Trigeminal Stimulus Menthol Masks Bitter Off-Flavor of Artificial Sweetener Acesulfame-K
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sensory Panels
2.3. Test Procedure
2.4. Test Evaluation
3. Results and Discussion
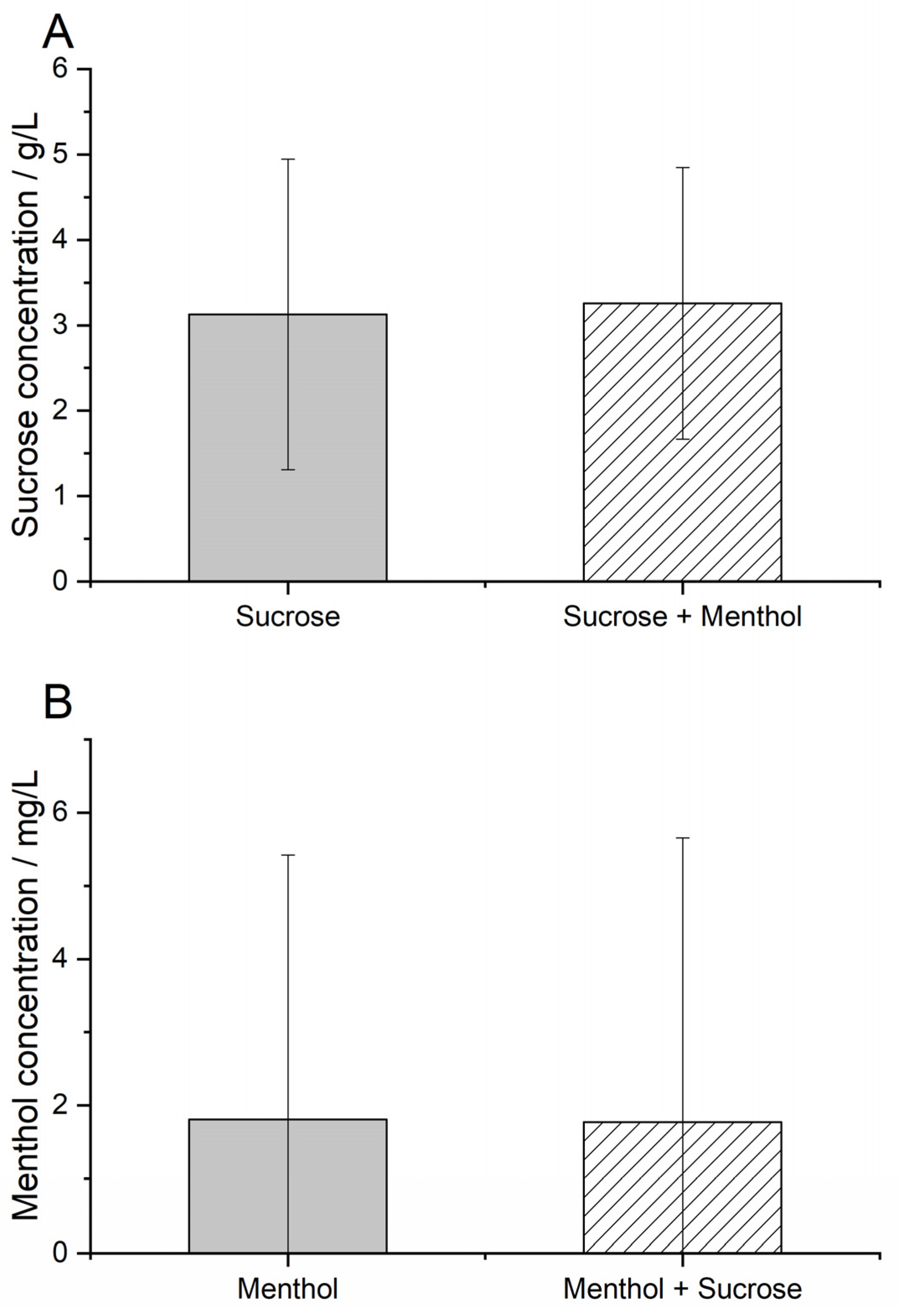
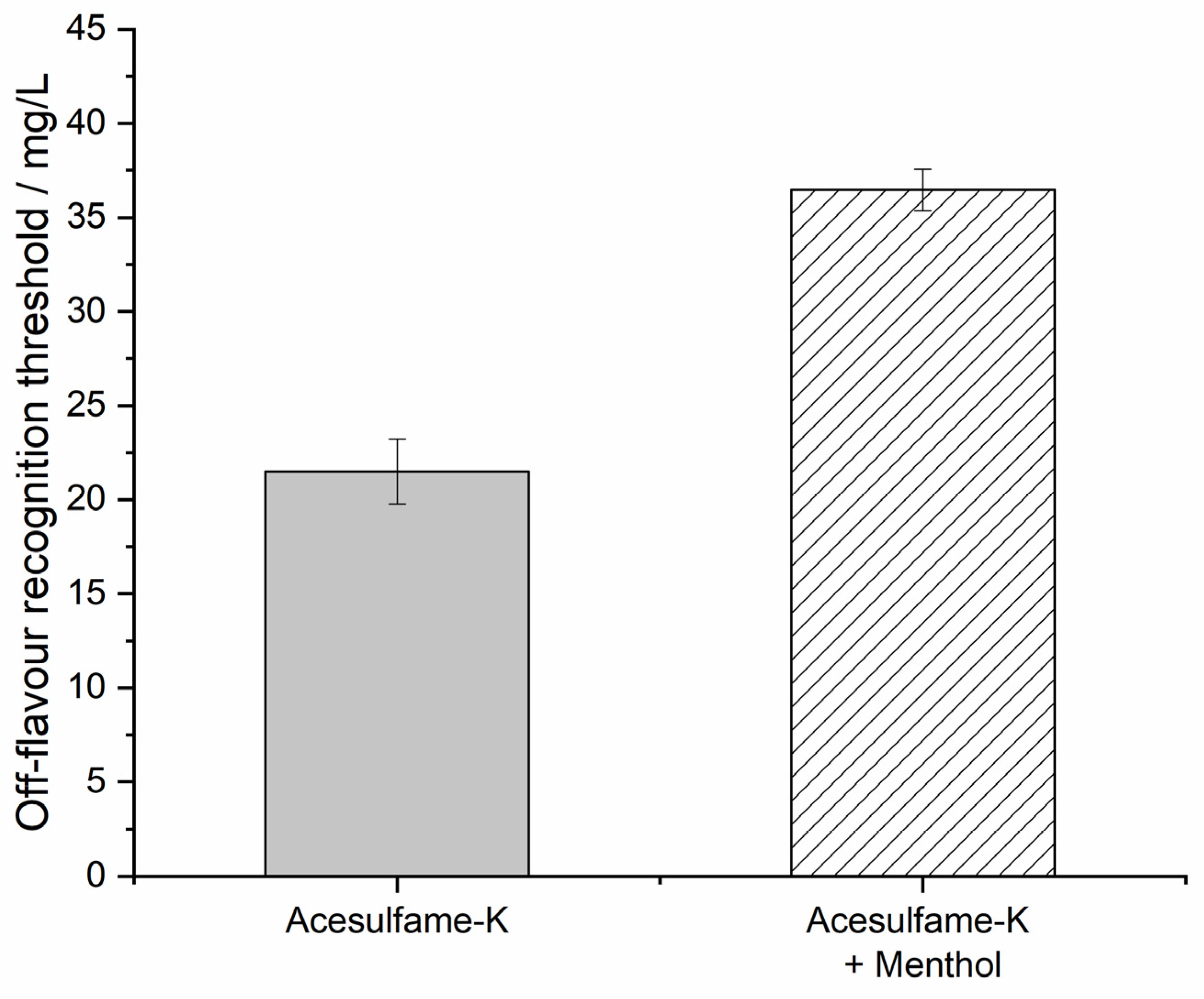
3.1. Recognition Threshold
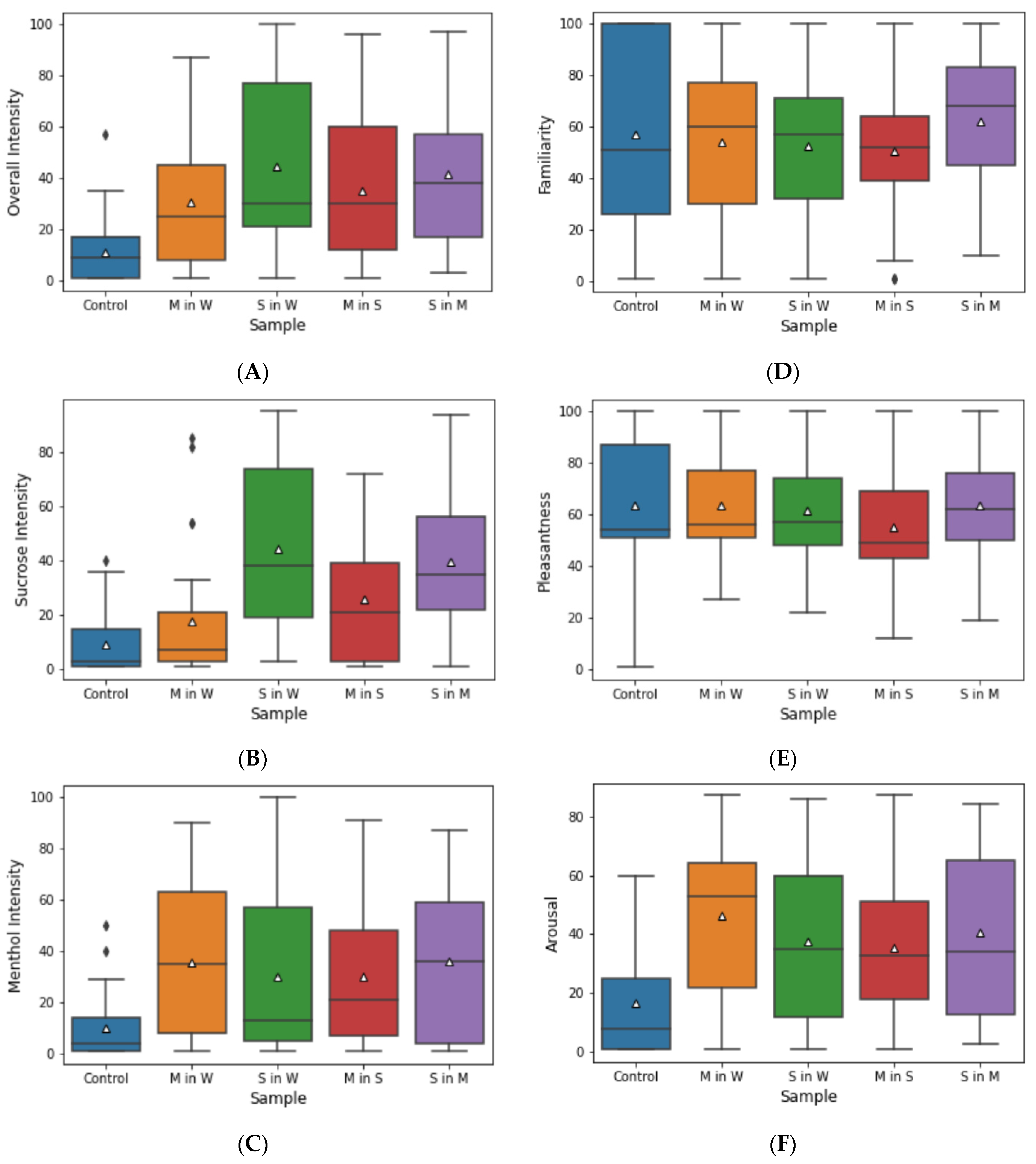
3.2. Perceptual Ratings
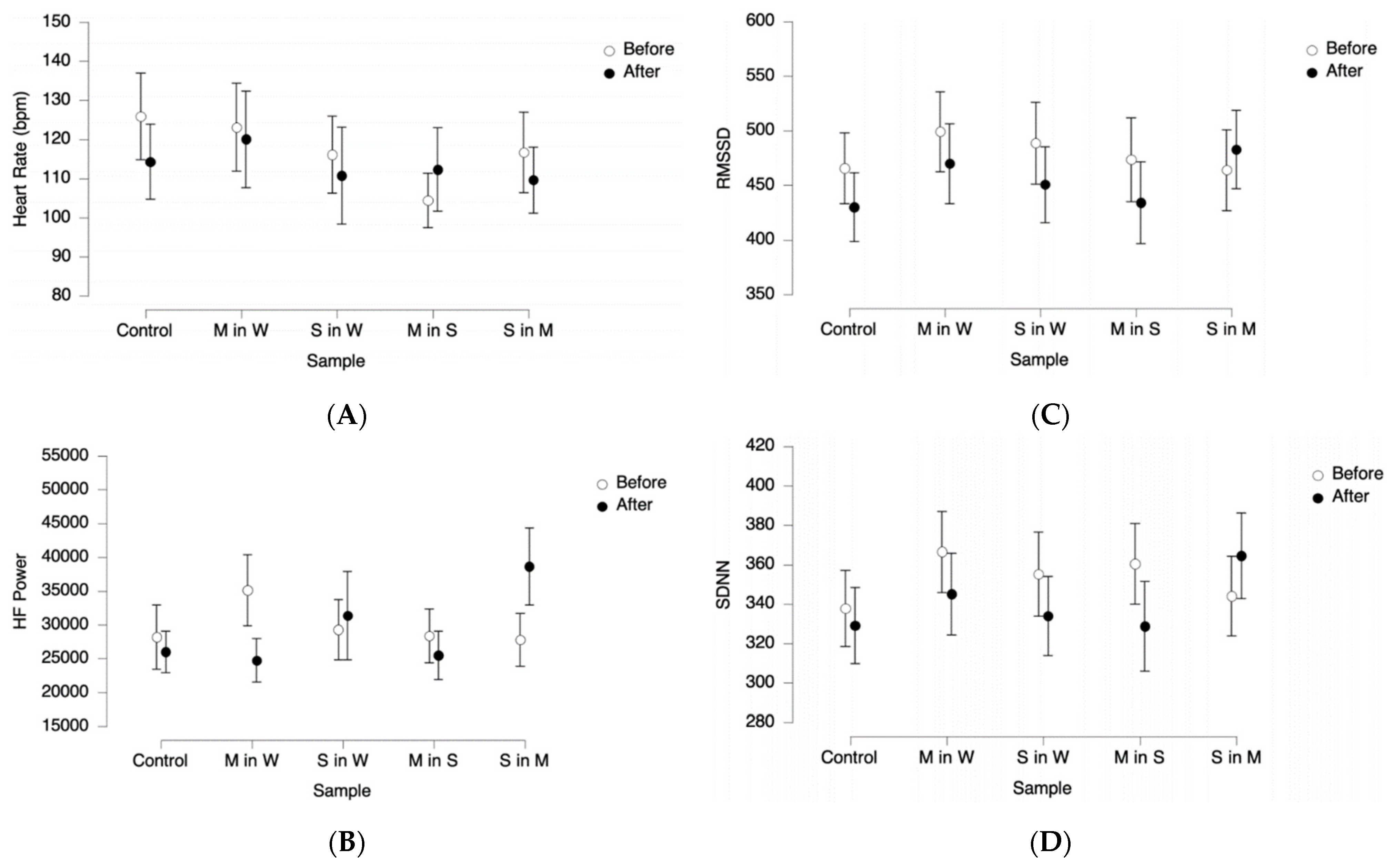
3.3. Psychophysiology—Unconscious Body Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahned, J.; Saeed, D.M.; Misra, S. Sugar-free Workplace: A Step for Fighting Obesity. Cureus 2019, 11, e6336. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; Le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A. Sugar-sweetened and artificially-sweetened beverages in relation to obesity risk. Adv. Nutr. 2014, 5, 797–808. [Google Scholar] [CrossRef] [PubMed]
- James, E.; Lajous, M.; Reich, M.R. The Politics of Taxes for Health: An Analysis of the Passage of the Sugar-Sweetened Beverage Tax in Mexico. Health Syst. Reform 2020, 6, e1669122. [Google Scholar] [CrossRef] [PubMed]
- Lyn, R.; Heath, E.; Dubhashi, J. Global Implementation of Obesity Prevention Policies: A Review of Progress, Politics, and the Path Forward. Curr. Obes. Rep. 2019, 8, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and artificially sweetened beverages linked to obesity: A systematic review and meta-analysis. QJM 2017, 110, 513–520. [Google Scholar] [CrossRef]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef]
- Monnard, C.R.; Grasser, E.K. Perspective: Cardiovascular Responses to Sugar-Sweetened Beverages in Humans: A Narrative Review with Potential Hemodynamic Mechanisms. Adv. Nutr. 2018, 9, 70–77. [Google Scholar] [CrossRef]
- Borges, M.C.; Louzada, M.L.; de Sá, T.H.; Laverty, A.A.; Parra, D.C.; Garzillo, J.; Monteiro, C.A.; Millett, C. Artificially sweetened beverages and the response to the global obesity crisis. PLoS Med. 2017, 14, e1002195. [Google Scholar] [CrossRef]
- Šedivá, A.; Panovská, Z.; Pokorný, J.A. Sensory Profiles of Sweeteners in Aqueous Solutions. Czech J. Food Sci. 2006, 24, 283–287. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.; Lawless, H.T.; Speirs, W.; Sposato, D. Bitter Taste of Saccharin and Acesulfame-K. Chem. Senses 2002, 27, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Auvray, M.; Spence, C. The multisensory perception of flavor. Conscious. Cogn. 2008, 17, 1016–1031. [Google Scholar] [CrossRef]
- Viana, F. Chemosensory properties of the trigeminal system. ACS Chem. Neurosci. 2011, 2, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Renner, B.; Schreiber, K. Olfactory and trigeminal interaction of menthol and nicotine in humans. Exp. Brain Res. 2012, 219, 13–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeffries, O.; Waldron, M. The effects of menthol on exercise performance and thermal sensation: A meta-analysis. J. Sci. Med. Sport 2019, 22, 707–715. [Google Scholar] [CrossRef]
- Abe, J.; Hosokawa, H.; Okazawa, M.; Kandachi, M.; Sawada, Y.; Yamanaka, K.; Matsumura, K.; Kobayashi, S. TRPM8 protein localization in trigeminal ganglion and taste papillae. Brain Res. Mol. Brain Res. 2005, 136, 91–98. [Google Scholar] [CrossRef]
- Bombicz, P.; Buschmann, J.; Luger, P.; Dung, N.X.; Nam, C.B. Crystal structure of (l1R,2S,5R)-2-isopropyl-5-methyl-cyclohexanol, (−)-menthol. Z. Krist. 1999, 214, 420–423. [Google Scholar]
- Koskinen, S.; Kälviäinen, N.; Tuorila, H. Perception of chemosensory stimuli and related responses to flavored yogurts in the young and elderly. Food Qual. Prefer. 2003, 14, 623–635. [Google Scholar] [CrossRef]
- Duizer, L.M.; Bloom, K.; Findlay, C.J. Dual-attribute Time-intensity Measurement of Sweetness and Peppermint Perception of Chewing Gum. J. Food Sci. 1996, 61, 636–638. [Google Scholar] [CrossRef]
- Davidson, J.M.; Linforth, R.S.; Hollowood, T.A.; Taylor, A.J. Effect of sucrose on the perceived flavor intensity of chewing gum. J. Agric. Food Chem. 1999, 47, 4336–4340. [Google Scholar] [CrossRef] [PubMed]
- Limpawattana, M. Sensory thresholds for natural flavoring extracts in different matrices. Rangsit J. Arts Sci. 2015, 5, 13–18. [Google Scholar] [CrossRef]
- Kivikangas, M.J.; Chanel, G.; Cowley, B.; Ekman, I.; Salminen, M.; Järvelä, S.; Ravaja, N. A review of the use of psychophysiological methods in game research. J. Gaming Virtual Worlds 2011, 3, 181–199. [Google Scholar] [CrossRef]
- Chambaron, S.; Chisin, Q.; Chabanet, C.; Issanchou, S.; Brand, G. Impact of olfactory and auditory priming on the attraction to foods with high energy density. Appetite 2015, 95, 74–80. [Google Scholar] [CrossRef]
- Kaneko, D.; Toet, A.; Brouwer, A.-M.; Kallen, V.; van Erp, J.B. Methods for evaluating emotions evoked by food experiences: A literature review. Front. Psychol. 2018, 9, 911. [Google Scholar]
- Baig, M.Z.; Kavakli, M. A Survey on Psycho-Physiological Analysis & Measurement Methods in Multimodal Systems. Multimodal Technol. Interact. 2019, 3, 37. [Google Scholar]
- Bensafi, M.; Rouby, C.; Farget, V.; Bertrand, B.; Vigouroux, M.; Holley, A. Autonomic nervous system responses to odours: The role of pleasantness and arousal. Chem. Senses 2002, 27, 703–709. [Google Scholar] [CrossRef]
- Dirican, A.C.; Göktürk, M. Psychophysiological measures of human cognitive states applied in human computer interaction. Procedia Comput. Sci. 2011, 3, 1361–1367. [Google Scholar] [CrossRef]
- Park, B. Psychophysiology as a tool for HCI research: Promises and pitfalls. In Proceedings of the International Conference on Human-Computer Interaction, Bonn, Germany, 15–18 September 2009. [Google Scholar]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar]
- Forte, G.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [PubMed]
- DIN ISO 3972:2013-12; Deutsches Institut für Normung. Sensorische Analyse—Methodologie—Bestimmung der Geschmacksempfindlichkeit, ISO_3972:2011_+_Cor._1:2012. Beuth Verlag GmbH: Berlin, Germany, 2013.
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use: Task force of the European Society of Cardiology and the North American Society for Pacing and Electrophysiology. Ann. Noninvasive Electrocardiol. 1996, 1, 151–181. [Google Scholar]
- Alf, E.F.; Grossberg, J.M. The geometric mean: Confidence limits and significance tests. Percept. Psychophys. 1979, 26, 419–421. [Google Scholar] [CrossRef]
- van Rossum, G.; Drake, F.L. Python 3 Reference Manual; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Won, E.; Kim, Y.-K. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr. Neuropharmacol. 2016, 14, 665–673. [Google Scholar] [CrossRef]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Michels, N.; Sioen, I.; Clays, E.; de Buyzere, M.; Ahrens, W.; Huybrechts, I.; Vanaelst, B.; de Henauw, S. Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biol. Psychol. 2013, 94, 433–440. [Google Scholar] [CrossRef]
- Peng, R.-C.; Yan, W.-R.; Zhou, X.-L.; Zhang, N.-L.; Lin, W.-H.; Zhang, Y.-T. Time-frequency analysis of heart rate variability during the cold pressor test using a time-varying autoregressive model. Physiol. Meas. 2015, 36, 441–452. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.; Jansen, A. Cephalic phase responses, craving and food intake in normal subjects. Appetite 2000, 35, 45–55. [Google Scholar] [CrossRef]
- Kilpatrick, L.A.; Coveleskie, K.; Connolly, L.; Labus, J.S.; Ebrat, B.; Stains, J.; Jiang, Z.; Suyenobu, B.Y.; Raybould, H.E.; Tillisch, K.; et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 2014, 146, 1212–1221. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büchner, K.; Haagen, J.; Sastrosubroto, A.; Kerpes, R.; Freiherr, J.; Becker, T. Trigeminal Stimulus Menthol Masks Bitter Off-Flavor of Artificial Sweetener Acesulfame-K. Foods 2022, 11, 2734. https://doi.org/10.3390/foods11182734
Büchner K, Haagen J, Sastrosubroto A, Kerpes R, Freiherr J, Becker T. Trigeminal Stimulus Menthol Masks Bitter Off-Flavor of Artificial Sweetener Acesulfame-K. Foods. 2022; 11(18):2734. https://doi.org/10.3390/foods11182734
Chicago/Turabian StyleBüchner, Kai, Jana Haagen, Ashtri Sastrosubroto, Roland Kerpes, Jessica Freiherr, and Thomas Becker. 2022. "Trigeminal Stimulus Menthol Masks Bitter Off-Flavor of Artificial Sweetener Acesulfame-K" Foods 11, no. 18: 2734. https://doi.org/10.3390/foods11182734
APA StyleBüchner, K., Haagen, J., Sastrosubroto, A., Kerpes, R., Freiherr, J., & Becker, T. (2022). Trigeminal Stimulus Menthol Masks Bitter Off-Flavor of Artificial Sweetener Acesulfame-K. Foods, 11(18), 2734. https://doi.org/10.3390/foods11182734





