Cocoa Spread with Grape Seed Oil and Encapsulated Grape Seed Extract: Impact on Physical Properties, Sensory Characteristics and Polyphenol Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plan of Experiments
2.3. Preparation of Grape Seed Encapsulate
2.4. Methods
2.4.1. Particle Size Distribution of Encapsulated Grape Seed Extract and Cocoa Spread Samples
2.4.2. Rheological Properties of Cocoa Spread Samples
2.4.3. Thermal Properties of Cocoa Spreads
2.4.4. Textural Properties of Cocoa Spreads
2.4.5. Sensory Analyses
2.4.6. Polyphenol Content and Antioxidant Activity in Cocoa Spread Samples
2.4.7. Statistical Analysis
3. Results and Discussion
3.1. The Impact of Grape Seed Oil and Encapsulated Grape Seed Extract on Particle Size Distribution and Rheological Properties of Cocoa Spreads
3.2. The Impact of Grape Seed Oil and Encapsulated Grape Seed Extract on Thermal and Textural Properties of Cocoa Spread
3.3. The Impact of Grape Seed Oil and Encapsulated Grape Seed Extract on Sensory Characteristics of Cocoa Spread
3.4. The Impact of Grape Seed Oil and Encapsulated Grape Seed Extract on Polyphenol Content of Cocoa Spread
3.5. The Impact of Grape Seed Oil and Encapsulated Grape Seed Extract on Antioxidant Activity of Cocoa Spread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illanes, A. Lactose: Production and upgrading. In Lactose-Derived Prebiotics: A Process Perspective; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–33. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data (accessed on 23 August 2022).
- Duba, K.S.; Fiori, L. Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. J. Supercrit. Fluids 2015, 98, 33–43. [Google Scholar] [CrossRef]
- Ruml, M.; Vuković, A.; Vujadinović, M.; Djurdjević, V.; Ranković-Vasić, Z.; Atanacković, Z.; Sivčev, B.; Marković, N.; Matijašević, S.; Petrović, N. On the use of regional climate models: Implications of climate change for viticulture in Serbia. Agric. For. Meteorol. 2012, 158–159, 53–62. [Google Scholar] [CrossRef]
- Dabetic, N.M.; Todorovic, V.M.; Djuricic, I.D.; Antic Stankovic, J.A.; Basic, Z.N.; Vujovic, D.S.; Sobajic, S.S. Grape Seed Oil Characterization: A Novel Approach for Oil Quality Assessment. Eur. J. Lipid Sci. Technol. 2020, 122, 1900447. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape Seed Oil Compounds: Biological and Chemical Actions for Health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef]
- Dimić, I.; Teslić, N.; Putnik, P.; Kovačević, D.B.; Zeković, Z.; Šojić, B.; Mrkonjić, Ž.; Čolović, D.; Montesano, D.; Pavlić, B. Innovative and conventional valorizations of grape seeds from winery by-products as sustainable source of lipophilic antioxidants. Antioxidants 2020, 9, 568. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Vidaković, A.; Vidović, S.; Velićanski, A.; Versari, A.; Radosavljević, R.; Zeković, Z. Sage processing from by-product to high quality powder: I. Bioactive potential. Ind. Crops Prod. 2017, 107, 81–89. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Olgun, E.O.; Canli, O.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Optimization of antioxidants recovery from wild thyme (Thymus serpyllum L.) by ultrasound-assisted extraction: Multi-response approach. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100333. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Böger, B.R.; Georgetti, S.R.; Kurozawa, L.E. Microencapsulation of grape seed oil by spray drying. Food Sci. Technol. 2018, 38, 263–270. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Petrović, J.; Zarić, D.; Sakač, M.; Torbica, A.; Lloyd, D.M.; Omorjan, R. The impact of sunflower and rapeseed lecithin on the rheological properties of spreadable cocoa cream. J. Food Eng. 2016, 171, 67–77. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Sakač, M.; Zarić, D.; Rakin, M.; Petrović, J.; Torbica, A. Influence of Rapeseed and Sesame Oil on Crystallization and Rheological Properties of Cocoa Cream Fat Phase and Quality of Final Product. J. Texture Stud. 2016, 47, 432–442. [Google Scholar] [CrossRef]
- Aydemir, O. Utilization of different oils and fats in cocoa hazelnut cream production. J. Food Process. Preserv. 2019, 43, e13930. [Google Scholar] [CrossRef]
- Guzmán, R.E.; Gómez, J.D.; Chocrón, S. Potential use of Sesame (Sesamum indicum L.) oil and sesame oil cake in the development of spreadable cocoa cream. Am. J. Food Sci. Nutr. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Aydemir, O.; Atalar, İ. Functionality of chestnut and fat/oil contents in cocoa chestnut cream production—A new product development. J. Food Process Eng. 2019, 42, e13222. [Google Scholar] [CrossRef]
- Aydemir, O.; Beşir, A.; Aden, H.M. Textural and rheological characteristics of cocoa hazelnut cream partially substituted with glucose syrup. Eur. Food Sci. Eng. 2021, 2, 13–17. [Google Scholar]
- Bascuas, S.; Espert, M.; Llorca, E.; Quiles, A.; Salvador, A.; Hernando, I. Structural and sensory studies on chocolate spreads with hydrocolloid-based oleogels as a fat alternative. LWT 2021, 135, 110228. [Google Scholar] [CrossRef]
- Akca, S.; Akpinar, A. The Effects of Grape, pomegranate, Sesame Seed Powder and Their Oils on Probiotic Ice Cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Acan, B.G.; Kilicli, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of grape pomace usage in chocolate spread formulation on textural, rheological and digestibility properties. LWT 2021, 138, 110451. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Cankurt, H.; Tornuk, F. Interaction Between Some Phenolic Compounds and Probiotic Bacterium in Functional Ice Cream Production. Food Bioprocess Technol. 2012, 5, 2964–2971. [Google Scholar] [CrossRef]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Ma, M.; Dolan, K.D. Effects of Spray Drying on Antioxidant Capacity and Anthocyanidin Content of Blueberry By-Products. J. Food Sci. 2011, 76, H156–H164. [Google Scholar] [CrossRef]
- IOCCC. Viscosity of Cocoa and Chocolate Products. Anal. Method 2000, 46, 1–7. [Google Scholar]
- Aidoo, R.P.; Afoakwa, E.O.; Dewettinck, K. Rheological properties, melting behaviours and physical quality characteristics of sugar-free chocolates processed using inulin/polydextrose bulking mixtures sweetened with stevia and thaumatin extracts. LWT—Food Sci. Technol. 2015, 62, 592–597. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Relationship between rheological, textural and melting properties of dark chocolate as influenced by particle size distribution and composition. Eur. Food Res. Technol. 2008, 227, 1215–1223. [Google Scholar] [CrossRef]
- ISO 8586; Sensory analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2012.
- ISO 4121; Sensory Analysis—Methodology—Evaluation of Food Product by Methods of Using Scales. ISO: Geneva, Switzerland, 2002.
- ISO 8589; Sensory Analysis-General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- Belščak, A.; Komes, D.; Horžić, D.; Ganić, K.K.; Karlović, D. Comparative study of commercially available cocoa products in terms of their bioactive composition. Food Res. Int. 2009, 42, 707–716. [Google Scholar] [CrossRef]
- Bolenz, S.; Holm, M.; Langkrär, C. Improving particle size distribution and flow properties of milk chocolate produced by ball mill and blending. Eur. Food Res. Technol. 2014, 238, 139–147. [Google Scholar] [CrossRef]
- Pajin, B.; Dokić, L.; Zarić, D.; Šoronja-Simović, D.; Lončarević, I.; Nikolić, I. Crystallization and rheological properties of soya milk chocolate produced in a ball mill. J. Food Eng. 2013, 114, 70–74. [Google Scholar] [CrossRef]
- Fasina, O.O.; Craig-Schmidt, M.; Colley, Z.; Hallman, H. Predicting melting characteristics of vegetable oils from fatty acid composition. LWT—Food Sci. Technol. 2008, 41, 1501–1505. [Google Scholar] [CrossRef]
- Hellwig, V.; Gasser, J. Polyphenols from waste streams of food industry: Valorisation of blanch water from marzipan production. Phytochem. Rev. 2020, 19, 1539–1546. [Google Scholar] [CrossRef] [Green Version]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Palma, A.S.; Teslić, N.; Brilli, C.; Pizzi, A.; Versari, A. Analytical profiling of food-grade extracts from grape (Vitis vinifera sp.) seeds and skins, green tea (Camellia sinensis) leaves and Limousin oak (Quercus robur) heartwood using MALDI-TOF-MS, ICP-MS and spectrophotometric methods. J. Food Compos. Anal. 2017, 59, 95–104. [Google Scholar] [CrossRef]
- Del Bo, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef]
- Lončarević, I.; Pajin, B.; Fišteš, A.; Tumbas Šaponjac, V.; Petrović, J.; Jovanović, P.; Vulić, J.; Zarić, D. Enrichment of white chocolate with blackberry juice encapsulate: Impact on physical properties, sensory characteristics and polyphenol content. LWT 2018, 92, 458–464. [Google Scholar] [CrossRef]
- Altınok, E.; Kurultay, S.; Konar, N.; Toker, O.S.; Kopuk, B.; Gunes, R.; Palabiyik, I. Utilising grape juice processing by-products as bulking and colouring agent in white chocolate. Int. J. Food Sci. Technol. 2022, 57, 4119–4128. [Google Scholar] [CrossRef]
- Bjelica, M.S.; Vujasinovi, V.; Rabrenovi, B.; Dimi, S.; Vujasinović, V.; Rabrenović, B.; Dimić, S. Some Chemical Characteristics and Oxidative Stability of Cold Pressed Grape Seed Oils Obtained from Different Winery Waste. Eur. J. Lipid Sci. Technol. 2019, 121, 1800416. [Google Scholar] [CrossRef]
- Kaur, M.; Kumar, S.; Bhat, Z.F.; Naqvi, Z.; Jayawardena, R. The impact of raspberry and blueberry extract on the microbial and lipid oxidative stability of calcium and chicken protein fortified composite chocolate. J. Food Process. Preserv. 2022, 46, e16216. [Google Scholar] [CrossRef]
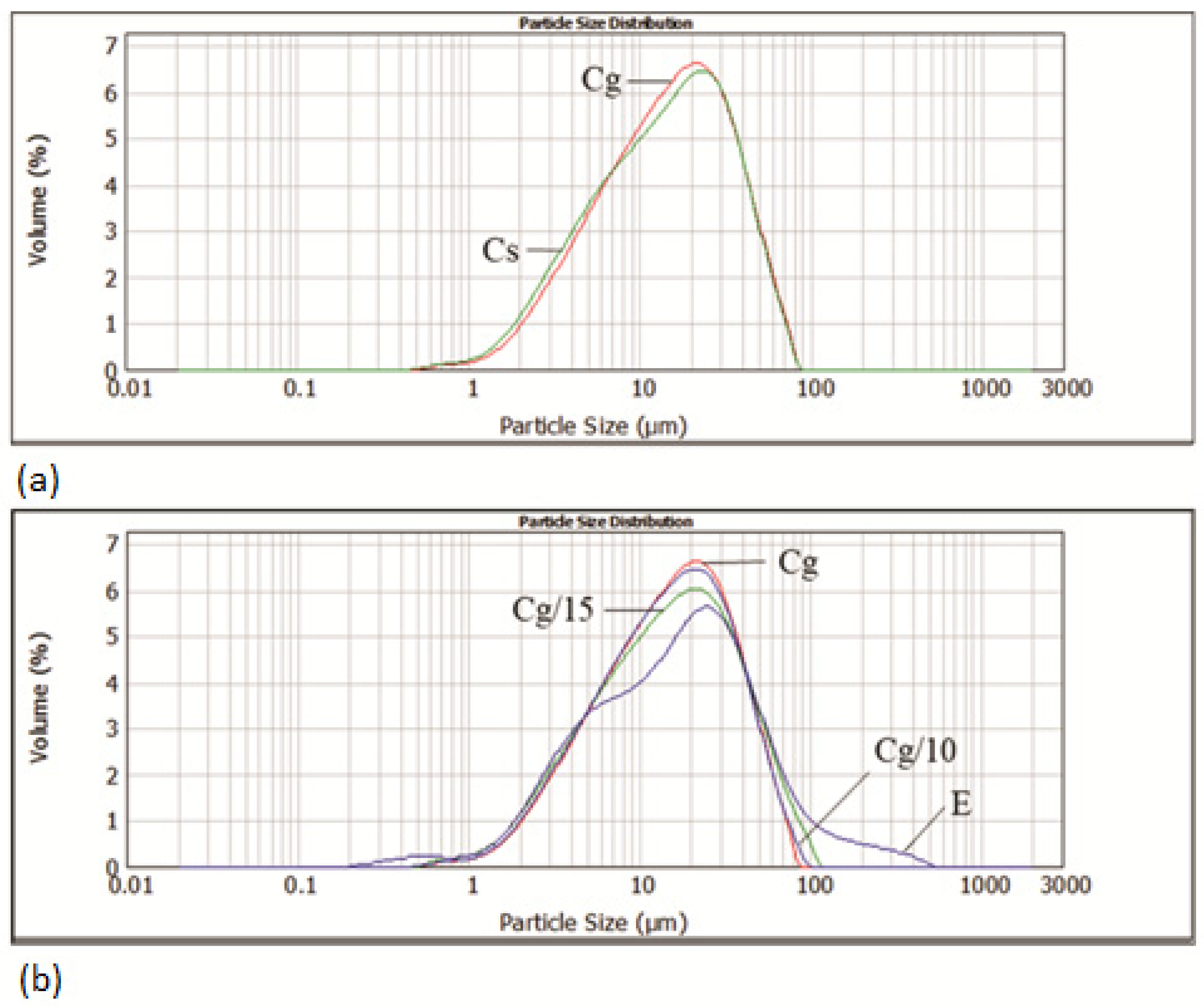
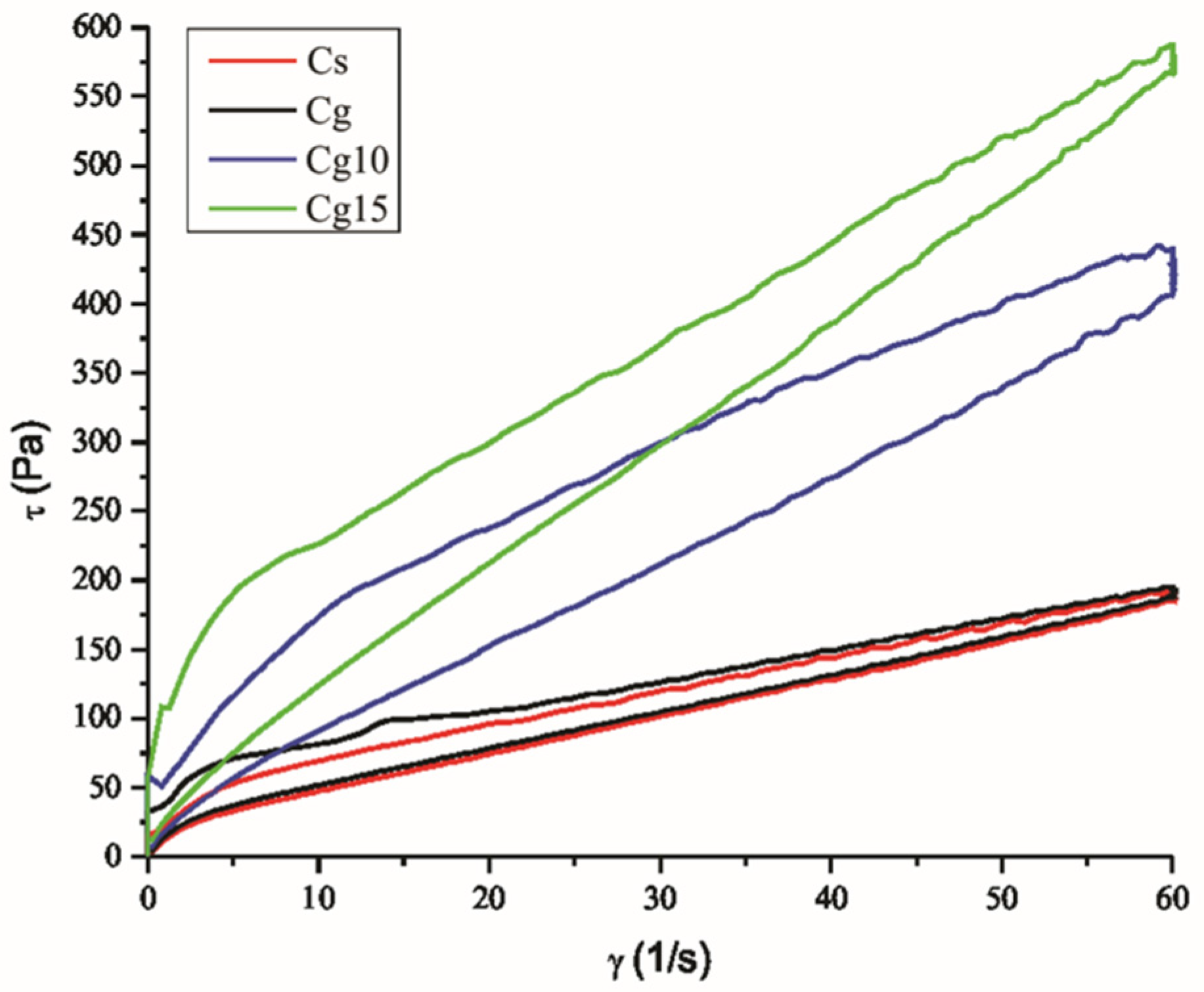
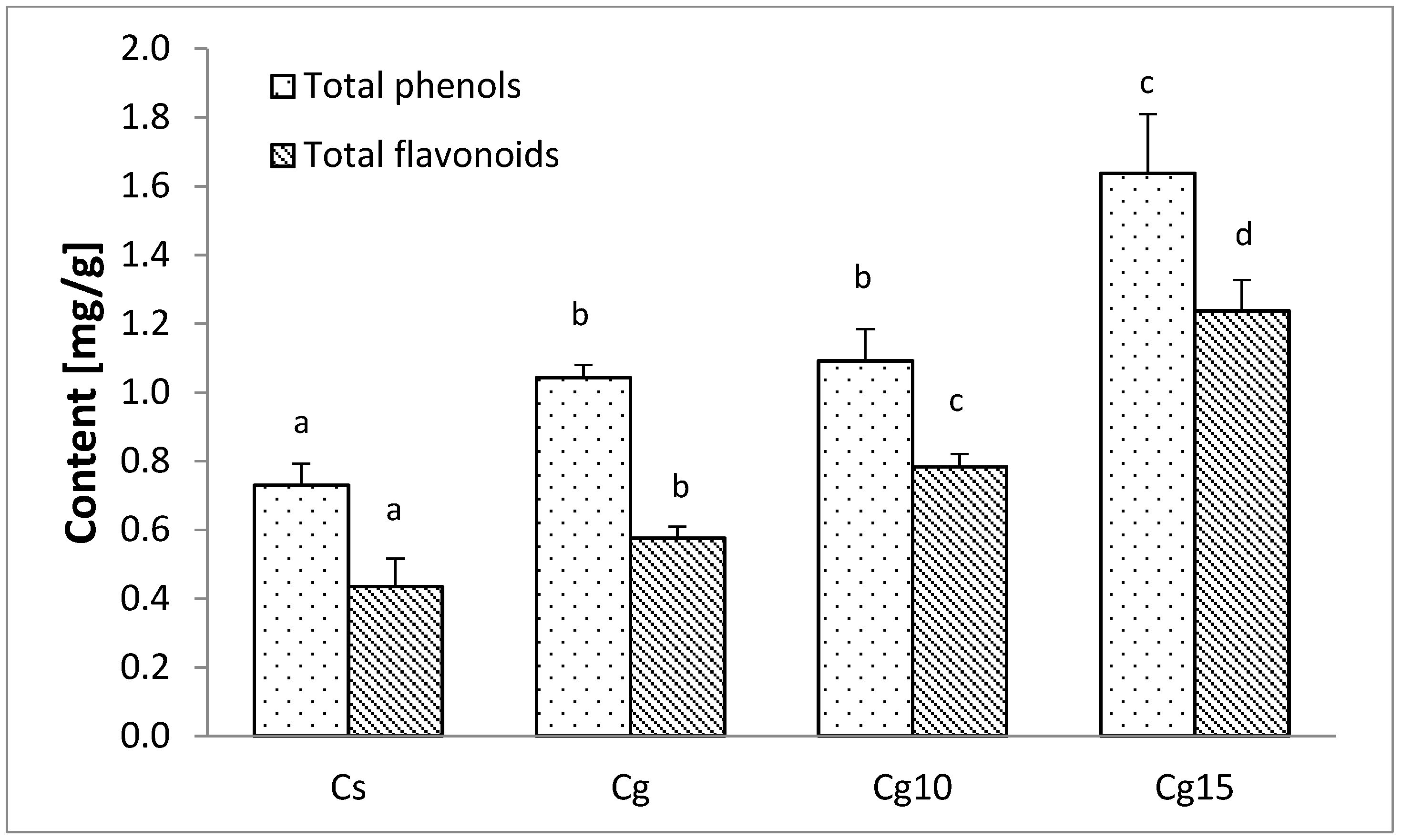
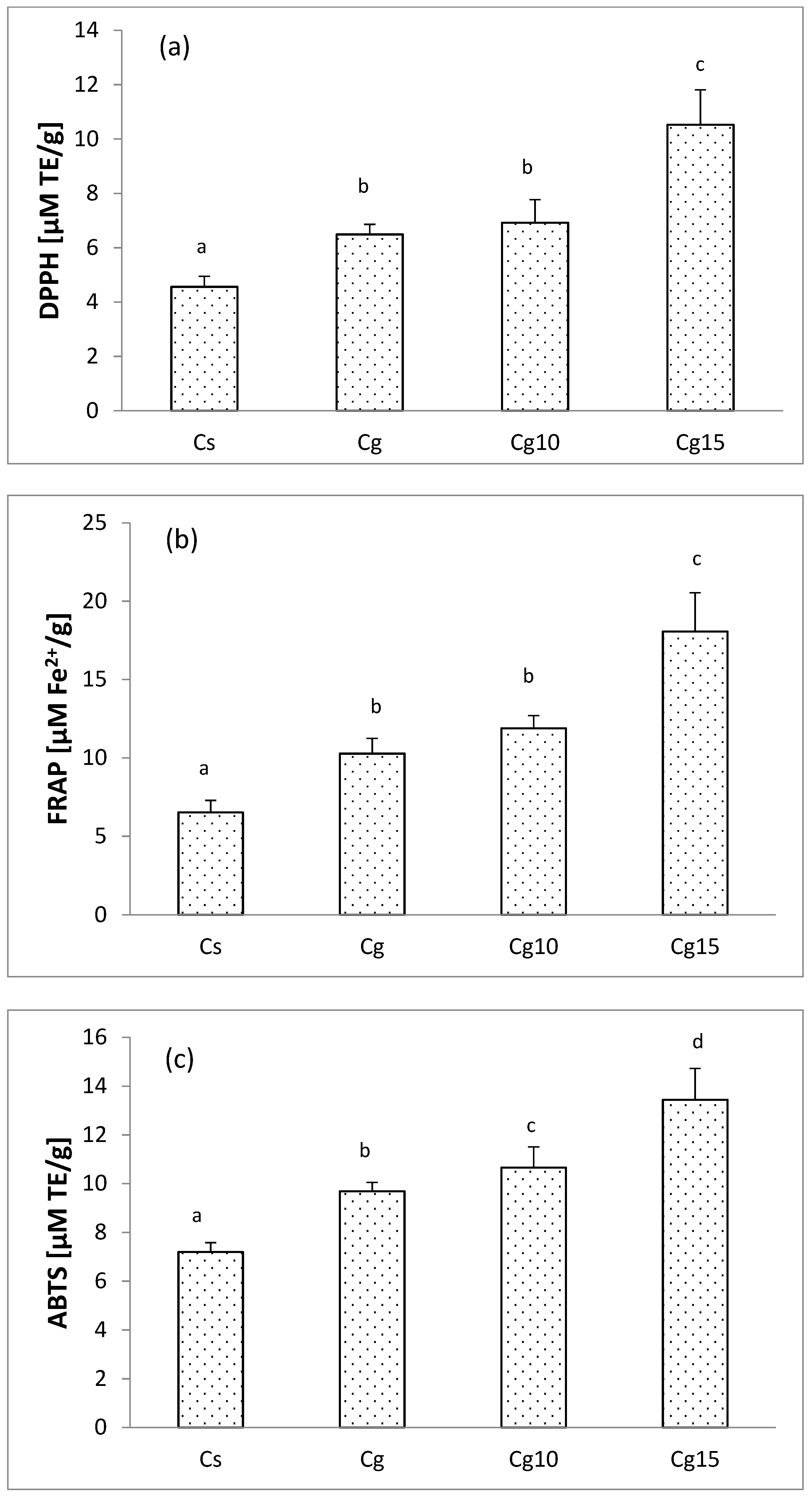
| Sample | Particle Size Parameters (µm) | |||
|---|---|---|---|---|
| d (0.1) | d (0.5) | d (0.9) | D [4,3] | |
| Cs | 3.56 ± 0.06 b | 14.85 ± 0.12 a | 41.44 ± 0.36 a | 19.17 ± 0.10 a |
| Cg | 3.96 ± 0.09 c | 15.32 ± 0.04 c | 41.48 ± 0.32 a | 19.60 ± 0.11 b |
| E | 3.30 ± 0.03 a | 17.06 ± 0.13 e | 62.65 ± 0.44 d | 30.67 ± 0.12 d |
| Cg10 | 3.85 ± 0.04 c | 15.09 ± 0.15 b | 42.40 ± 0.28 b | 19.71 ± 0.14 b |
| Cg15 | 3.55 ± 0.07 b | 15.24 ± 0.04 b,c | 46.94 ± 0.16 c | 21.04 ± 0.06 c |
| Sample | Casson Yield Stress (Pa) | Casson Viscosity (Pa·s) | Thixotropic Curve Area (Pa/s) |
|---|---|---|---|
| Cs | 9.90 ± 0.25 a | 1.86 ± 0.18 a | 1127 ± 19.85 a |
| Cg | 16.41 ± 0.43 b | 1.58 ± 0.13 a | 1482 ± 23.11 b |
| Cg10 | 23.90 ± 0.65 c | 4.11 ± 0.33 b | 5352 ± 77.65 c |
| Cg15 | 29.45 ± 0.82 d | 5.70 ± 0.31 c | 6085 ± 86.52 d |
| Sample | Thermal Properties | Textural Parameters | |||
|---|---|---|---|---|---|
| Tonset (°C) | Tpeak (°C) | Tend (°C) | Hardness (kg) | Work of Shear (kg·s) | |
| Cs | 31.62 ± 0.32 a | 35.15 ± 0.21 a,b | 42.82 ± 0.29 b | 3.88 ± 0.18 a | 3.02 ± 0.22 a |
| Cg | 32.06 ± 0.17 b | 35.48 ± 0.38 b,c | 42.19 ± 0.21 a | 3.71 ± 0.24 a | 2.99 ± 0.18 a |
| Cg10 | 32.32 ± 0.25 b | 35.72 ± 0.21 c | 42.47 ± 0.29 a,b | 8.81 ± 0.35 b | 7.58 ± 0.31 b |
| Cg15 | 32.47 ± 0.36 b | 35.41 ± 0.21 b,c | 42.83 ± 0.28 b | 20.31 ± 0.71 c | 20.39 ± 0.64 c |
| Sensory Parameter | Sample | |||
|---|---|---|---|---|
| Cs | Cg | Cg10 | Cg15 | |
| Color uniformity | 6.72 ± 0.44 c | 6.28 ± 0.26 b,c | 6.17 ± 0.35 a,b | 5.78 ± 0.44 a |
| Glow | 3.94 ± 0.30 c | 3.80 ± 0.25 c | 2.85 ± 0.24 b | 1.89 ± 0.22 a |
| Hardness | 2.18 ± 0.21 a | 2.30 ± 0.15 a | 4.08 ± 0.22 b | 6.32 ± 0.35 c |
| Graininess | 1.61 ± 0.22 a | 1.78 ± 0.26 a | 2.39 ± 0.42 b | 4.33 ± 0.25 c |
| Melting | 1.72 ± 0.26 a | 2.39 ± 1.20 a,b | 2.83 ± 0.25 b,c | 4.55 ± 0.30 d |
| Cocoa flavor | 5.22 ± 0.36 c,d | 5.11 ± 0.22 b,c | 4.77 ± 0.26 b | 3.59 ± 0.49 a |
| Grape seed oil flavor | 1.00 ± 0.00 a | 6.02 ± 0.15 d | 5.50 ± 0.22 c | 4.84 ± 0.24 b |
| Sweetness | 6.04 ± 0.25 c | 5.80 ± 0.40 c | 4.76 ± 0.25 b | 4.10 ± 0.22 a |
| Grape seed oil taste | 1.00 ± 0.00 a | 6.32 ± 0.30 d | 5.25 ± 0.26 c | 4.22 ± 0.21 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarević, I.; Petrović, J.; Teslić, N.; Nikolić, I.; Maravić, N.; Pajin, B.; Pavlić, B. Cocoa Spread with Grape Seed Oil and Encapsulated Grape Seed Extract: Impact on Physical Properties, Sensory Characteristics and Polyphenol Content. Foods 2022, 11, 2730. https://doi.org/10.3390/foods11182730
Lončarević I, Petrović J, Teslić N, Nikolić I, Maravić N, Pajin B, Pavlić B. Cocoa Spread with Grape Seed Oil and Encapsulated Grape Seed Extract: Impact on Physical Properties, Sensory Characteristics and Polyphenol Content. Foods. 2022; 11(18):2730. https://doi.org/10.3390/foods11182730
Chicago/Turabian StyleLončarević, Ivana, Jovana Petrović, Nemanja Teslić, Ivana Nikolić, Nikola Maravić, Biljana Pajin, and Branimir Pavlić. 2022. "Cocoa Spread with Grape Seed Oil and Encapsulated Grape Seed Extract: Impact on Physical Properties, Sensory Characteristics and Polyphenol Content" Foods 11, no. 18: 2730. https://doi.org/10.3390/foods11182730
APA StyleLončarević, I., Petrović, J., Teslić, N., Nikolić, I., Maravić, N., Pajin, B., & Pavlić, B. (2022). Cocoa Spread with Grape Seed Oil and Encapsulated Grape Seed Extract: Impact on Physical Properties, Sensory Characteristics and Polyphenol Content. Foods, 11(18), 2730. https://doi.org/10.3390/foods11182730









