Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Reagents and Chemicals
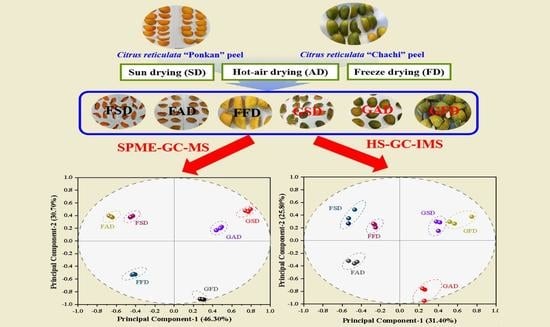
2.3. Drying of the Fresh Citrus Peels
2.4. Analysis of Volatile Compounds
2.4.1. HS-SPME-GC-MS Analysis
2.4.2. HS-GC-IMS Analysis
2.4.3. Calculation of Odor Activity Values (OAVs)
2.5. Statistical Analysis
3. Results and Discussion
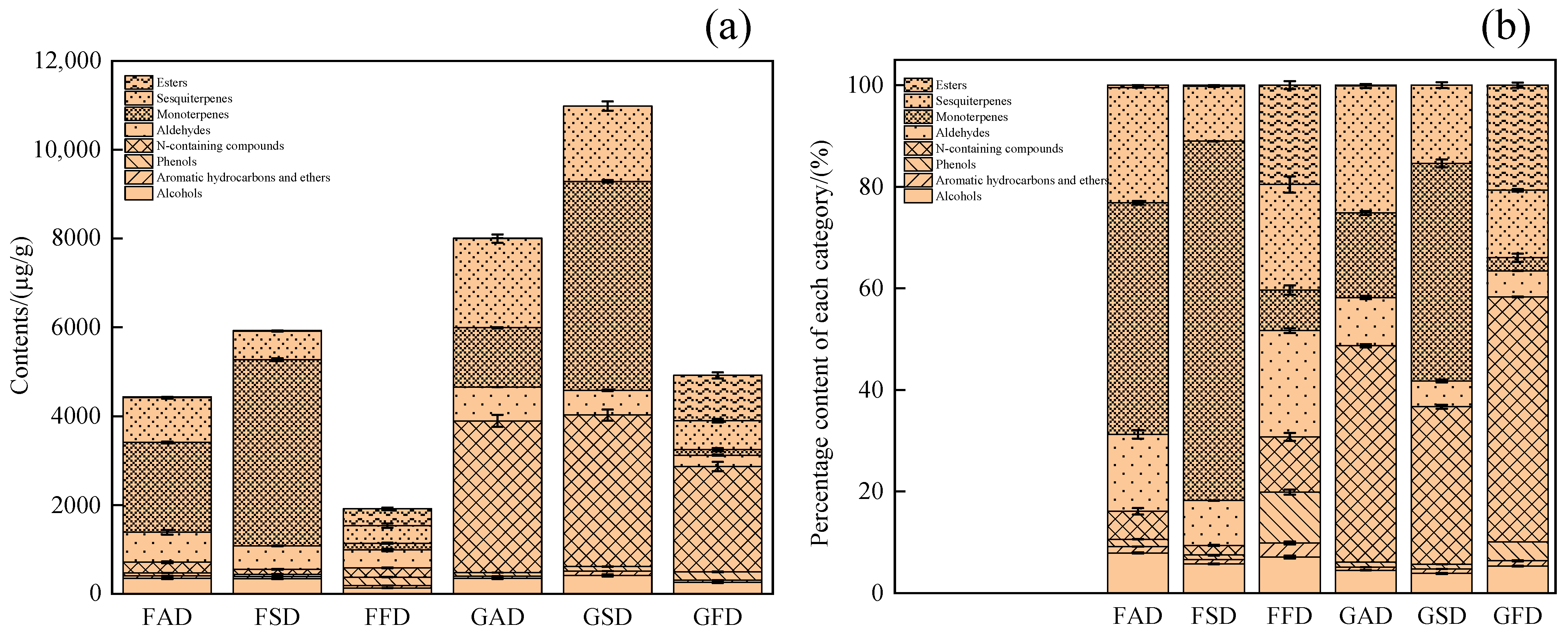
3.1. GC-MS Analysis of Volatile Profile of Dried Citrus Peel as Effected by Drying Methods
3.1.1. Alcohols
3.1.2. Aromatic Hydrocarbons and Ethers
3.1.3. Phenols
3.1.4. N-Containing Compounds
3.1.5. Aldehydes
3.1.6. Monoterpenes
3.1.7. Sesquiterpenes
3.1.8. Esters
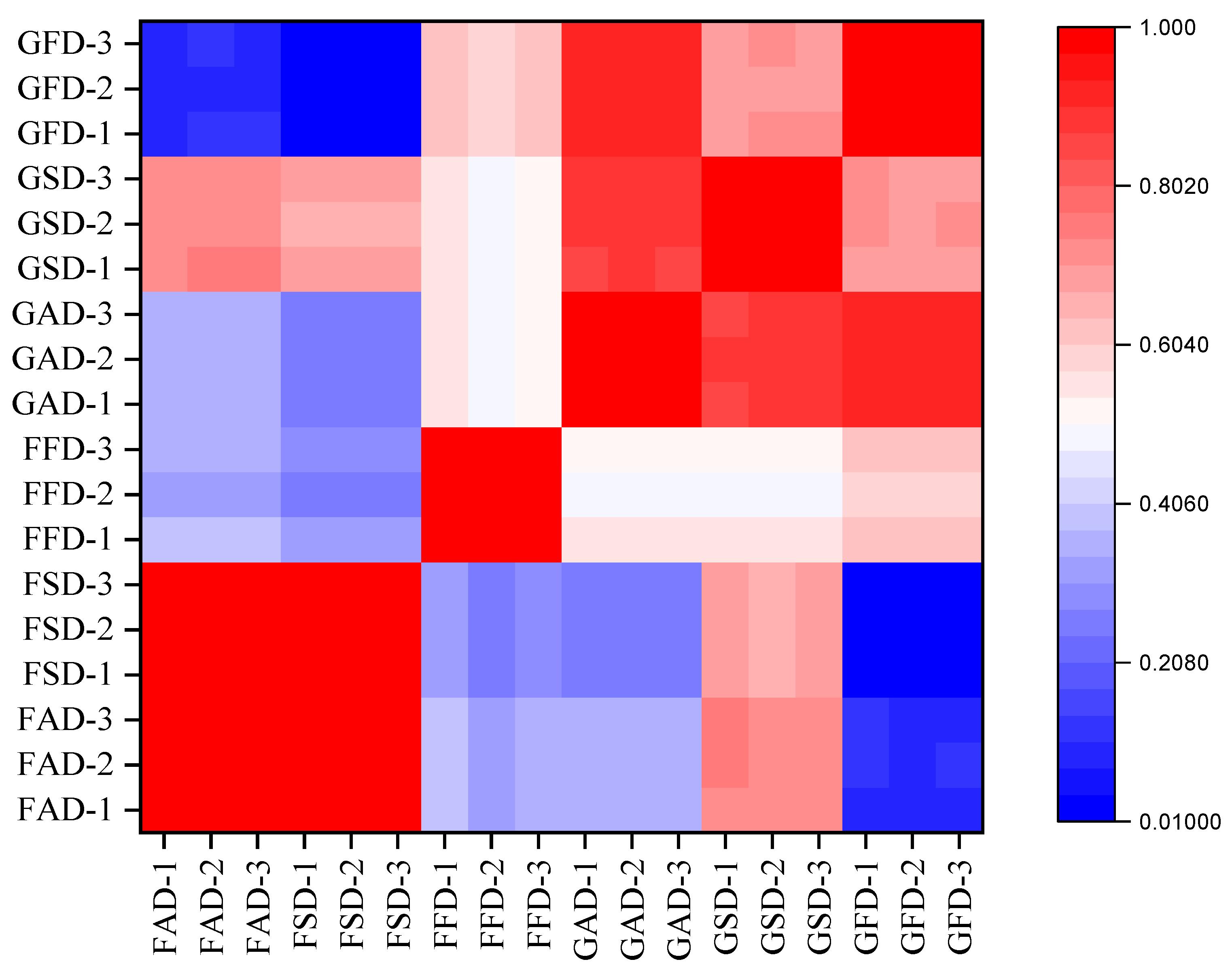
3.1.9. Correlation and PCA Analysis
3.2. OAVs and Sensory Attributes
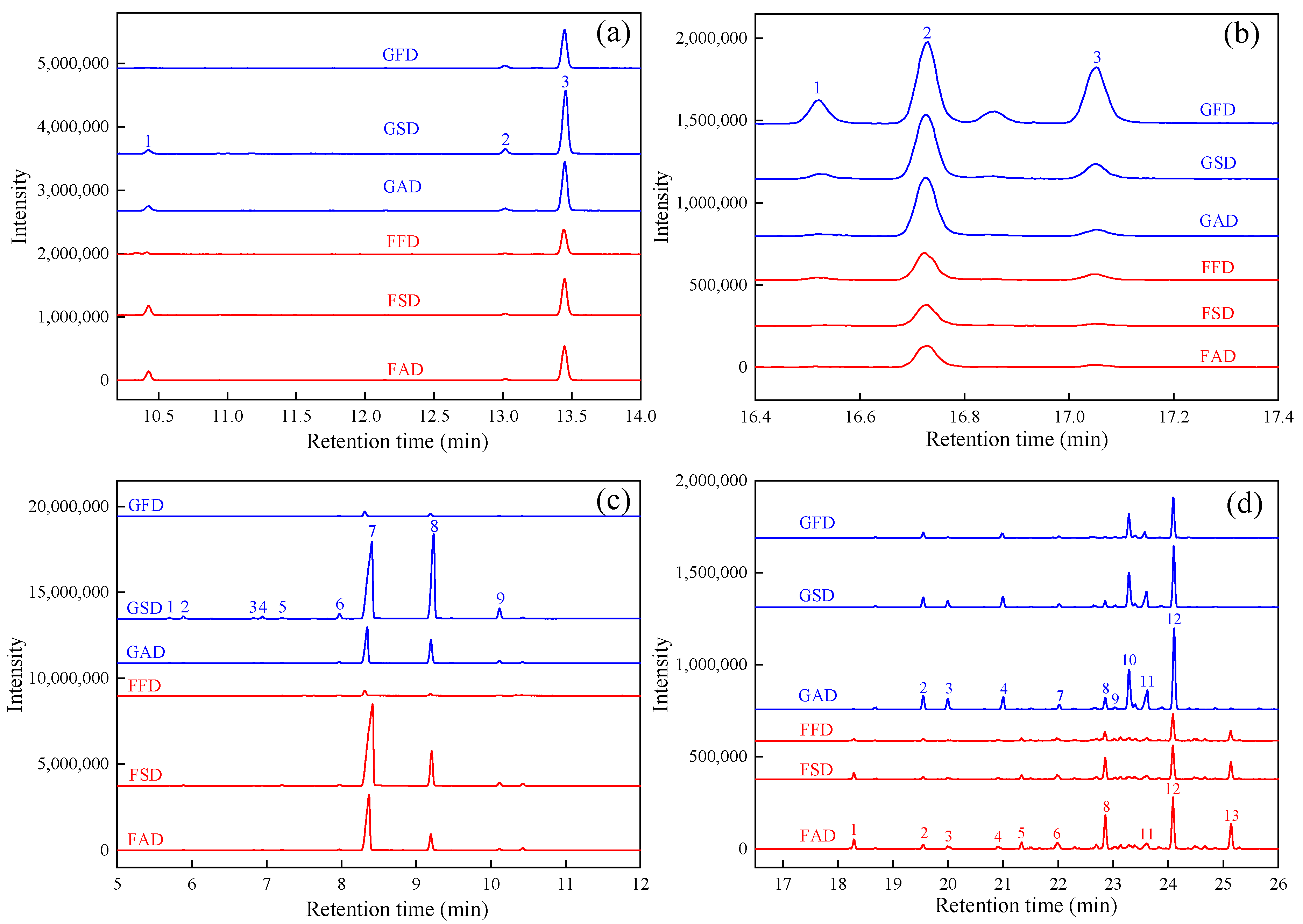
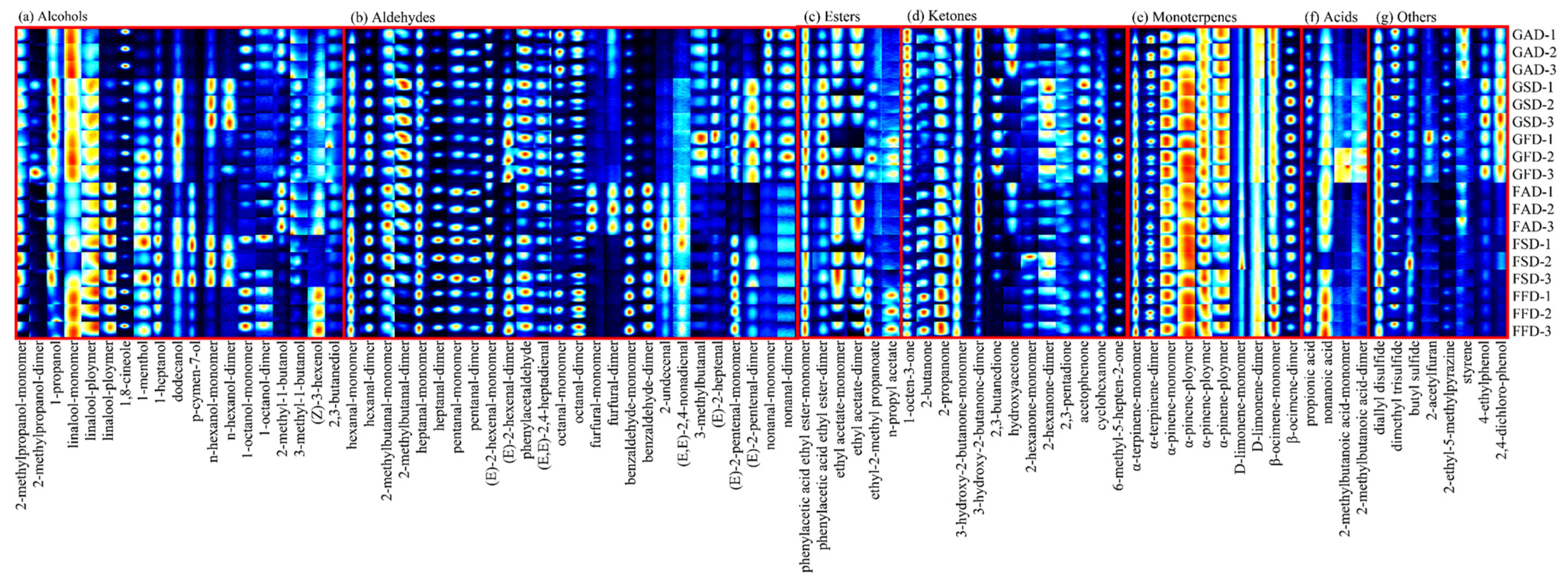
3.3. GC-IMS Analysis of Volatile Profile of Dried Citrus Peel as Effected by Drying Methods
3.3.1. Aldehydes and Alcohols
3.3.2. Monoterpenes
3.3.3. Esters, Ketones, Acids, and Others
3.3.4. Correlation and PCA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0D0K&sj=2021 (accessed on 21 August 2022).
- Kamal, G.M.; Anwar, F.; Hussain, A.I.; Sarri, N.; Ashraf, M.Y. Yield and chemical composition of citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011, 18, 1275–1282. [Google Scholar]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Olasehinde, T.A.; Adeoyo, O.O. From folk medicine to functional food: A review on the bioactive components and pharmacological properties of citrus peels. Orient. Pharm. Exp. Med. 2018, 18, 9–20. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Li, M.; Li, G.; Yang, Y.; Luo, H.; Huang, W.; Chung, H.; Ye, W.; Wang, G.; et al. Antiviral activity of polymethoxylated flavones from “Guangchenpi”, the edible and medicinal pericarps of Citrus reticulata ‘Chachi’. J. Agric. Food Chem. 2014, 62, 2182–2189. [Google Scholar] [CrossRef]
- Tian, C.; Xu, H.; Li, J.; Han, Z. Characteristics and intestinal immunomodulating activities of water-soluble pectic polysaccharides from Chenpi with different storage periods. J. Sci. Food Agric. 2018, 98, 3752–3757. [Google Scholar] [CrossRef]
- Guo, J.; Tao, H.; Cao, Y.; Ho, C.T.; Jin, S.; Huang, Q. Prevention of obesity and type 2 diabetes with aged citrus peel (chenpi) extract. J. Agric. Food Chem. 2016, 64, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Garza, G.; Antonyan, N.; Loera-Hernandez, I. Orange peel dehydration and creation of new edible products. Int. J. Food Eng. 2018, 4, 327–331. [Google Scholar] [CrossRef]
- National Pharmacopoeia Commission. Pharmacopoeia of People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Fu, Y.; Dou, X.; Zhang, L.; Qin, J.; Yang, M.; Luo, J. A comprehensive analysis of 201 pesticides for different herbal species-ready application using gas chromatography–tandem mass spectrometry coupled with quechers. J. Chromatogr. B 2019, 1125, 121730. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Guo, Y.; Liu, Y.; Yang, D.; Li, G.; Lü, S. Citri Reticulatae Pericarpium (Chenpi): Botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018, 220, 265–282. [Google Scholar] [CrossRef]
- Abd-Elwahab, S.M.; El-Tanbouly, N.D.; Moussa, M.Y.; Abdel-Monem, A.R.; Fayek, N.M. Antimicrobial and antiradical potential of four agro-waste citrus peels cultivars. J. Essent. Oil Bear. Plants 2016, 19, 1932–1942. [Google Scholar] [CrossRef]
- Bacanli, M.; Başaran, A.A.; Başaran, N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem. Toxicol. 2015, 81, 160–170. [Google Scholar] [CrossRef]
- Luo, M.; Luo, H.; Hu, P.; Yang, Y.; Wu, B.; Zheng, G. Evaluation of chemical components in Citri Reticulatae Pericarpium of different cultivars collected from different regions by GC-MS and HPLC. Food Sci. Nutr. 2018, 6, 400–416. [Google Scholar] [CrossRef]
- Qin, K.; Zheng, L.; Cai, H.; Cao, G.; Lou, Y.; Lu, T.; Shu, Y.; Zhou, W.; Cai, B. Characterization of chemical composition of pericarpium citri reticulatae volatile oil by comprehensive two-dimensional gas chromatography with high-resolution time-of-flight mass spectrometry. Evid.-Based Complementary Altern. Med. 2013, 2013, 237541. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in Citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lei, Z.; Zhang, G.; Cesar, A.; David, P. Metabolite profiles of essential oils in citrus peels and their taxonomic implications. Metabolomics 2015, 11, 952–963. [Google Scholar] [CrossRef]
- Fu, M.; Xu, Y.; Chen, Y.; Wu, J.; Yu, Y.; Zou, B.; An, K.; Xiao, G. Evaluation of bioactive flavonoids and antioxidant activity in Pericarpium Citri Reticulatae (Citrus reticulata ‘Chachi’) during storage. Food Chem. 2017, 230, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhao, P.; Duan, L.; Zheng, G.; Guo, L.; Yang, H.; Li, P. Simultaneous determination of six bioactive flavonoids in Citri Reticulatae Pericarpium by rapid resolution liquid chromatography coupled with triple quadrupole electrospray tandem mass spectrometry. Food Chem. 2013, 141, 3977–3983. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Dou, L.; Zhou, C.; Xu, F.; Zheng, G.; Li, P.; Liu, E. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’ by gas chromatograph-mass spectrometry based metabolomics approach. Food Chem. 2016, 212, 123–127. [Google Scholar] [CrossRef]
- Li, S.; Guan, X.; Gao, Z.; Lan, H.; Yin, Q.; Chu, C.; Yang, D.; Liu, E.; Zhou, P. A simple method to discriminate Guangchenpi and Chenpi by high-performance thin-layer chromatography and high-performance liquid chromatography based on analysis of dimethyl anthranilate. J. Chromatogr. B 2019, 1126, 121736. [Google Scholar] [CrossRef]
- Ni, L.; Zhang, F.; Han, M.; Zhang, L.; Luan, S.; Li, W.; Deng, H.; Lan, Z.; Wu, Z.; Luo, X.; et al. Qualitative analysis of the roots of Salvia miltiorrhiza and Salvia yunnanensis based on NIR, UHPLC and LC–MS-MS. J. Pharm. Biomed. Anal. 2019, 170, 295–304. [Google Scholar] [CrossRef]
- Yildiz, O.; Gurkan, H.; Sahingil, D.; Degirmenci, A.; Kemal, M.E.; Kolayli, S.; Hayaloglu, A.A. Floral authentication of some monofloral honeys based on volatile composition and physicochemical parameters. Eur. Food Res. Technol. 2022, 248, 2145–2155. [Google Scholar] [CrossRef]
- Karpinski, P.; Kruszewski, B.; Stachelska, M.A.; Szablowska, E. Development of volatile profile of kumpiak podlaski dry-cured ham during traditional ripening. Int. J. Food Sci. Technol. 2020, 55, 3630–3638. [Google Scholar] [CrossRef]
- Wroniak, M.; Raczyk, M.; Kruszewski, B.; Symoniuk, E.; Dach, D. Effect of deep frying of potatoes and tofu on thermo-oxidative changes of cold pressed rapeseed oil, cold pressed high oleic rapeseed oil and palm olein. Antioxidants 2021, 10, 1637. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, T.; He, L.; Zheng, J.; Sun, S.; Cao, Y. Analysis of the volatile compounds in dried tangerine peel of different years by optimized Solid Phase Micro-Extraction/Gas Chromatography-Mass Spectrometry (in Chinese). Mod. Food Sci. Technol. 2017, 33, 238–244. [Google Scholar]
- Majlát, P.; Erdös, Z.; Takás, J. Calculation and application of the retention indices in programmed-temperature gas chromatography. J. Chromatogr. A 1974, 91, 89–103. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, B.; Fu, Y.; Shi, Y.-G.; Chen, F.-L.; Guan, H.-N.; Liu, L.-L.; Zhang, C.-Y.; Zhu, P.-Y.; Liu, Y.; et al. HS-GC-IMS with PCA to analyze volatile flavor compounds across different production stages of fermented soybean whey tofu. Food Chem. 2021, 346, 128880. [Google Scholar] [CrossRef]
- Van Gemert, L.J. Odour Thresholds-Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners: Utrecht, The Netherlands, 2003. [Google Scholar]
- Ren, J.; Tai, Y.; Dong, M.; Shao, J.H.; Yang, S.Z.; Pan, S.Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef]
- Lin, J.; Rouseff, R.L. Characterization of aroma-impact compounds in cold-pressed grapefruit oil using time–intensity GC–olfactometry and GC–MS. Flavour Fragr. J. 2010, 16, 457–463. [Google Scholar] [CrossRef]
- Högnadóttir, A.; Rouseff, R.L. Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 998, 201–211. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, Y.; Zhang, H.; Deng, X.; Chen, F.; Xu, J. Volatile constituents of wild citrus Mangshanyegan (Citrus nobilis Lauriro) peel oil. J. Agric. Food Chem. 2012, 60, 2617–2628. [Google Scholar] [CrossRef]
- Fischer, A.; Grab, W.; Schieberle, P. Characterisation of the most odour-active compounds in a peel oil extract from pontianak oranges (Citrus nobilis var. Lour. microcarpa Hassk.). Eur. Food Res. Technol. 2008, 227, 735–744. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Li, Y.; Li, L. Research progress on the chemical composition of Citri reticulatae of different regions and different storage time (in Chinese). J. Food. Saf. Qual. 2020, 11, 3809–3817. [Google Scholar]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef]
- Ding, Y.; Huffaker, A.; Kollner, T.G.; Weckwerth, P.; Robert, C.A.M.; Spencer, J.L.; Lipka, A.E.; Schmelz, E.A. Selinene volatiles are essential precursors for maize defense promoting fungal pathogen resistance. Plant Physiol. 2017, 175, 1455–1468. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Li, F.; Chen, Z.; Li, X.; Zhu, L.; Wang, G.; Yu, J.; Huang, D.; Lang, Z. The maize transcription factor EREB58 mediates the jasmonate-induced production of sesquiterpene volatiles. Plant J. 2015, 84, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Deng, L.; Zhou, Y.; Yao, S.; Zeng, K. Analysis of changes in volatile constituents and expression of genes involved in terpenoid metabolism in oleocellosis peel. Food Chem. 2018, 243, 269–276. [Google Scholar] [CrossRef]
- Murat, G.; Tulin, O.A.; Ebru, K. Comparison of lipids, fatty acids and volatile compounds of various kumquat species using HS/GC/MS/FID techniques. J. Sci. Food Agric. 2015, 95, 1268–1273. [Google Scholar]
- Fan, X.; Jiao, X.; Liu, J.; Jia, M.; Blanchard, C.; Zhou, Z. Characterizing the volatile compounds of different sorghum cultivars by both GC-MS and HS-GC-IMS. Food Res. Int. 2021, 140, 109975. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Q.; Xiang, L.; Liang, Y. Recent advances in volatiles of teas. Molecules 2016, 21, 338. [Google Scholar] [CrossRef] [PubMed]
- Flavornet. Available online: http://www.flavornet.org/flavornet.html (accessed on 20 August 2022).
- Qiao, Y.; Xie, B.J.; Zhang, Y.; Zhang, Y.; Fan, G.; Yao, X.L.; Pan, S.Y. Characterization of aroma active compounds in fruit juice and peel oil of jinchen sweet orange fruit (Citrus sinensis (L.) Osbeck) by GC-MS and GC-O. Molecules 2008, 13, 1333–1344. [Google Scholar] [CrossRef]
- Bazemore, R.; Goodner, K.; Rouseff, R. Volatiles from unpasteurized and excessively heated orange juice analyzed with solid phase microextraction and GC-olfactometry. J. Food Sci. 1999, 64, 800–803. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, M.; Hu, J.; Zeng, S.; Bai, X. Influence of pH on the formation and radical scavenging activity of volatile compounds produced by heating glucose with histidine/tyrosine. Eur. Food Res. Technol. 2012, 234, 333–343. [Google Scholar] [CrossRef]
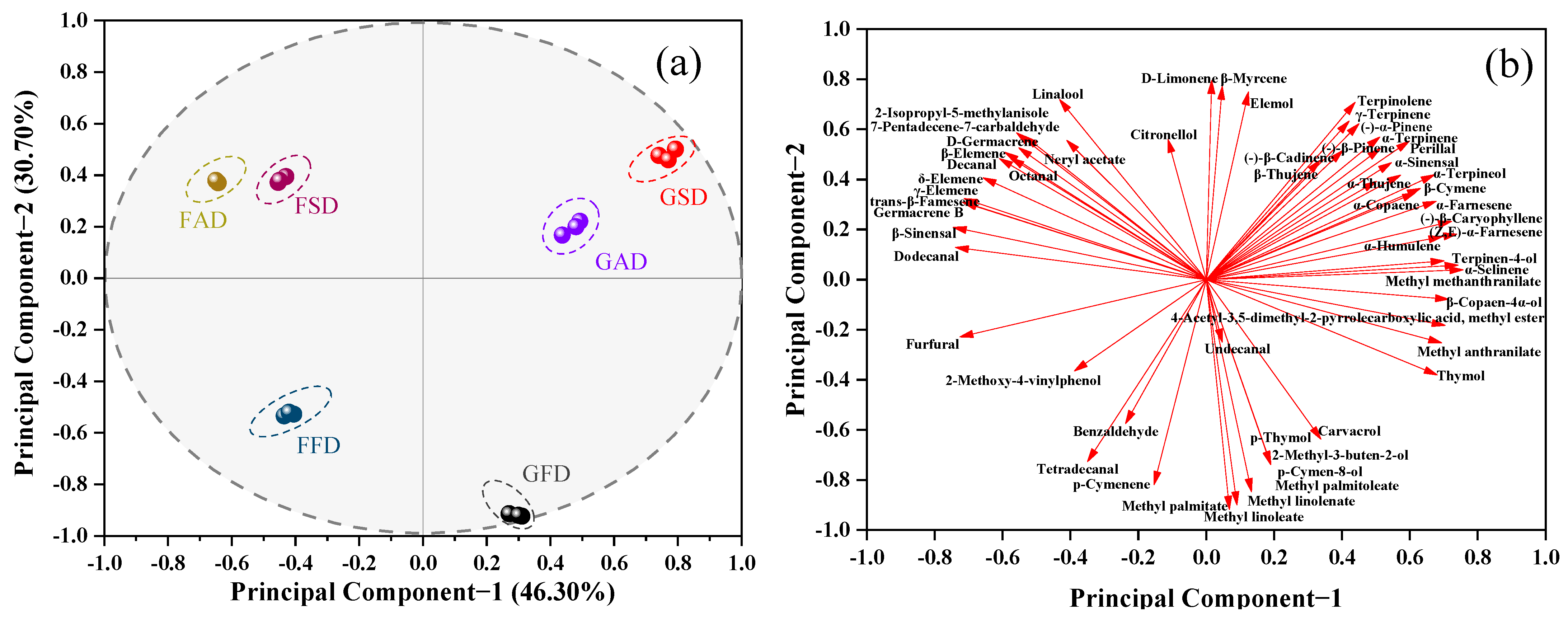
| Compound | CAS | Identification 2 | LRI 1 | Formula | FAD | FSD | FFD | GAD | GSD | GFD |
|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||||
| 2-Methyl-3-buten-2-ol | 115-18-4 | MS + LRI | <800 | C5H10O | nd c | nd | nd | nd | nd | 14.84 ± 0.33 |
| Linalool | 78-70-6 | MS + LRI * | 1101 | C10H18O | 170.40 ± 4.76 a | 175.11 ± 4.40 a | 29.35 ± 0.59 d | 74.41 ± 2.07 b | 67.17 ± 2.08 c | 13.27 ± 0.51 e |
| Terpinen-4-ol | 562-74-3 | MS + LRI * | 1181 | C10H18O | 19.18 ± 0.51 d | 22.45 ± 0.26 d | 22.39 ± 0.48 d | 36.18 ± 1.95 c | 73.19 ± 5.83 a | 45.94 ± 2.23 b |
| p-Cymen-8-ol | 1197-01-9 | MS + LRI | 1188 | C10H14O | nd | nd | nd | nd | nd | 14.24 ± 0.50 |
| α-Terpineol | 98-55-5 | MS + LRI * | 1194 | C10H18O | 112.70 ± 3.88 d | 116.38 ± 3.58 d | 85.02 ± 0.04 e | 156.23 ± 4.28 b | 215.32 ± 8.42 a | 130.94 ± 7.13 c |
| Citronellol | 106-22-9 | MS + LRI * | 1230 | C10H20O | 26.22 ± 1.55 a | 15.65 ± 0.94 b | nd | 13.36 + 3.11 b | 14.09 ± 0.22 b | 12.10 ± 2.40 b |
| Elemol | 639-99-6 | MS + LRI | 1552 | C15H26O | 23.46 ± 0.70 b | 12.28 ± 0.92 d | nd | 29.45 ± 0.69 a | 16.78 ± 0.72 c | nd |
| β-Copaen-4α-ol | 124753-76-0 | MS + LRI | 1588 | C15H24O | nd | nd | nd | 43.46 ± 1.43 a | 30.73 ± 4.05 b | 30.74 ± 0.71 b |
| Sum | 351.96 ± 11.39 b | 341.88 ± 6.38 b | 136.77 ± 0.07 d | 353.10 ± 12.15 b | 417.28 ± 21.32 a | 262.07 ± 13.81 c | ||||
| Aromatic hydrocarbons and ethers | ||||||||||
| β-Cymene | 535-77-3 | MS + LRI * | 1027 | C10H14 | 13.73 ± 0.28 d | 16.74 ± 0.41 d | 10.58 ± 1.46 e | 32.50 ± 0.00 b | 97.20 ± 0.71 a | 27.63 ± 2.54 c |
| p-Cymenene | 1195-32-0 | MS + LRI | 1092 | C10H12 | nd | nd | 41.96 ± 2.28 a | nd | nd | 24.85 ± 0.17 b |
| 2-Isopropyl-5-methylanisole | 1076-56-8 | MS + LRI * | 1236 | C11H16O | 41.20 ± 2.02 a | 31.03 ± 0.12 b | nd | 14.64 ± 1.00 c | nd | nd |
| Sum | 54.93 ± 2.30 b | 47.77 ± 0.29 c | 52.54 ± 3.74 bc | 47.14 ± 1.00 c | 97.20 ± 0.71 a | 52.48 ± 2.37 bc | ||||
| Phenols | ||||||||||
| p-Thymol | 3228-02-2 | MS | 1287 | C10H14O | nd | nd | nd | nd | nd | 21.79 ± 0.65 |
| Thymol | 89-83-8 | MS + LRI * | 1293 | C10H14O | 27.81 ± 0.06 e | 27.61 ± 2.94 e | 35.05 ± 1.79 d | 67.64 ± 2.25 c | 74.64 ± 3.39 b | 91.74 ± 3.04 a |
| Carvacrol | 499-75-2 | MS + LRI * | 1302 | C10H14O | nd | nd | nd | nd | 15.23 ± 2.48 b | 53.96 ± 2.48 a |
| 2-Methoxy-4-vinylphenol | 7786-61-0 | MS + LRI | 1316 | C9H10O2 | 37.48 ± 1.58 b | 27.08 ± 0.73 c | 156.97 ± 0.17 a | 12.15 ± 0.14 d | 14.41 ± 2.56 d | 14.65 ± 1.35 d |
| Sum | 65.28 ± 1.64 e | 54.69 ± 3.67 f | 192.02 ± 1.96 a | 79.79 ± 2.39 d | 104.29 ± 3.32 c | 182.14 ± 7.52 b | ||||
| N-containing compounds | ||||||||||
| Methyl anthranilate | 134-20-3 | MS + LRI * | 1345 | C8H9NO2 | nd | nd | nd | 11.76 ± 0.37 c | 22.01 ± 0.61 b | 23.61 ± 0.32 a |
| Methyl methanthranilate | 85-91-6 | MS + LRI * | 1411 | C9H11NO2 | 242.36 ± 23.30 c | 113.66 ± 6.84 c | 207.97 ± 6.47 c | 3393.14 ± 137.30 a | 3376.18 ± 124.59 a | 2337.90 ± 102.47 b |
| 4-Acetyl-3,5-dimethyl-2-pyrrolecarboxylic acid, methyl ester | 89909-47-7 | MS | 1644 | C10H13NO3 | nd | nd | nd | 12.58 ± 2.52 a | 9.72 ± 0.01 a | 11.60 ± 0.76 a |
| Sum | 242.36 ± 23.30 c | 113.66 ± 6.84 c | 207.97 ± 6.47 c | 3417.47 ± 134.41 a | 3407.91 ± 125.20 a | 2373.11 ± 102.91 b | ||||
| Aldehydes | ||||||||||
| Furfural | 98-01-1 | MS + LRI * | 832 | C5H4O2 | 69.68 ± 11.16 a | 54.02 ± 0.45 b | 76.90 ± 6.41 a | 28.14 ± 3.56 c | 13.30 ± 0.24 d | 39.24 ± 0.66 c |
| Benzaldehyde | 100-52-7 | MS + LRI * | 964 | C7H6O | nd | nd | 24.72 ± 0.90 a | nd | nd | 8.84 ± 1.89 b |
| Octanal | 124-13-0 | MS + LRI * | 1004 | C8H16O | 12.56 ± 0.20 a | 10.97 ± 0.20 a | nd | nd | nd | nd |
| Decanal | 112-31-2 | MS + LRI * | 1206 | C10H20O | 78.74 ± 1.46 a | 66.15 ± 0.86 b | 36.84 ± 0.17 c | 38.24 ± 0.80 c | 30.23 ± 0.30 d | 30.39 ± 1.84 d |
| Perillal | 2111-75-3 | MS + LRI | 1277 | C10H14O | 30.27 ± 0.03 c | 31.44 ± 0.97 c | nd | 61.20 ± 2.69 b | 68.11 ± 0.43 a | 29.54 ± 1.42 c |
| Undecanal | 112-44-7 | MS + LRI * | 1308 | C11H22O | 10.63 ± 0.83 a | nd | nd | 10.12 ± 0.37 a | nd | 11.48 ± 0.76 a |
| Dodecanal | 112-54-9 | MS + LRI * | 1407 | C12H24O | 80.41 ± 0.91 a | 49.26 ± 2.50 c | 66.26 ± 0.10 b | nd | nd | nd |
| Tetradecanal | 124-25-4 | MS + LRI | 1611 | C14H28O | 11.51 ± 1.59 c | nd | 23.10 ± 0.93 a | nd | nd | 15.19 ± 0.43 b |
| β-Sinensal | 60066-88-8 | MS + LRI | 1700 | C15H22O | 136.96 ± 11.34 a | 94.58 ± 0.65 b | 93.48 ± 7.54 b | nd | nd | nd |
| α-Sinensal | 17909-77-2 | MS + LRI | 1758 | C15H22O | 178.01 ± 17.33 c | 140.49 ± 1.45 d | 68.38 ± 9.69 e | 610.97 ± 6.81 a | 428.20 ± 16.82 b | 118.09 ± 13.40 d |
| 7-Pentadecene-7-carbaldehyde | - | MS + LRI | 1762 | C16H30O | 64.42 ± 5.15 b | 76.56 ± 2.05 a | 11.24 ± 0.97 c | 10.92 ± 2.48 c | 16.65 ± 0.38 c | nd |
| Sum | 673.18 ± 49.99 b | 523.47 ± 7.01 c | 400.93 ± 24.86 d | 759.60 ± 1.86 a | 556.50 ± 16.22 c | 252.77 ± 11.62 e | ||||
| Monoterpenes | ||||||||||
| α-Thujene | 2867-05-2 | MS + LRI | 929 | C10H16 | nd | nd | nd | nd | 29.81 ± 0.24 | nd |
| (-)-α-Pinene | 7785-26-4 | MS + LRI * | 937 | C10H16 | 13.07 ± 0.21 d | 36.80 ± 0.20 b | nd | 18.71 ± 0.21 c | 94.63 ± 0.48 a | nd |
| β-Thujene | 28634-89-1 | MS + LRI | 976 | C10H16 | nd | 9.46 ± 0.26 a | nd | nd | 10.84 ± 0.09 a | nd |
| (-)-β-Pinene | 18172-67-3 | MS + LRI * | 981 | C10H16 | nd | 17.60 ± 0.21 b | nd | 13.48 ± 0.18 c | 71.60 ± 0.02 a | nd |
| β-Myrcene | 123-35-3 | MS + LRI * | 993 | C10H16 | 40.43 ± 0.87 c | 95.94 ± 0.56 a | nd | 21.81 ± 0.08 d | 86.64 ± 0.32 b | nd |
| α-Terpinene | 99-86-5 | MS + LRI * | 1019 | C10H16 | nd | 16.25 ± 0.60 b | nd | 13.24 ± 0.05 c | 47.09 ± 1.40 a | nd |
| D-Limonene | 5989-27-5 | MS * | 1033 | C10H16 | 1768.96 ± 18.75 c | 3599.53 ± 29.64 a | 131.65 ± 10.49 e | 975.48 ± 8.37 d | 3159.90 ± 12.33 b | 98.57 ± 29.02 f |
| γ-Terpinene | 99-85-4 | MS + LRI * | 1061 | C10H16 | 176.09 ± 2.20 d | 382.14 ± 3.12 b | 20.90 ± 1.12 e | 260.37 ± 0.95 c | 1108.98 ± 10.89 a | 26.77 ± 5.69 e |
| Terpinolene | 586-62-9 | MS + LRI * | 1091 | C10H16 | 23.33 ± 0.41 c | 34.07 ± 1.04 b | nd | 32.57 ± 0.81 b | 93.36 ± 3.94 a | nd |
| Sum | 2021.88 ± 22.44 c | 4191.78 ± 33.17 b | 152.54 ± 11.62 e | 1335.66 ± 10.12 d | 4702.86 ± 28.73 a | 125.34 ± 34.71 e | ||||
| Sesquiterpenes | ||||||||||
| δ-Elemene | 20307-84-0 | MS + LRI | 1341 | C15H24 | 162.62 ± 2.75 a | 104.24 ± 2.66 b | 36.24 ± 4.84 c | 9.05 ± 0.97 d | nd | nd |
| α-Copaene | 3856-25-5 | MS + LRI | 1380 | C15H24 | 22.11 ± 0.49 c | 12.31 ± 0.23 d | 9.81 ± 0.29 d | 62.78 ± 3.87 a | 47.80 ± 2.29 b | 24.78 ± 0.89 c |
| β-Elemene | 515-13-9 | MS + LRI | 1393 | C15H24 | 93.99 ± 2.69 a | 60.92 ± 2.53 b | 29.10 ± 1.75 c | 29.92 ± 0.99 c | 20.27 ± 0.99 d | 13.40 ± 0.76 e |
| (-)-β-Caryophyllene | 87-44-5 | MS + LRI | 1422 | C15H24 | 22.28 ± 2.48 c | 11.96 ± 1.07 c | 9.93 ± 1.02 c | 225.01 ± 10.12 a | 214.38 ± 13.52 a | 94.71 ± 4.94 b |
| γ-Elemene | 29873-99-2 | MS + LRI | 1433 | C15H24 | 78.73 ± 1.31 a | 52.65 ± 1.03 b | 32.88 ± 4.17 c | nd | nd | nd |
| trans-β-Famesene | 18794-84-8 | MS + LRI | 1454 | C15H24 | 103.68 ± 1.54 a | 64.67 ± 2.22 b | 44.62 ± 3.88 c | nd | nd | nd |
| α-Humulene | 6753-98-6 | MS + LRI | 1456 | C15H24 | nd | nd | nd | 39.47 ± 2.61 a | 28.45 ± 1.78 b | 13.90 ± 0.96 c |
| D-Germacrene | 23986-74-5 | MS + LRI | 1482 | C15H24 | 143.79 ± 4.65 a | 89.04 ± 1.03 b | 39.97 ± 5.03 d | 49.94 ± 3.12 c | 27.32 ± 1.76 e | nd |
| (Z,E)-α-Farnesene | 26560-14-5 | MS + LRI | 1492 | C15H24 | nd | nd | nd | 36.96 ± 1.38 a | 35.68 ± 1.87 a | 16.06 ± 0.63 b |
| α-Selinene | 473-13-2 | MS + LRI | 1500 | C15H24 | nd | nd | 16.94 ± 1.60 d | 140.77 ± 7.32 a | 124.67 ± 6.18 b | 74.27 ± 3.95 c |
| α-Farnesene | 502-61-4 | MS + LRI * | 1510 | C15H24 | 143.47 ± 0.26 d | 88.93 ± 0.20 d | 60.82 ± 6.99 d | 1291.76 ± 62.20 a | 1101.39 ± 67.64 b | 353.83 ± 23.95 c |
| (-)-β-Cadinene | 523-47-7 | MS + LRI | 1527 | C15H24 | 89.05 ± 3.30 b | 59.20 ± 0.67 cd | 52.09 ± 3.13 d | 119.63 ± 4.85 a | 94.10 ± 5.94 b | 62.66 ± 3.51 c |
| Germacrene B | 15423-57-1 | MS + LRI | 1562 | C15H24 | 147.90 ± 4.10 a | 98.68 ± 0.96 b | 66.13 ± 14.52 c | nd | nd | nd |
| Sum | 1007.63 ± 18.60 c | 642.60 ± 12.19 d | 398.52 ± 46.64 e | 2005.29 ± 95.50 a | 1694.05 ± 101.97 b | 653.59 ± 39.59 d | ||||
| Esters | ||||||||||
| Neryl acetate | 141-12-8 | MS + LRI * | 1365 | C12H20O2 | 20.22 ± 0.00 a | 11.60 ± 0.21 b | nd | 11.48 ± 0.39 b | nd | nd |
| Methyl palmitoleate | 1120-25-8 | MS + LRI | 1902 | C17H32O2 | nd | nd | nd | nd | nd | 22.83 ± 1.94 |
| Methyl palmitate | 112-39-0 | MS + LRI * | 1925 | C17H34O2 | nd | nd | 232.79 ± 12.51 b | nd | nd | 527.87 ± 37.65 a |
| Methyl linoleate | 112-63-0 | MS + LRI * | 2094 | C19H34O2 | nd | nd | 100.03 ± 12.25 b | nd | nd | 275.04 ± 13.19 a |
| Methyl linolenate | 301-00-8 | MS + LRI | 2101 | C19H32O2 | nd | nd | 42.04 ± 5.94 b | nd | nd | 192.85 ± 15.55 a |
| Sum | 20.22 ± 0.00 c | 11.60 ± 0.21 c | 374.86 ± 30.70 b | 11.48 ± 0.39 c | nd | 1018.60 ± 68.32 a | ||||
| Total | 4437.44 ± 83.05 e | 5927.43 ± 48.32 c | 1916.16 ± 78.47 f | 8009.52 ± 254.11 b | 10980.09 ± 265.03 a | 4920.10 ± 206.68 d | ||||
| Compound | Threshold in Water from the Literature (mg/kg) | OAVs | |||||
|---|---|---|---|---|---|---|---|
| FAD | FSD | FFD | GAD | GSD | GFD | ||
| Alcohols | |||||||
| Linalool | 0.028 | 6086 | 6254 | 1048 | 2658 | 2399 | 474 |
| Terpinen-4-ol | 6.4 | 3 | 4 | 4 | 6 | 11 | 7 |
| α-Terpineol | 10 | 11 | 12 | 9 | 16 | 22 | 13 |
| Citronellol | 0.062 | 423 | 252 | __ | 216 | 227 | 195 |
| Elemol | 0.1 | 235 | 123 | __ | 295 | 168 | __ |
| Aromatic hydrocarbons and ethers | |||||||
| β-Cymene | 0.8 | 17 | 21 | 13 | 41 | 121 | 35 |
| p-Cymenene | 0.665 | __ | __ | 63 | __ | __ | 37 |
| Phenols | |||||||
| Thymol | 0.79 | 35 | 35 | 44 | 86 | 94 | 116 |
| Carvacrol | 2.29 | __ | __ | __ | __ | 7 | 24 |
| 2-Methoxy-4-vinylphenol | 0.003 | 12,493 | 9028 | 52,323 | 4050 | 4805 | 4883 |
| N-containing compounds | |||||||
| Methyl anthranilate | 0.003 | __ | __ | __ | 3919 | 7336 | 7872 |
| Methyl methanthranilate | 0.349 | 694 | 326 | 596 | 9722 | 9674 | 6699 |
| Aldehydes | |||||||
| Furfural | 3 | 23 | 18 | 26 | 9 | 4 | 13 |
| Benzaldehyde | 1 | __ | __ | 25 | __ | __ | 9 |
| Octanal | 0.08 | 157 | 137 | __ | __ | __ | __ |
| Decanal | 0.07 | 1125 | 945 | 526 | 546 | 432 | 434 |
| Perillal | 0.03 | 1009 | 1048 | __ | 2040 | 2270 | 985 |
| Undecanal | 0.04 | 266 | __ | __ | 253 | __ | 287 |
| Dodecanal | 0.055 | 1462 | 896 | 1205 | __ | __ | __ |
| Tetradecanal | 0.067 | 172 | __ | 345 | __ | __ | 227 |
| α-Sinensal | 0.22 | 809 | 639 | 311 | 2777 | 1946 | 537 |
| Monoterpenes | |||||||
| (-)-α-Pinene | 0.1 | 131 | 368 | __ | 187 | 946 | __ |
| (-)-β-Pinene | 4.16 | __ | 4 | __ | 3 | 17 | __ |
| β-Myrcene | 0.099 | 408 | 969 | __ | 220 | 875 | __ |
| α-Terpinene | 0.08 | __ | 203 | __ | 166 | 589 | __ |
| D-limonene | 1.2 | 1474 | 3000 | 110 | 813 | 2633 | 82 |
| γ-Terpinene | 0.6 | 293 | 637 | 35 | 434 | 1848 | 45 |
| Terpinolene | 0.041 | 569 | 831 | __ | 794 | 2277 | __ |
| Sesquiterpenes | |||||||
| (-)-β-caryophyllene | 1.54 | 14 | 8 | 6 | 146 | 139 | 62 |
| α-humulene | 0.39 | __ | __ | __ | 101 | 73 | 36 |
| Compound | CAS | Formula | MW | RI | RT (s) | DT (ms) | Comment |
|---|---|---|---|---|---|---|---|
| Alcohols | |||||||
| 2-Methylpropanol | 78-83-1 | C4H10O | 74.1 | 655.3 | 178.498 | 1.1728 | Monomer |
| 2-Methylpropanol | 78-83-1 | C4H10O | 74.1 | 638.2 | 170.541 | 1.3634 | Dimer |
| 1-Propanol | 71-23-8 | C3H8O | 60.1 | 562.9 | 141.808 | 1.1113 | |
| Linalool | 78-70-6 | C10H18O | 154.3 | 1098.9 | 806.393 | 1.2181 | Monomer |
| Linalool | 78-70-6 | C10H18O | 154.3 | 1099.4 | 807.109 | 1.6902 | Polymer |
| Linalool | 78-70-6 | C10H18O | 154.3 | 1108.2 | 821.018 | 2.2416 | Polymer |
| 1-Menthol | 2216-51-5 | C10H20O | 156.3 | 1176.5 | 928.37 | 1.8827 | |
| 1,8-Cineole | 470-82-6 | C10H18O | 154.3 | 1024 | 683.053 | 1.2952 | |
| 1-Heptanol | 111-70-6 | C7H16O | 116.2 | 967.5 | 570.796 | 1.3964 | |
| Dodecanol | 112-53-8 | C12H26O | 186.3 | 1495.5 | 1430.277 | 1.6136 | |
| p-Cymen-7-ol | 536-60-7 | C10H14O | 150.2 | 1314.5 | 1145.531 | 1.3218 | |
| n-Hexanol | 111-27-3 | C6H14O | 102.2 | 875.6 | 394.101 | 1.3286 | Monomer |
| n-Hexanol | 111-27-3 | C6H14O | 102.2 | 874.4 | 392.236 | 1.6395 | Dimer |
| 1-Octanol | 111-87-5 | C8H18O | 130.2 | 1077.4 | 772.375 | 1.474 | Monomer |
| 1-Octanol | 111-87-5 | C8H18O | 130.2 | 1075.3 | 769.058 | 1.8822 | Dimer |
| 2-Methyl-1-butanol | 137-32-6 | C5H12O | 88.1 | 712.1 | 215.353 | 1.2361 | |
| 3-Methyl-1-butanol | 123-51-3 | C5H12O | 88.1 | 732.7 | 233.387 | 1.2497 | |
| (Z)-3-Hexenol | 928-96-1 | C6H12O | 100.2 | 868.3 | 383.342 | 1.2298 | |
| 2,3-Butanediol | 513-85-9 | C4H10O2 | 90.1 | 791.1 | 291.806 | 1.3693 | |
| Aldehydes | |||||||
| Hexanal | 66-25-1 | C6H12O | 100.2 | 793.9 | 294.682 | 1.2569 | Monomer |
| Hexanal | 66-25-1 | C6H12O | 100.2 | 794.5 | 295.284 | 1.5665 | Dimer |
| 2-Methylbutanal | 96-17-3 | C5H10O | 86.1 | 671.2 | 186.926 | 1.1585 | Monomer |
| 2-Methylbutanal | 96-17-3 | C5H10O | 86.1 | 664.6 | 183.314 | 1.4033 | Dimer |
| Heptanal | 111-71-7 | C7H14O | 114.2 | 901.1 | 435.987 | 1.3322 | Monomer |
| Heptanal | 111-71-7 | C7H14O | 114.2 | 900.4 | 434.707 | 1.6959 | Dimer |
| Pentanal | 110-62-3 | C5H10O | 86.1 | 699.5 | 205.405 | 1.1864 | Monomer |
| Pentanal | 110-62-3 | C5H10O | 86.1 | 697.4 | 203.832 | 1.4221 | Dimer |
| (E)-2-Hexenal | 6728-26-3 | C6H10O | 98.1 | 845.6 | 352.95 | 1.1803 | Monomer |
| (E)-2-Hexenal | 6728-26-3 | C6H10O | 98.1 | 852.6 | 361.851 | 1.5176 | Dimer |
| Phenylacetaldehyde | 122-78-1 | C8H8O | 120.2 | 1048.9 | 726.125 | 1.2491 | |
| (E,E)-2,4-Heptadienal | 4313-03-5 | C7H10O | 110.2 | 1018.1 | 672.456 | 1.1866 | |
| Octanal | 124-13-0 | C8H16O | 128.2 | 1012.2 | 661.438 | 1.4035 | Monomer |
| Octanal | 124-13-0 | C8H16O | 128.2 | 1007.9 | 653.298 | 1.8273 | Dimer |
| Furfural | 98-01-1 | C5H4O2 | 96.1 | 831.6 | 335.997 | 1.0834 | Monomer |
| Furfural | 98-01-1 | C5H4O2 | 96.1 | 830.7 | 334.948 | 1.3324 | Dimer |
| Benzaldehyde | 100-52-7 | C7H6O | 106.1 | 956.6 | 547.609 | 1.1494 | Monomer |
| Benzaldehyde | 100-52-7 | C7H6O | 106.1 | 956.6 | 547.609 | 1.465 | Dimer |
| 2-Undecenal | 2463-77-6 | C11H20O | 168.3 | 1380.8 | 1249.854 | 1.4952 | |
| (E,E)-2,4-Nonadienal | 5910-87-2 | C9H14O | 138.2 | 1240.1 | 1028.477 | 1.3537 | |
| 3-Methylbutanal | 590-86-3 | C5H10O | 86.1 | 655.1 | 178.408 | 1.1959 | |
| (E)-2-Heptenal | 18829-55-5 | C7H12O | 112.2 | 954.8 | 543.803 | 1.2541 | |
| (E)-2-Pentenal | 1576-87-0 | C5H8O | 84.1 | 754.1 | 253.909 | 1.1022 | Monomer |
| (E)-2-pentenal | 1576-87-0 | C5H8O | 84.1 | 754.2 | 253.99 | 1.3603 | Dimer |
| Nonanal | 124-19-6 | C9H18O | 142.2 | 1115.4 | 832.379 | 1.4722 | Monomer |
| Nonanal | 124-19-6 | C9H18O | 142.2 | 1113.9 | 829.928 | 1.9474 | Dimer |
| Esters | |||||||
| Phenylacetic acid ethyl ester | 101-97-3 | C10H12O2 | 164.2 | 1225.5 | 1005.553 | 1.2959 | Monomer |
| Phenylacetic acid ethyl ester | 101-97-3 | C10H12O2 | 164.2 | 1224.3 | 1003.65 | 1.7843 | Dimer |
| Ethyl acetate | 141-78-6 | C4H8O2 | 88.1 | 601.9 | 156.127 | 1.0953 | Monomer |
| Ethyl acetate | 141-78-6 | C4H8O2 | 88.1 | 605.4 | 157.428 | 1.3376 | Dimer |
| Ethyl-2-methyl propanoate | 97-62-1 | C6H12O2 | 116.2 | 727.2 | 228.365 | 1.1956 | |
| n-Propyl acetate | 109-60-4 | C5H10O2 | 102.1 | 733.2 | 233.845 | 1.1685 | |
| Ketones | |||||||
| 1-Octen-3-one | 4312-99-6 | C8H14O | 126.2 | 995.3 | 628.789 | 1.2859 | |
| 2-Butanone | 78-93-3 | C4H8O | 72.1 | 595.1 | 153.636 | 1.0617 | |
| 2-Propanone | 67-64-1 | C3H6O | 58.1 | 509.2 | 122.061 | 1.1191 | |
| 3-Hydroxy-2-butanone | 513-86-0 | C4H8O2 | 88.1 | 722.9 | 224.501 | 1.0599 | Monomer |
| 3-Hydroxy-2-butanone | 513-86-0 | C4H8O2 | 88.1 | 717.7 | 220.027 | 1.328 | Dimer |
| 2,3-Butanedione | 431-03-8 | C4H6O2 | 86.1 | 633 | 168.329 | 1.1685 | |
| Hydroxyacetone | 116-09-6 | C3H6O2 | 74.1 | 638.3 | 170.599 | 1.0427 | |
| 2-Hexanone | 591-78-6 | C6H12O | 100.2 | 786.1 | 286.633 | 1.1901 | Monomer |
| 2-Hexanone | 591-78-6 | C6H12O | 100.2 | 787.2 | 287.739 | 1.5056 | Dimer |
| 2,3-Pentadione | 600-14-6 | C5H8O2 | 100.1 | 660.2 | 180.929 | 1.2261 | |
| Acetophenone | 98-86-2 | C8H8O | 120.2 | 1067.6 | 756.873 | 1.191 | |
| Cyclohexanone | 108-94-1 | C6H10O | 98.1 | 897.6 | 429.786 | 1.1484 | |
| 6-Methyl-5-hepten-2-one | 110-93-0 | C8H14O | 126.2 | 990.1 | 618.121 | 1.173 | |
| Monoterpenes | |||||||
| α-Terpinene | 99-86-5 | C10H16 | 136.2 | 1017 | 670.467 | 1.2204 | Monomer |
| α-Terpinene | 99-86-5 | C10H16 | 136.2 | 1017.4 | 671.183 | 1.7217 | Dimer |
| α-Pinene | 80-56-8 | C10H16 | 136.2 | 942 | 516.604 | 1.217 | Monomer |
| α-Pinene | 80-56-8 | C10H16 | 136.2 | 940 | 512.312 | 1.2954 | Polymer |
| α-Pinene | 80-56-8 | C10H16 | 136.2 | 938.3 | 508.735 | 1.6688 | Polymer |
| α-Pinene | 80-56-8 | C10H16 | 136.2 | 936.5 | 505.158 | 1.7341 | Polymer |
| D-Limonene | 5989-27-5 | C10H16 | 136.2 | 1040.8 | 712.473 | 1.2165 | Monomer |
| D-Limonene | 5989-27-5 | C10H16 | 136.2 | 1038.9 | 709.238 | 1.2987 | Polymer |
| β-Ocimene | 13877-91-3 | C10H16 | 136.2 | 1057.2 | 739.969 | 1.2143 | Monomer |
| β-Ocimene | 13877-91-3 | C10H16 | 136.2 | 1057.7 | 740.777 | 1.6943 | Dimer |
| Acids | |||||||
| Propionic acid | 79-09-4 | C3H6O2 | 74.1 | 704.6 | 209.337 | 1.1119 | |
| Nonanoic acid | 112-05-0 | C9H18O2 | 158.2 | 1279.5 | 1090.406 | 1.5442 | |
| 2-Methylbutanoic acid | 116-53-0 | C5H10O2 | 102.1 | 890.7 | 417.914 | 1.2097 | Monomer |
| 2-Methylbutanoic acid | 116-53-0 | C5H10O2 | 102.1 | 890.7 | 417.914 | 1.4764 | Dimer |
| Others | |||||||
| Diallyl disulfide | 2179-57-9 | C6H10S2 | 146.3 | 1090.7 | 793.377 | 1.1873 | |
| Dimethyl trisulfide | 3658-80-8 | C2H6S3 | 126.3 | 978.3 | 593.635 | 1.2952 | |
| Butyl sulfide | 544-40-1 | C8H18S | 146.3 | 1085.6 | 785.453 | 1.3011 | |
| 2-Acetylfuran | 1192-62-7 | C6H6O2 | 110.1 | 909.8 | 452.045 | 1.1171 | |
| 2-Ethyl-5-methylpyrazine | 13360-64-0 | C7H10N2 | 122.2 | 1002.1 | 642.196 | 1.1768 | |
| Styrene | 100-42-5 | C8H8 | 104.2 | 904.6 | 442.242 | 1.0594 | |
| 4-Ethylphenol | 123-07-9 | C8H10O | 122.2 | 1198.2 | 962.542 | 1.1888 | |
| 2,4-Dichloro-phenol | 120-83-2 | C6H4Cl2O | 163 | 1140.7 | 872.045 | 1.1901 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Chen, X.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods 2022, 11, 2662. https://doi.org/10.3390/foods11172662
Yu X, Chen X, Li Y, Li L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods. 2022; 11(17):2662. https://doi.org/10.3390/foods11172662
Chicago/Turabian StyleYu, Xiangying, Xiaochun Chen, Yuting Li, and Lin Li. 2022. "Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS" Foods 11, no. 17: 2662. https://doi.org/10.3390/foods11172662
APA StyleYu, X., Chen, X., Li, Y., & Li, L. (2022). Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods, 11(17), 2662. https://doi.org/10.3390/foods11172662