercial feed for chicken farms, as shown in
Table S1. From the results of the analysis of heavy metals in animal feed, it was not found that it exceeded the standard limit but should be critically controlled for levels of heavy metals in animal feed and water sources as well as monitored regularly to assess the risks.
3.1.2. Area > 25 km away from the Gold Mine
On the contrary, the average Pb concentration in chicken eggs in an area >25 km away (57.03 ± 17.50 μg kg
−1 dry weight) was significantly higher than in an area <25 km away (44.38 ± 10.44 μg kg
−1 dry weight) at
p < 0.05, as shown in
Table 1. Surprisingly, the concentration of Hg and Cd in soil from the chicken farm was also significantly higher than in an area <25 km away, as shown in
Table 9. Pb is primarily derived from particular anthropogenic sources, such as traffic, agriculture, and coal burning. Pb exposure occurs through the production and use of Pb-containing products such as Pb gasoline, paint, and Pb pipes in water-distribution systems, indicated to be an important source of potential exposure to general organisms [
58,
84,
85]. Zarcinas et al. (2004) reported that Cd concentrations in soil in Thailand were strongly correlated with organic matter and attributed to the input of contaminants in agricultural fertilizers and soil amendments (e.g., manures, composts) [
86]. Moreover, the mobilization of Pb and Cd in soil depends on the persistence of the metal-containing particles in the atmosphere [
87]. The location of chicken farms >25 km away was mainly located 100% within the community, with the soil on the farm being dug up and brought back to make manure at 83.33%, whereas in areas <25 km was located within the community at 83.33%, with the soil on the farm dug up and brought back to make manure at 66.66%, as shown in
Table S1. The location and utilization of soil on farms was the main factor causing the Pb and Cd contamination in the >25 km area to be higher than the <25 km area. However, the concentrations of Hg, Pb, Cd, and Mn in both areas did not exceed the standards in soil for residential and agricultural uses, suggesting that the farming areas in Phichit were still safe and suitable for use in agriculture and farming. Hg and Cd contamination in soil and water may cause a significant accumulation in chicken and duck tissues, such as that found in kidneys, liver, and muscles in Spain and China [
88,
89]. On chicken farms, a correlation was found between Hg concentration in drinking water, eggs (r
2 = 0.41), and blood (r
2 = 0.25) at
p < 0.05. In addition, we found a significant correlation between Pb concentration in drinking water and chicken blood (r
2 = 0.31;
p < 0.05), as shown in
Table 6. Our study also indicated that Hg and Pb contamination in drinking water may result from tap water since 83.33% of the farmers used it to supply water to their animals (
Table S1). Although the concentration of heavy metals in water on chicken farms located >25 km away from the mine did not exceed the standard limit, monitoring tap-water quality should be carried out regularly to assess the risks.
Table 4.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry feed (mgkg−1 dry weight).
Table 4.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in poultry feed (mgkg−1 dry weight).
| Metals | Chicken | Duck Farm | Free-Grazing Duck | * Mineral Tolerance of Poultry |
|---|
| <25 km | >25 km | <25 km | >25 km | <25 km | >25 km |
|---|
| | Mean ± SD | 0.0024 ± 0.0009 | 0.0027 ± 0.0017 | 0.0045 ± 0.0028 | 0.0040 ± 0.0007 | - | - | 5 |
| Hg | Median | 0.0024 | 0.0021 | 0.0045 | 0.0043 | - | - |
| Min | 0.0013 | 0.0013 | 0.0026 | 0.0031 | - | - |
| Max | 0.0036 | 0.0059 | 0.0065 | 0.0044 | - | - |
| | Mean ± SD | 0.16 ± 0.09 | 0.14 ± 0.11 | 0.18 ± 0.12 | 0.35 ± 0.38 | - | - | 10 |
| Pb | Median | 0.13 | 0.12 | 0.18 | 0.14 | - | - |
| Min | 0.08 | 0.04 | 0.10 | 0.12 | - | - |
| Max | 0.33 | 0.27 | 0.27 | 0.79 | - | - |
| | Mean ± SD | 0.22 ± 0.12 | 0.14 ± 0.03 | 0.18 ± 0.03 | 0.15 ± 0.05 | - | - | 10 |
| Cd | Median | 0.16 | 0.15 | 0.18 | 0.12 | - | - |
| Min | 0.11 | 0.08 | 0.16 | 0.11 | - | - |
| Max | 0.44 | 0.17 | 0.20 | 0.20 | - | - |
| | Mean ± SD | 102.74 ± 28.90 | 114.50 ± 13.96 | 124.75 ± 15.75 | 130.04 ± 34.62 | - | - | 2000 |
| Mn | Median | 104.41 | 113.54 | 124.75 | 125.92 | - | - |
| Min | 57.81 | 96.90 | 113.61 | 96.90 | - | - |
| Max | 147.62 | 136.09 | 135.89 | 136.09 | - | - |
Table 5.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in drinking water (µL L−1).
Table 5.
Mean ± SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in drinking water (µL L−1).
| Metals | Chicken | Duck Farm | Free-Grazing Duck | Water Standards for Animal Consumption [91,92,93] |
|---|
| <25 km | >25 km | <25 km | >25 km | <25 km | >25 km |
|---|
| Hg | Mean ± SD | 0.0293 ± 0.0239 | 0.0176 ± 0.0095 | 0.0021 ± 0.0018 | 0.0456 ± 0.0789 | 0.0356 ± 0.0329 | 0.0125 ± 0.0042 | 10 |
| Median | 0.0225 | 0.0175 | 0.0021 | ND | 0.0200 | 0.0125 |
| Min | 0.0033 | 0.0058 | 0.0008 | ND | 0.0133 | 0.0083 |
| Max | 0.0675 | 0.0325 | 0.0033 | 0.1367 | 0.0733 | 0.0167 |
| Pb | Mean ± SD | 1.10 ± 1.30 | 0.54 ± 0.77 | 0.05 ± 0.01 | 0.19 ± 0.08 | 1.10 ± 1.01 | 1.14 ± 0.75 | 100 |
| Median | 0.44 | 0.11 | 0.05 | 0.24 | 0.54 | 1.04 |
| Min | 0.10 | 0.07 | 0.04 | 0.10 | 0.49 | 0.44 |
| Max | 3.36 | 1.95 | 0.05 | 0.24 | 2.26 | 1.94 |
| Cd | Mean ± SD | 0.12 ± 0.05 * | 0.06 ± 0.03 * | 0.21 ± 0.22 | 0.18 ± 0.13 | 0.12 ± 0.05 | 0.72 ± 0.74 | 50 |
| Median | 0.10 | 0.07 | 0.21 | 0.23 | 0.15 | 0.59 |
| Min | 0.07 | 0.01 | 0.06 | 0.03 | 0.07 | 0.05 |
| Max | 0.21 | 0.09 | 0.37 | 0.28 | 0.15 | 1.52 |
| Mn | Mean ± SD | 7.10 ± 5.09 | 17.04 ± 18.51 | 287.67 ± 393.37 | 330.43 ± 315.00 | 288.67 ± 231.63 | 560.91 ± 307.24 | 50 |
| Median | 5.19 | 10.42 | 287.67 | 360.56 | 188.10 | 729.99 |
| Min | 3.72 | 0.67 | 9.51 | 1.45 | 124.34 | 206.27 |
| Max | 17.23 | 51.17 | 565.82 | 629.28 | 553.59 | 746.48 |
Table 6.
Correlations between Hg, Pb, Cd, and Mn in eggs, blood, and drinking water (r2-value).
Table 6.
Correlations between Hg, Pb, Cd, and Mn in eggs, blood, and drinking water (r2-value).
| | | Metals | Drinking Water |
|---|
| <25 km | >25 km |
|---|
| Hg | Pb | Cd | Mn | p-Value | Hg | Pb | Cd | Mn | p-Value |
|---|
| Eggs | Chicken | Hg | 0.001 | | | | 0.9041 | 0.41 * | | | | 0.0040 |
| Pb | | 0.01 | | | 0.7151 | | 0.001 | | | 0.9050 |
| Cd | | | 0.18 | | 0.0769 | | | 0.04 | | 0.4527 |
| Mn | | | | 0.09 | 0.2293 | | | | 0.03 | 0.5100 |
| Duck farm | Hg | 0.10 | | | | 0.5639 | 0.02 | | | | 0.7435 |
| Pb | | 0.14 | | | 0.4972 | | 0.01 | | | 0.7435 |
| Cd | | | 0.69 | | 0.0583 | | | 0.37 | | 0.0857 |
| Mn | | | | 0.04 | 0.7139 | | | | 0.16 | 0.2912 |
| Free-grazing duck | Hg | 0.11 | | | | 0.3496 | 0.003 | | | | 0.8840 |
| Pb | | 0.21 | | | 0.1808 | | 0.06 | | | 0.5292 |
| Cd | | | 0.06 | | 0.5109 | | | 0.03 | | 0.6682 |
| Mn | | | | 0.04 | 0.5563 | | | | 0.19 | 0.2440 |
| Blood | Chicken | Hg | 0.06 | | | | 0.3120 | 0.25 * | | | | 0.0331 |
| Pb | | 0.002 | | | 0.8571 | | 0.31 * | | | 0.0157 |
| Cd | | | 0.002 | | 0.8480 | | | 0.001 | | 0.8804 |
| Mn | | | | 0.08 | 0.2610 | | | | 0.18 | 0.0762 |
| Duck farm | Hg | 0.01 | | | | 0.9194 | 0.08 | | | | 0.4630 |
| Pb | | 0.19 | | | 0.4194 | | 0.01 | | | 0.8100 |
| Cd | | | 0.04 | | 0.7139 | | | 0.04 | | 0.6134 |
| Mn | | | | 0.01 | 0.9194 | | | | 0.001 | 0.9484 |
| Free-grazing duck | Hg | 0.17 | | | | 0.2359 | 0.13 | | | | 0.3309 |
| Pb | | 0.84 * | | | 0.0002 | | 0.003 | | | 0.8979 |
| Cd | | | 0.42 * | | 0.0443 | | | 0.28 | | 0.1392 |
| Mn | | | | 0.01 | 0.8287 | | | | 0.08 | 0.4600 |
Table 7.
Correlations between Hg, Pb, Cd, and Mn in eggs, blood, and soil (r2-value).
Table 7.
Correlations between Hg, Pb, Cd, and Mn in eggs, blood, and soil (r2-value).
| | | Metals | Soil |
|---|
| <25 km | >25 km |
|---|
| Hg | Pb | Cd | Mn | P-value | Hg | Pb | Cd | Mn | p-value |
|---|
| Eggs | Chicken | Hg | 0.12 | | | | 0.1575 | 0.02 | | | | 0.5977 |
| Pb | | 0.55 * | | | 0.0004 | | 0.02 | | | 0.5479 |
| Cd | | | 0.001 | | 0.8997 | | | 0.0003 | | 0.9449 |
| Mn | | | | 0.01 | 0.7664 | | | | 0.04 | 0.4136 |
| Duck farm | Hg | 0.14 | | | | 0.4972 | 0.02 | | | | 0.7435 |
| Pb | | 0.01 | | | 0.9194 | | 0.02 | | | 0.7435 |
| Cd | | | 0.10 | | 0.5639 | | | 0.25 | | 0.1777 |
| Mn | | | | 0.43 | 0.1750 | | | | 0.13 | 0.3363 |
| Free-grazing duck | Hg | 0.004 | | | | 0.8687 | 0.08 | | | | 0.4630 |
| Pb | | 0.05 | | | 0.5809 | | 0.28 | | | 0.1475 |
| Cd | | | 0.05 | | 0.5809 | | | 0.05 | | 0.5809 |
| Mn | | | | 0.11 | 0.3853 | | | | 0.003 | 0.9116 |
| Blood | Chicken | Hg | 0.01 | | | | 0.7507 | 0.12 | | | | 0.1659 |
| Pb | | 0.02 | | | 0.6042 | | 0.05 | | | 0.3667 |
| Cd | | | 0.01 | | 0.7109 | | | 0.09 | | 0.2260 |
| Mn | | | | 0.32 * | 0.0147 | | | | 0.04 | 0.4184 |
| Duck farm | Hg | 0.001 | | | | 1.0000 | 0.01 | | | | 0.8100 |
| Pb | | 0.10 | | | 0.5639 | | 0.05 | | | 0.5517 |
| Cd | | | 0.02 | | 0.8028 | | | 0.12 | | 0.3586 |
| Mn | | | | 0.36 | 0.2417 | | | | 0.08 | 0.4630 |
| Free-grazing duck | Hg | 0.06 | | | | 0.5206 | 0.09 | | | | 0.4366 |
| Pb | | 0.23 | | | 0.1938 | | 0.004 | | | 0.8801 |
| Cd | | | 0.01 | | 0.8432 | | | 0.08 | | 0.4630 |
| Mn | | | | 0.04 | 0.6134 | | | | 0.02 | 0.7435 |
Table 8.
Correlations between THg, Pb, Cd, and Mn in eggs, blood, and feed (r2-value).
Table 8.
Correlations between THg, Pb, Cd, and Mn in eggs, blood, and feed (r2-value).
| Metals | Feed |
|---|
| <25 km | >25 km |
|---|
| Hg | Pb | Cd | Mn | p-Value | Hg | Pb | Cd | Mn | p-Value |
|---|
| Eggs | Chicken | Hg | 0.14 | | | | 0.1328 | 0.19 | | | | 0.0675 |
| Pb | | 0.13 | | | 0.1370 | | 0.02 | | | 0.5416 |
| Cd | | | 8.6×10−5 | | 0.9708 | | | 0.10 | | 0.2033 |
| Mn | | | | 0.07 | 0.2993 | | | | 0.20 | 0.0630 |
| Duck farm | Hg | 0.001 | | | | 1.0000 | 0.22 | | | | 0.2125 |
| Pb | | 0.29 | | | 0.2972 | | 0.19 | | | 0.2499 |
| Cd | | | 0.24 | | 0.3556 | | | 0.01 | | 0.7756 |
| Mn | | | | 0.43 | 0.1750 | | | | 0.02 | 0.7081 |
| Blood | Chicken | Hg | 0.003 | | | | 0.8293 | 0.19 | | | | 0.0707 |
| Pb | | 0.01 | | | 0.6625 | | 0.03 | | | 0.4616 |
| Cd | | | 0.06 | | 0.3365 | | | 0.05 | | 0.3495 |
| Mn | | | | 0.24 * | 0.0399 | | | | 0.0005 | 0.9320 |
| Duck farm | Hg | 0.001 | | | | 1.0000 | 0.06 | | | | 0.5517 |
| Pb | | 0.001 | | | 1.0000 | | 0.06 | | | 0.5206 |
| Cd | | | 0.95 * | | 0.0010 | | | 0.02 | | 0.7081 |
| Mn | | | | 0.07 | 0.6583 | | | | 0.13 | 0.3363 |
Table 9.
Mean ±SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in soil (mg kg−1).
Table 9.
Mean ±SD, the median, minimum, and maximum values of Hg, Pb, Cd, and Mn concentrations in soil (mg kg−1).
| Metals | Chicken | Duck Farm | Free-Grazing Duck | ** Soil Standard Limit |
|---|
| <25 km | >25 km | <25 km | >25 km | <25 km | >25 km |
|---|
| Hg | Mean ± SD | 0.0115 ± 0.0034 * | 0.0318 ± 0.0238 * | 0.0100 ± 0.0017 | 0.0207 ± 0.0008 | 0.0177 ± 0.0007 | 0.0236 ± 0.0024 | 22 |
| Median | 0.0105 | 0.0232 | 0.0100 | 0.0206 | 0.0176 | 0.0246 |
| Min | 0.0091 | 0.0158 | 0.0087 | 0.0200 | 0.0171 | 0.0209 |
| Max | 0.0183 | 0.0792 | 0.0112 | 0.0216 | 0.0185 | 0.0253 |
| Pb | Mean ± SD | 5.99 ± 2.04 | 9.75 ± 4.28 | 6.58 ± 7.58 | 5.39 ± 2.27 | 7.41 ± 5.14 | 14.62 ± 3.31 | 400 |
| Median | 5.78 | 10.75 | 6.58 | 5.66 | 10.05 | 14.57 |
| Min | 2.62 | 4.27 | 1.22 | 3.00 | 1.49 | 11.33 |
| Max | 8.16 | 14.12 | 11.93 | 7.51 | 10.70 | 17.95 |
| Cd | Mean ± SD | 0.16 ± 0.05 * | 0.28 ± 0.09 * | 0.17 ± 0.09 | 0.36 ± 0.15 | 0.11 ± 0.02 | 0.19 ± 0.08 | 67 |
| Median | 0.14 | 0.30 | 0.17 | 0.29 | 0.12 | 0.21 |
| Min | 0.11 | 0.15 | 0.11 | 0.24 | 0.09 | 0.10 |
| Max | 0.25 | 0.41 | 0.24 | 0.53 | 0.12 | 0.26 |
| Mn | Mean ± SD | 502.19 ± 237.46 | 509.55 ± 37.81 | 483.10 ± 192.66 | 631.64 ± 212.81 | 304.86 ± 106.95 | 449.89 ± 175.51 | 1710 |
| Median | 450.62 | 523.89 | 483.10 | 608.46 | 340.52 | 421.19 |
| Min | 257.34 | 437.28 | 346.87 | 431.37 | 184.64 | 290.51 |
| Max | 859.13 | 536.73 | 619.33 | 855.10 | 389.43 | 637.99 |