Comprehensive Quality and Bioactive Constituent Analysis of Celery Juice Made from Different Cultivars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Juice
2.2. Physical Properties
2.3. Basic Nutritional Components
2.4. Antioxidant Properties
2.5. Secondary Metabolites
2.6. Volatile Flavor Compounds
2.7. Statistical Analysis
3. Results and Discussion
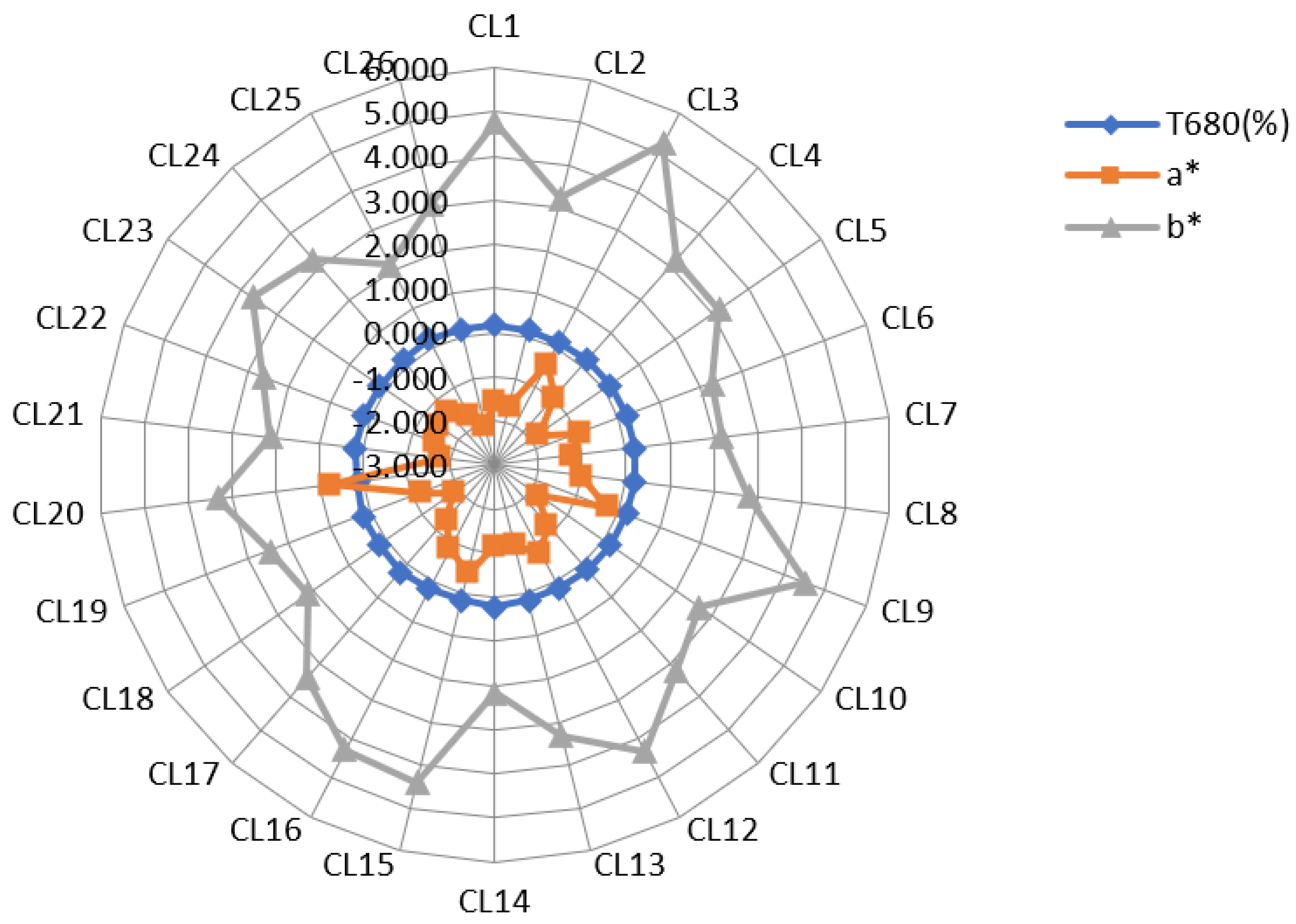
3.1. Basic Physical and Chemical Properties
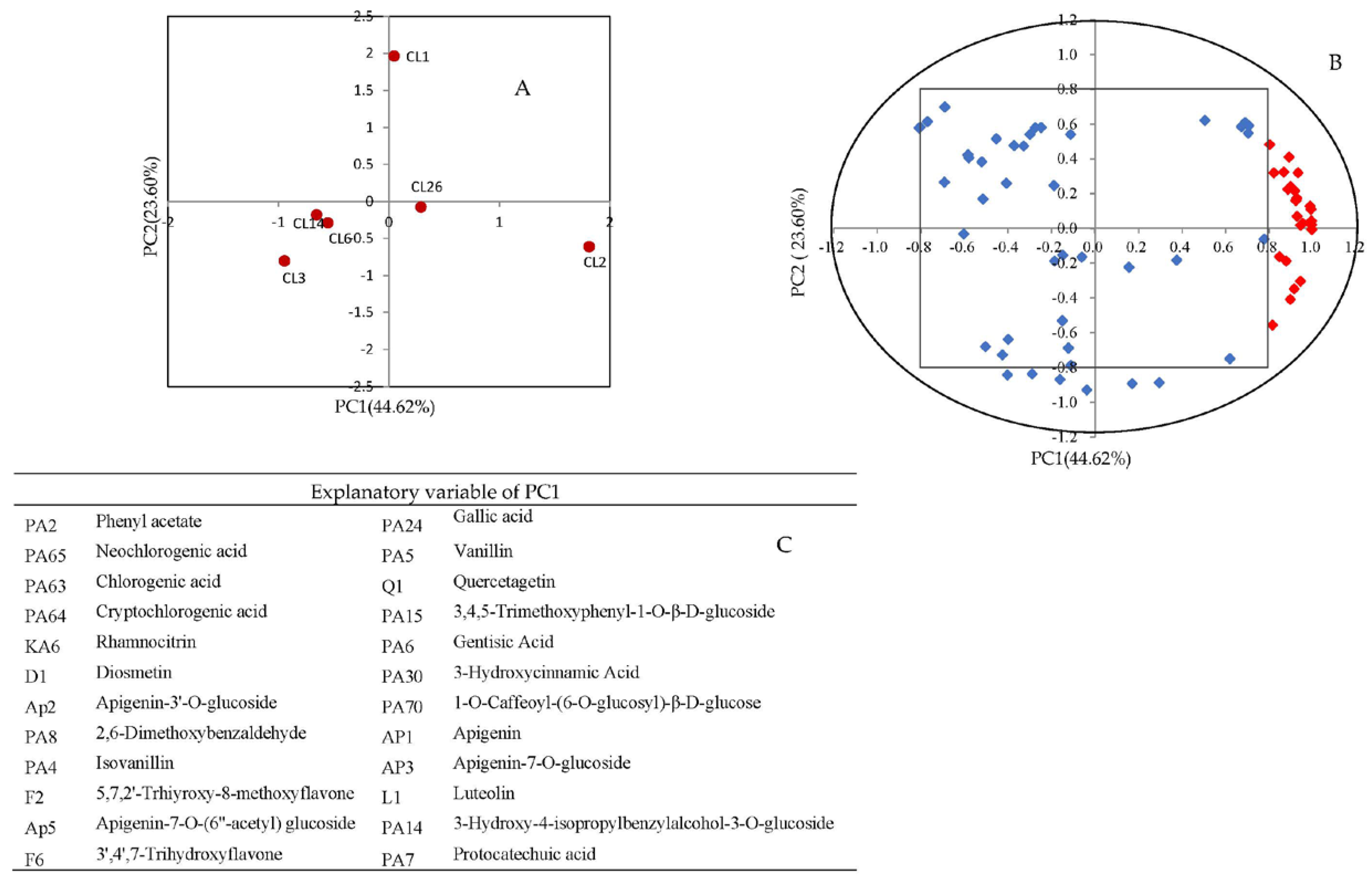
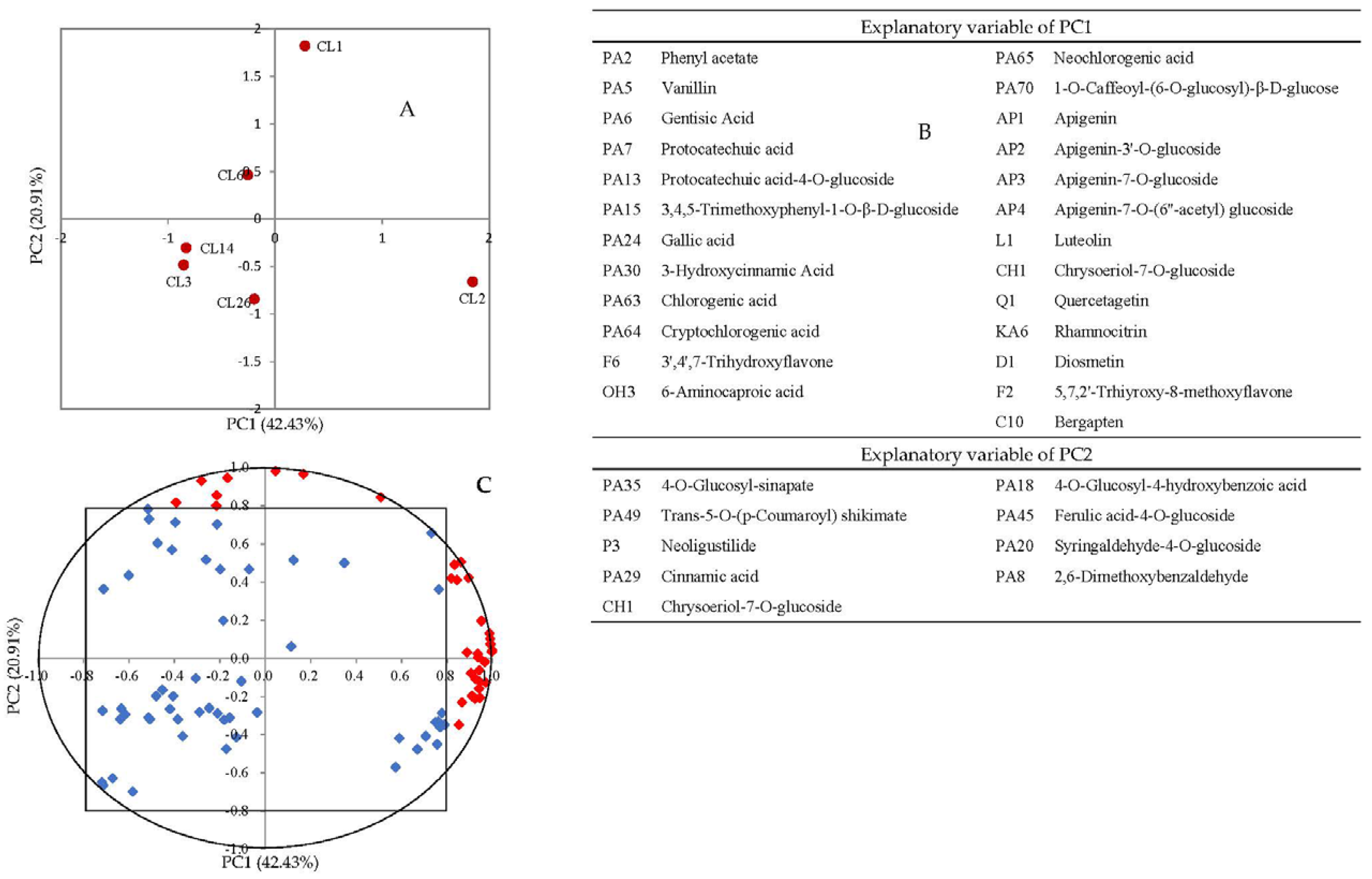
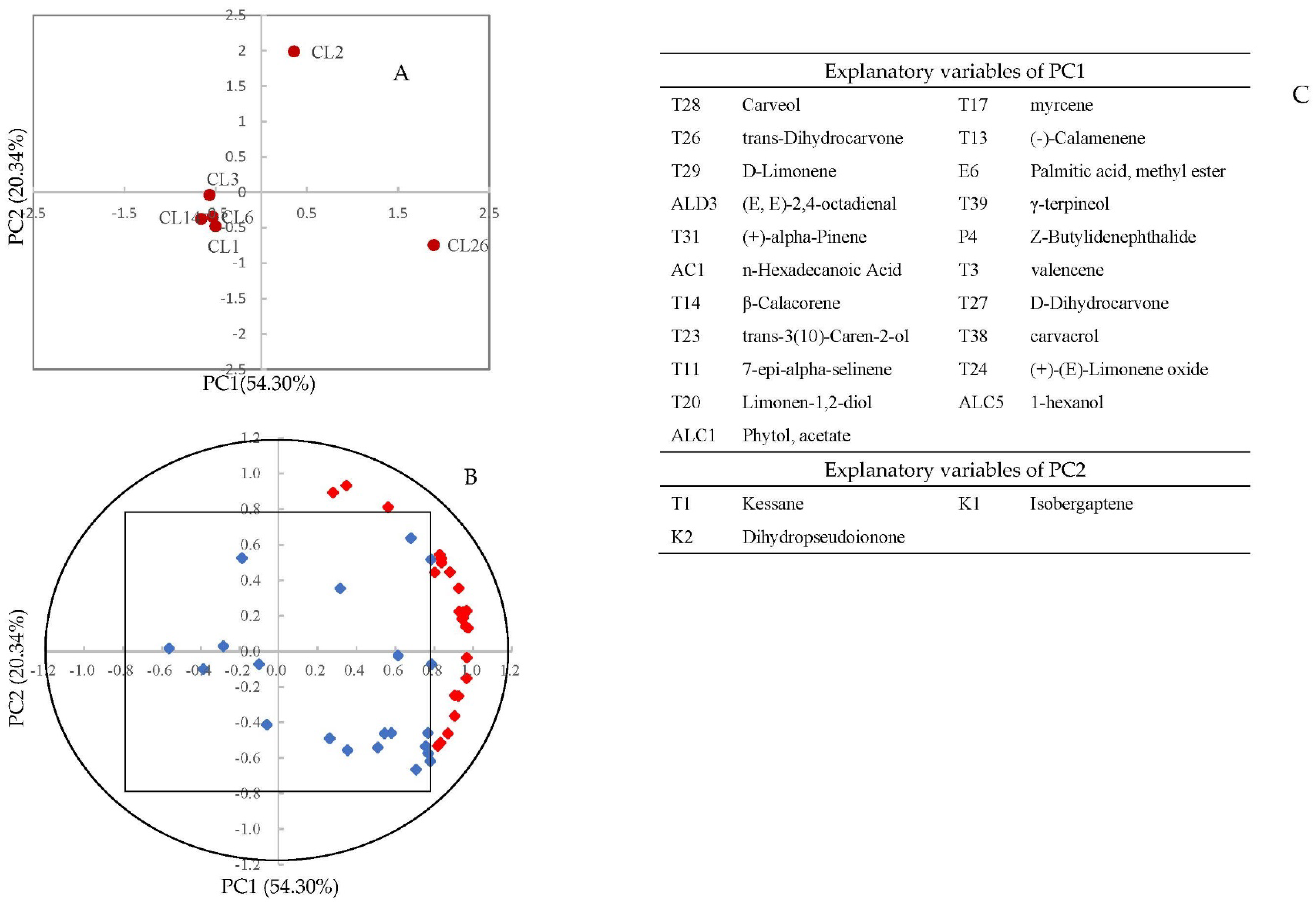
3.2. Principal Component Analysis (PCA)
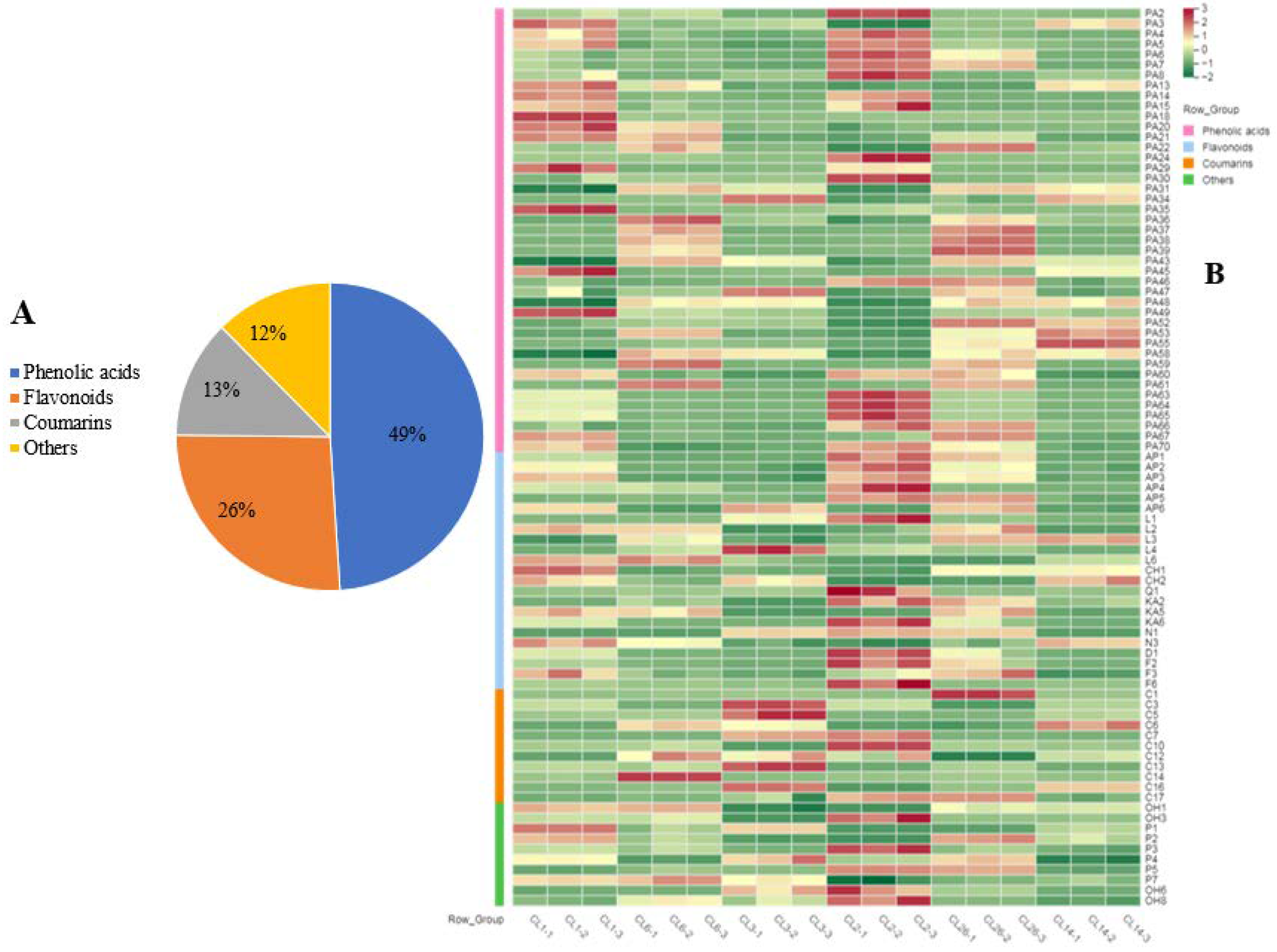
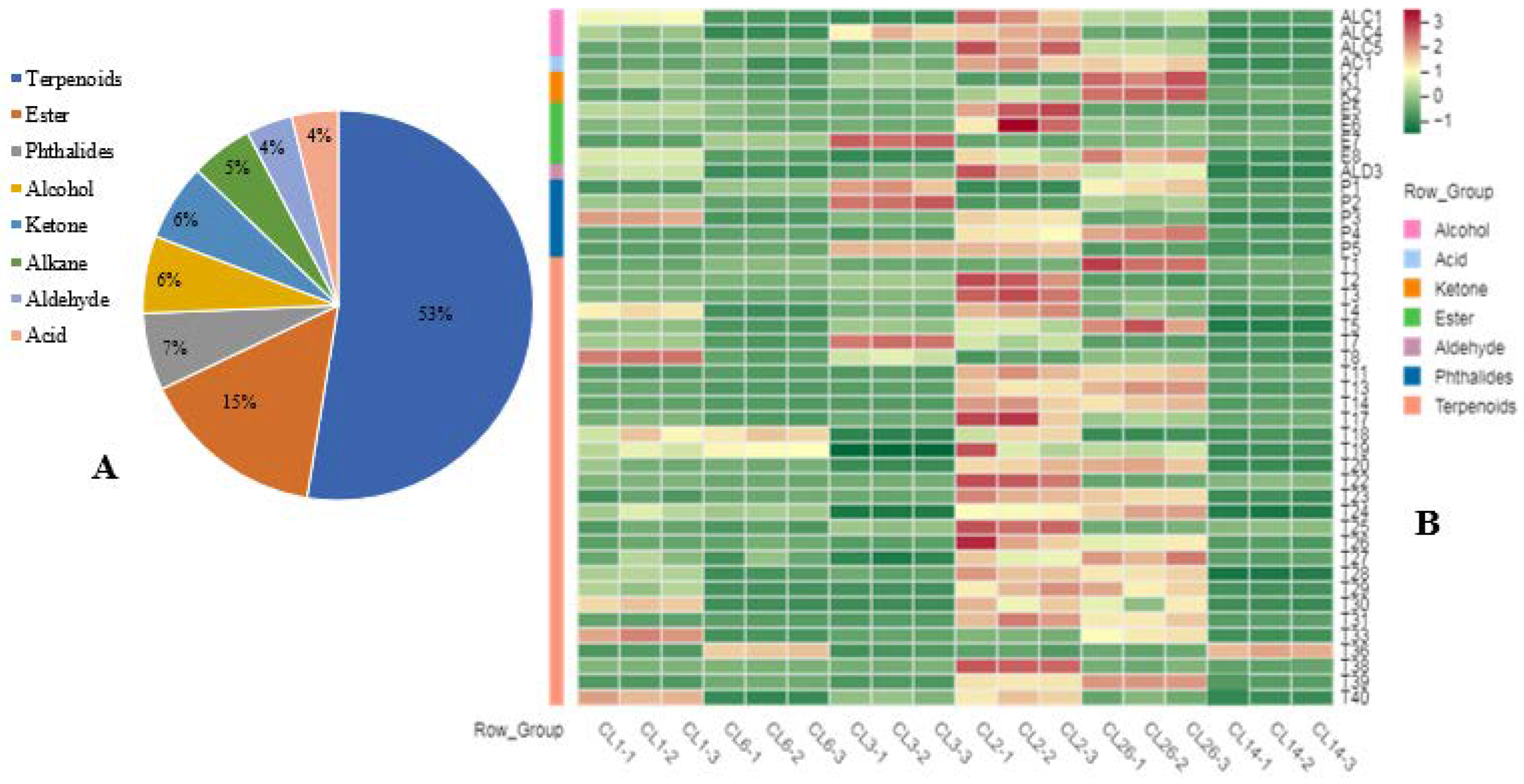
3.3. Secondary Metabolites
3.4. Volatile Flavor Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sowbhagya, H.B. Chemistry, Technology, and Nutraceutical Functions of Celery (Apium graveolens L.): An Overview. Crit. Rev. Food Sci. 2014, 54, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.J.; Chou, S.C. The Structural Diversity of Phthalides from the Apiaceae. J. Nat. Prod. 2007, 70, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Khairullah, A.R.; Solikhah, T.I.; Ansori, A.N.; Hidayatullah, A.R.; Hartadi, E.B.; Ramandinianto, S.C.; Fadholly, A. Review on the Pharmacological and Health Aspects of Apium Graveolens or Celery: An Update. Syst. Rev. Pharm. 2021, 12, 606–612. [Google Scholar]
- Jiang, L.L.; Lu, M.; Rao, T.Z.; Liu, Z.Y.; Wu, X.M.; An, H.M. Comparative analysis of fruit metabolome using widely targeted metabolomics reveals nutritional characteristics of different Rosa roxburghii genotypes. Foods 2022, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Hostetler, G.L.; Riedl, K.M.; Schwartz, S.J. Endogenous Enzymes, Heat, and pH Affect Flavone Profiles in Parsley (Petroselinum crispum var. neapolitanum) and Celery (Apium graveolens) during Juice Processing. J. Agric. Food Chem. 2012, 60, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Lasria, S.; Srilina, B.P.; Zulkarnain, B.B. The Comparison Study of Celery Leaves in Juice and Celery Boiled Water to Reduce of Blood Pressure on Elderly Patients with Hypertension. Adv. Soc. Sci. Educ. Humanit. Res. 2021, 521, 189–195. [Google Scholar] [CrossRef]
- Illes, J.D. Blood Pressure Change After Celery Juice Ingestion in a Hypertensive Elderly Male. J. Chiropr. Med. 2021, 20, 90–94. [Google Scholar] [CrossRef]
- Kolarovic, J.; Popovic, M.; Mikov, M.; Mitic, R.; Gvozdenovic, L. Protective Effects of Celery Juice in Treatments with Doxorubicin. Molecules 2009, 14, 1627–1638. [Google Scholar] [CrossRef]
- Shilpa, S.S.; Han-Seok, S. Influences of sensory attribute intensity, emotional responses, and nonsensory factors on purchase intent toward mixed-vegetable juice productsunder informed tasting condition. Food Res. Int. 2020, 132, 109095. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xu, S.; Gu, W.H.; Zhu, W.M. Apigenin accumulation and expression analysis of apigenin biosynthesis relative genes in celery. Sci. Hortic. 2014, 165, 218–224. [Google Scholar] [CrossRef]
- Obeng, E.; Kpodo, F.M.; Tettey, C.O.; Essuman, E.K.; Adzinyo, O.A. Antioxidant, total phenols, and proximate constituents of four tropical leafy vegetables. Sci. Afr. 2020, 7, e00227. [Google Scholar] [CrossRef]
- Hmidani, A.; Bouhlali, E.D.T.; Khouya, T.; Ramchoun, M.; Filali-zegzouti, Y.; Benlyas, M.; Alem, C. Effect of extraction methods on antioxidant and anticoagulant activities of Thymus atlanticus aerial part. Sci. Afr. 2019, 5, e00143. [Google Scholar] [CrossRef]
- Liu, F.X.; Fu, S.F.; Bi, X.F.; Chen, F.; Liao, X.J.; Hu, X.S.; Wu, J.H. Physico-chemical and antioxidant properties of four mango (Mangifera indica L.) cultivars in China. Food Chem. 2013, 138, 396–405. [Google Scholar] [CrossRef]
- Lim, K.T.; Hu, C.; Kitts, D.D. Antioxidant activity of a Rhus verniciflua stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power"; the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Buttkus, H.A. Celery Leaf Juice: Evaluation and Utilization of a Product from Harvest Debris. J. Agric. Food Chem. 1978, 26, 827–830. [Google Scholar] [CrossRef]
- Shahkoom ahally, S.; Khadivi, A.; Brecht, J.K.; Sarkhosh, A. Chemical and physical attributes of fruit juice and peel of pomegranate genotypes grown in Florida, USA. Food Chem. 2021, 342, 128302. [Google Scholar] [CrossRef]
- Tschida, A.; Stadlbauer, V.; Schwarzinger, B.; Maier, M.; Pitsch, J.; Stübl, F.; Müller, U.; Lanzerstorfer, P.; Himmelsbach, M.; Wruss, J.; et al. Nutrients, bioactive compounds, and minerals in the juices of 16 varieties of apple (Malus domestica) harvested in Austria: A four-year study investigating putative correlations with weather conditions during ripening. Food Chem. 2021, 338, 128065. [Google Scholar] [CrossRef]
- Heredia, F.J.; González-Miret, M.L.; Meléndez-Martínez, A.J.; Vicario, I.M. 19-Instrumental assessment of the sensory quality of juices, Instrumental Assessment of Food Sensory Quality (A Practical Guide). In Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 565–609, 610e. [Google Scholar]
- Liu, X.Y.; Li, X.Y.; Li, G.; Song, J.J.; Chen, A.J.; Tang, R. Comparison of Juicing Performance of Different Varieties of Sweet Potato (Ipomoea batatas L.). Sci. Technol. Food Ind. 2018, 24, 72–79. [Google Scholar]
- Mezeyová, I.; Hegedűsová, A.; Mezey, J.; Šlosár, M.; Farkaš, J. Evaluation of quantitative and qualitative characteristics of selected celery (Apium Graveolens var. Dulce) varieties in the context of juices production. Potravin. Slovak J. Food Sci. 2018, 12, 173–179. [Google Scholar] [CrossRef]
- Kurek, M.A.; Karp, S.; Wyrwisz, J.; Niu, Y. Physicochemical properties of dietary fibers extracted from gluten-free sources: Quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll. 2018, 85, 321–330. [Google Scholar] [CrossRef]
- Yildiz, L.; Baskan, K.S.; Tutem, E.T.; Apak, R. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta 2008, 77, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, A.B.; Landete, M.P.; Martinez, M.I.D.; Moya, M.S.P.; Garcia, A.V. Optimization of volatile compounds extraction from industrial celery (Apium graveolens) by-products by using response surface methodology and study of their potential as antioxidant sources. Foods 2021, 10, 2664. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.K.; Xu, C.C.; Zhang, L.; Ma, H.; Chen, X.J.; Sui, Y.C.; Zhang, H.Z. Evaluation of bioactive components and antioxidant capacity of four celery (Apium graveolens L.) leaves and petioles. Int. J. Food Prop. 2020, 23, 1097–1109. [Google Scholar] [CrossRef]
- Yao, Y.; Ren, G.X. Effect of thermal treatment on phenolic composition and antioxidant activities of two celery cultivars. LWT Food Sci. Technol. 2010, 44, 181–185. [Google Scholar] [CrossRef]
- Li, Q.L.; Sun, J.J.; Shan, Y.; Fu, F.H.; Yang, Y.; Liu, W. Suitability Evaluation of Different Citrus Varieties for Whole Fruit Juice Processing. Food Sci. 2019, 40, 36–44. [Google Scholar]
- Topalović, A.; Knežević, M.; Gačnik, S.; Mikulic-Petkovsek, M. Detailed chemical composition of juice from autochthonous pomegranate genotypes (Punica granatum L.) grown in different locations in Montenegro. Food Chem. 2020, 330, 127261. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.Y.; Bishop, K.S.; Zhang, J.Y.; Chen, D.L.; Quek, S.Y. Characterization of phenolic compounds and aroma active compounds in feijoa juice from four New Zealand grown cultivars by LC-MS and HS-SPME-GC-O-MS. Food Res. Int. 2020, 129, 108873. [Google Scholar] [CrossRef]
- Bello, C.; Maldini, M.; Baima, S.; Scaccini, C.; Natella, F. Glucoraphanin and sulforaphane evolution during juice preparation from broccoli sprouts. Food Chem. 2018, 268, 249–256. [Google Scholar] [CrossRef]
- Kooti, W.; Daraei, N. A Review of the Antioxidant Activity of Celery (Apium graveolens L). J. Evid. Based Complementary Altern. Med. 2017, 22, 1029–1033. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xu, S.; Wang, Y.; Shen, J.H.; Zhu, W.M. Assay and Evaluation of Flavonoid Content in Chinese Celery. Agric. Sci. Technol. 2014, 15, 1200–1204. [Google Scholar]
- Ali, F.; Naz, R.F.; Jyoti, S.; Siddique, Y.H. Health functionality of apigenin: A review. Int. J. Food Prop. 2017, 20, 1197–1238. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective Growth-Inhibitory, Cell-Cycle Deregulatory and Apoptotic Response of Apigenin in Normal Versus Human Prostate Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001, 87, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, W.B.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef]
- Akkol, E.K.; Genç, Y.; Karpuz, B.; Sobarzo-Sánchez, E.; Capasso, R. Coumarins and Coumarin-Related Compounds in Pharmacotherapy of Cancer. Cancers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Beier, R.C.; Oertli, E.H. Psoralen and other linear furocoumarins as phytoalexins in celery. Phytochemistry 1983, 22, 2595–2597. [Google Scholar] [CrossRef]
- Guo, L.Q.; Taniguchi, M.; Xiao, Y.Q.; Baba, K.; Ohta, T.; Yamazoe, Y. Inhibitory effect of natural furanocoumarins on human microsomal cytochrome P450 3A activity. Jpn. J. Pharm. 2000, 82, 122–129. [Google Scholar] [CrossRef]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the factors that influence the aroma profile of Apium graveolens: A. review. Food Chem. 2021, 345, 128673. [Google Scholar] [CrossRef]
- Turner, L.; Dawda, D.; Wagstaff, C.; Gawthrop, F.; Lignou, S. Influence of harvest maturity on the aroma quality of two celery (Apium graveolens) genotypes. Food Chem. 2021, 365, 130515. [Google Scholar] [CrossRef]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the Relationship of Genotype and Climate Conditions on the Volatile Composition and Sensory Profile of Celery (Apium graveolens). Foods 2021, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Turner, L.; Lignou, S.; Gawthrop, F.; Wagstaff, C. Investigating the Relationship of Genotype and Geographical Location on Volatile Composition and Sensory Profifile of Celery (Apium graveolens). Int. J. Mol. Sci. 2021, 22, 12016. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.F.; Qu, J.F.; Yin, H.Q.; Li, L.Y.; Zhi, Y.Y.; Liu, Y.P.; Fang, Z.H.; Hao, E. Apoptotic cell death induced by Z-Ligustilidein human ovarian cancer cells and role of NRF2. Food Chem. Toxicol. 2018, 121, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Deng, Y.F.; Hu, X.Y.; Ni, J.N.; Jiang, M.; Bai, G. Phthalides, senkyunolide A and ligustilide, show immunomodulatory effect in improving atherosclerosis, through inhibiting AP-1 and NF-κB expression. Biomed. Pharmacother. 2019, 117, 109074. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, M.Q.; Chen, C.; Du, J.R. Phthalide derivative CD21 ameliorates ischemic brain injury in a mouse model of global cerebral ischemia: Involvement of inhibition of NLRP3. Int. Immunopharmacol. 2020, 86, 106714. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lin, S.Z.; Dass, K.T.P.; Lin, W.J.; Liu, S.P.; Harn, H.J. 3-N-butylphthalide protects against high-fat-diet-induced obesity in C57BL/6 mice and increases metabolism in lipid-accumulating cells. Biomed. Pharmacother. 2021, 139, 111687. [Google Scholar] [CrossRef]

| Number | Variety Name | Origin |
|---|---|---|
| CL1 | Huangxin Qin | Shanghai, China |
| CL2 | Tie gan qin cai | Hebei, China |
| CL3 | White petiole Qin | Tianjin, China |
| CL4 | Jin nan shi qin No. 1 | Tianjin, China |
| CL5 | Si ji qing xiang little Qin | Tianjin, China |
| CL6 | Ventura | America |
| CL7 | Tango | Netherlands |
| CL8 | Queen | France |
| CL9 | Shen Qin No.2 | Shanghai, China |
| CL10 | Betty | America |
| CL11 | Fast-growing celery | Japan |
| CL12 | Golden Plume 4162 | America |
| CL13 | Hong cheng hollow Qin | Tianjin, China |
| CL14 | Dutch giant celery | Netherlands |
| CL15 | Xue bai qin cai | Sichuan, China |
| CL16 | Yellow seedling Qin | Tianjin, China |
| CL17 | Huang nen celery | France |
| CL18 | California King | America |
| CL19 | Bo li cui shi qin | Henan, China |
| CL20 | Purple petiole Qin | Shanxi, China |
| CL21 | Emperor | France |
| CL22 | Summer celery | Italy |
| CL23 | Jin nan shi qin No. 3 | Tianjin, China |
| CL24 | Shang nong yu qin | Shanghai, China |
| CL25 | Tall Utah 52–70 R | America |
| CL26 | Zhang qiu Bao Qin | Shandong, China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Yang, X.; He, L.; Huang, Z.; Zhu, M.; Fan, L.; Li, H.; Wu, L.; Yu, L.; Zhu, W. Comprehensive Quality and Bioactive Constituent Analysis of Celery Juice Made from Different Cultivars. Foods 2022, 11, 2719. https://doi.org/10.3390/foods11182719
Yan J, Yang X, He L, Huang Z, Zhu M, Fan L, Li H, Wu L, Yu L, Zhu W. Comprehensive Quality and Bioactive Constituent Analysis of Celery Juice Made from Different Cultivars. Foods. 2022; 11(18):2719. https://doi.org/10.3390/foods11182719
Chicago/Turabian StyleYan, Jun, Xiaofeng Yang, Lizhong He, Zhiwu Huang, Mingfen Zhu, Linhua Fan, Han Li, Lingyun Wu, Li Yu, and Weimin Zhu. 2022. "Comprehensive Quality and Bioactive Constituent Analysis of Celery Juice Made from Different Cultivars" Foods 11, no. 18: 2719. https://doi.org/10.3390/foods11182719
APA StyleYan, J., Yang, X., He, L., Huang, Z., Zhu, M., Fan, L., Li, H., Wu, L., Yu, L., & Zhu, W. (2022). Comprehensive Quality and Bioactive Constituent Analysis of Celery Juice Made from Different Cultivars. Foods, 11(18), 2719. https://doi.org/10.3390/foods11182719





