Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate
Abstract
:1. Introduction
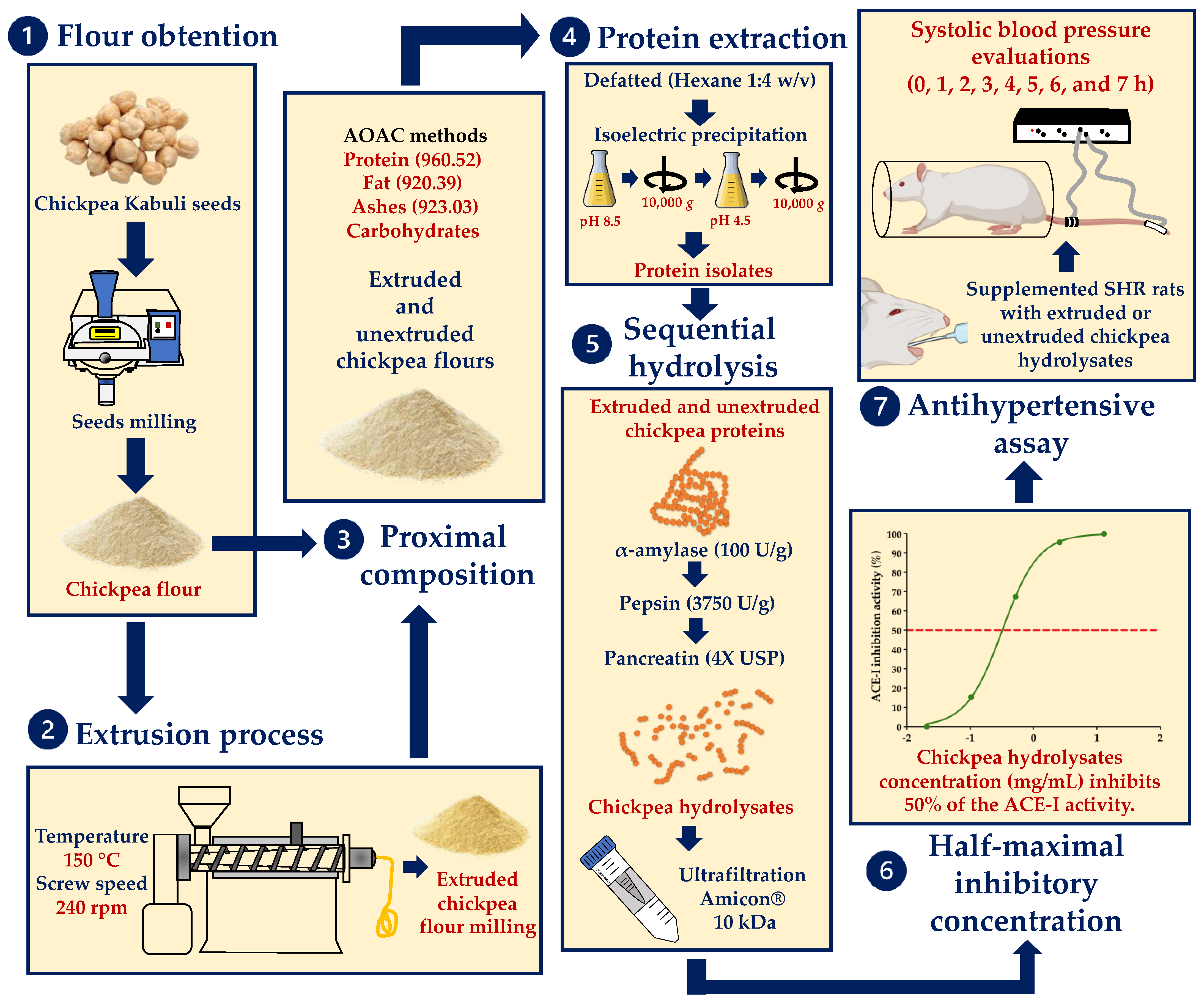
2. Materials and Methods
2.1. Chickpea Seeds
2.2. Chickpea Flour Preparation
2.3. Extrusion Process
2.4. Extruded and Unextruded Chickpea Flours Proximate Composition
2.5. Extraction and Concentration of Chickpea Protein
2.6. Hydrolysis and Fractioning of Chickpea Protein
2.7. Half-Maximal Inhibitory Concentration
2.8. Animals and Ethical Aspects
2.9. Effect of Chickpea Hydrolysates on Blood Pressure in SHRs
2.10. Statistical Analysis
3. Results and Discussion
3.1. Protein Extraction and Proximate Composition
3.2. Determination of the IC50
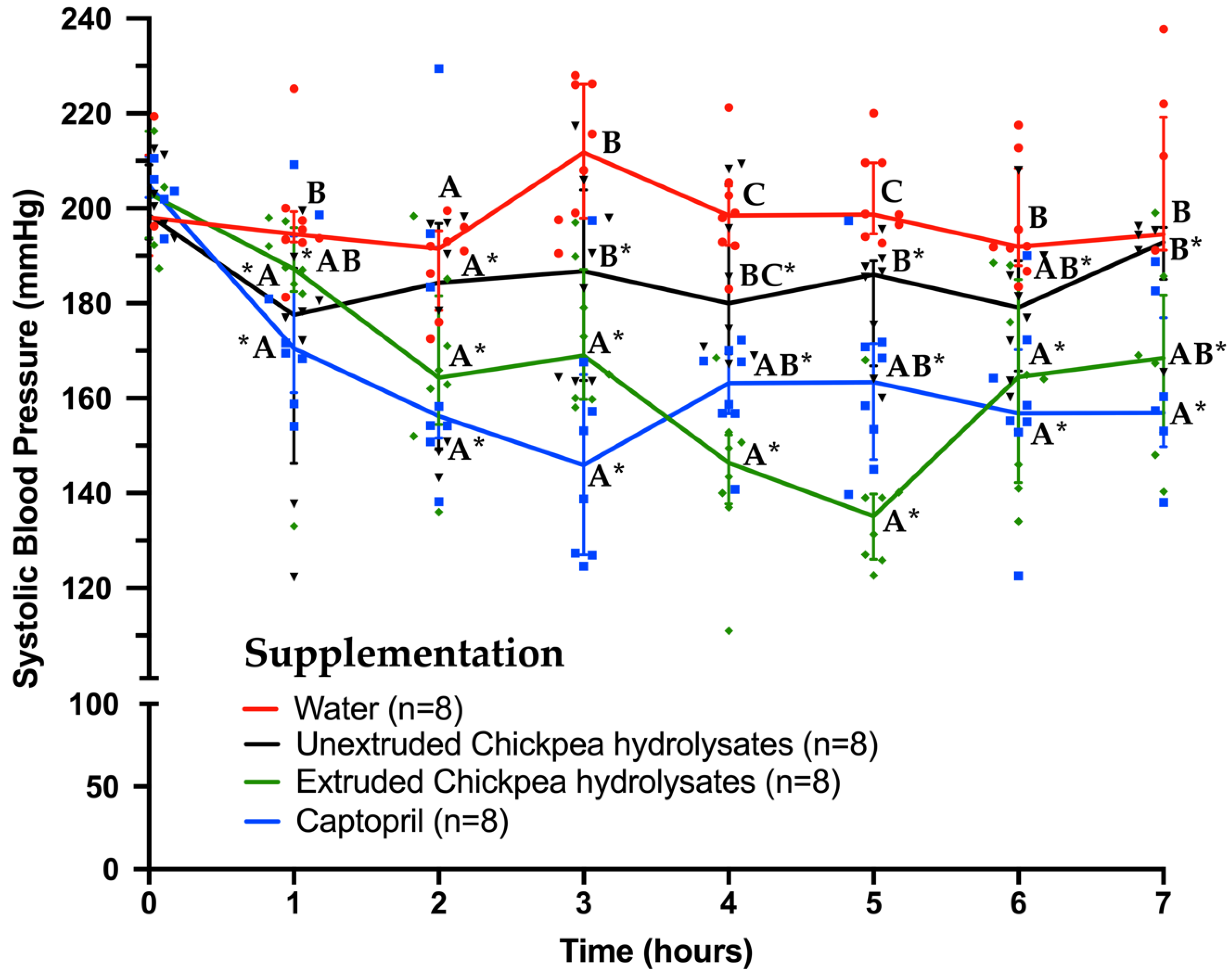
3.3. Effect of Unextruded and Extruded Chickpea Protein Hydrolysates on Blood Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Bovée, D.M.; Danser, A.J. The Renin-Angiotensin-Aldosterone System and Its Therapeutic Targets. Exp. Eye Res. 2019, 186, 107680. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Meza, E.E.; Raymundo, A.; Figueroa-Salcido, O.G.; Ramírez-Torres, G.I.; Fradinho, P.; Oliveira, S.; de Sousa, I.; Suárez-Jiménez, M.; Cárdenas-Torres, F.I.; Islas-Rubio, A.R.; et al. Pasta Enrichment with an Amaranth Hydrolysate Affects the Overall Acceptability While Maintaining Antihypertensive Properties. Foods 2019, 8, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez-Torres, G.; Ontiveros, N.; Lopez-Teros, V.; Ibarra-Diarte, J.A.; Reyes-Moreno, C.; Cuevas-Rodríguez, E.O.; Cabrera-Chávez, F. Amaranth Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Molecules 2017, 22, 1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahandideh, F.; Chakrabarti, S.; Majumder, K.; Li, Q.; Panahi, S.; Morton, J.S.; Davidge, S.T.; Wu, J. Egg White Protein Hydrolysate Reduces Blood Pressure, Improves Vascular Relaxation and Modifies Aortic Angiotensin II Receptors Expression in Spontaneously Hypertensive Rats. J. Funct. Foods 2016, 27, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Jiang, B.; Miao, M.; Mu, W.; Li, Y. Combined Effects of High-Pressure and Enzymatic Treatments on the Hydrolysis of Chickpea Protein Isolates and Antioxidant Activity of the Hydrolysates. Food Chem. 2012, 135, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, A.M.; Sila, A.; Przybylski, R.; Nedjar-Arroume, N.; Makhlouf, I.; Blecker, C.; Attia, H.; Dhulster, P.; Bougatef, A.; Besbes, S. Purification and Identification of Novel Antioxidant Peptides from Enzymatic Hydrolysate of Chickpea (Cicer arietinum L.) Protein Concentrate. J. Funct. Foods 2015, 12, 516–525. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Technological, Processing and Nutritional Aspects of Chickpea (Cicer arietinum)—A Review. Trends Food Sci. Technol 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Arámburo-Gálvez, J.G.; Arvizu-Flores, A.A.; Cárdenas-Torres, F.I.; Cabrera-Chávez, F.; Ramírez-Torres, G.I.; Flores-Mendoza, L.K.; Gastelum-Acosta, P.E.; Figueroa-Salcido, O.G.; Ontiveros, N. Prediction of ACE-I Inhibitory Peptides Derived from Chickpea (Cicer arietinum L.): In Silico Assessments Using Simulated Enzymatic Hydrolysis, Molecular Docking and ADMET Evaluation. Foods 2022, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Medina-Godoy, S.; Ambriz-Pérez, D.L.; Fuentes-Gutiérrez, C.I.; Germán-Báez, L.J.; Gutiérrez-Dorado, R.; Reyes-Moreno, C.; Valdez-Ortiz, A. Angiotensin-Converting Enzyme Inhibitory and Antioxidative Activities and Functional Characterization of Protein Hydrolysates of Hard-to-Cook Chickpeas. J. Sci. Food Agric. 2012, 92, 1974–1981. [Google Scholar] [CrossRef]
- Yust, M.M.; Pedroche, J.; Giron-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of Ace Inhibitory Peptides by Digestion of Chickpea Legumin with Alcalase. Food Chem. 2003, 81, 363–369. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-Converting Enzyme Inhibitory Activity of Chickpea and Pea Protein Hydrolysates. Food Res. Int. 2010, 43, 1642–1649. [Google Scholar] [CrossRef]
- Xu, Y.; Galanopoulos, M.; Sismour, E.; Ren, S.; Mersha, Z.; Lynch, P.; Almutaimi, A. Effect of Enzymatic Hydrolysis Using Endo-and Exo-Proteases on Secondary Structure, Functional, and Antioxidant Properties of Chickpea Protein Hydrolysates. J. Food Meas. Charact. 2020, 14, 343–352. [Google Scholar] [CrossRef]
- Vagadia, B.H.; Vanga, S.K.; Raghavan, V. Inactivation Methods of Soybean Trypsin Inhibitor–A Review. Trends Food Sci. Technol. 2017, 64, 115–125. [Google Scholar] [CrossRef]
- Berrios, J.D.J.; Losso, J.N.; Albertos, I. Extrusion Processing of Dry Beans and Pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 225–246. [Google Scholar] [CrossRef]
- Silvestre-De-León, R.; Espinosa-Ramírez, J.; Heredia-Olea, E.; Pérez-Carrillo, E.; Serna-Saldívar, S.O. Biocatalytic Degradation of Proteins and Starch of Extruded Whole Chickpea Flours. Food Bioproc. Tech. 2020, 13, 1703–1716. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Reyes-Moreno, C.; Camacho-Hernández, I.L.; Rouzaud-Sandez, O. Optimisation of Extrusion Process to Transform Hardened Chickpeas (Cicer arietinum L) into a Useful Product. J. Sci. Food Agric. 2002, 82, 1718–1728. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Megías, C.; Cortés-Giraldo, I.; Alaiz, M.; Vioque, J.; Girón-Calle, J. Isoflavones in Chickpea (Cicer arietinum) Protein Concentrates. J. Funct. Foods 2016, 21, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Milán-Noris, A.K.; Gutiérrez-Uribe, J.A.; Santacruz, A.; Serna-Saldívar, S.O.; Martínez-Villaluenga, C. Peptides and Isoflavones in Gastrointestinal Digests Contribute to the Anti-Inflammatory Potential of Cooked or Germinated Desi and Kabuli Chickpea (Cicer arietinum L.). Food Chem. 2018, 268, 66–76. [Google Scholar] [CrossRef]
- Guldiken, B.; Yovchev, A.; Nosworthy, M.G.; Stone, A.K.; House, J.D.; Hood-Niefer, S.; Nickerson, M.T. Effect of Extrusion Conditions on the Physical Properties of Desi Chickpea-Barley Extrudates and Quality Attributes of Their Resulting Flours. J. Texture Stud. 2020, 51, 300–307. [Google Scholar] [CrossRef]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Affinity Purification and Characterisation of Chelating Peptides from Chickpea Protein Hydrolysates. Food Chem. 2011, 129, 485–490. [Google Scholar] [CrossRef]
- Sánchez-Chino, X.M.; Jiménez Martínez, C.; León-Espinosa, E.B.; Garduño-Siciliano, L.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Dávila-Ortiz, G. Protective Effect of Chickpea Protein Hydrolysates on Colon Carcinogenesis Associated with a Hypercaloric Diet. J. Am. Coll Nutr 2019, 38, 162–170. [Google Scholar] [CrossRef]
- Milán-Noris, A.K.; De la Rosa-Millan, J.; Serna-Saldivar, S.O. Comparative Analysis of Techno-Functional Properties, Starch Digestion and Protein Quality of Pigmented Chickpea Flours. Int. J. Food Sci. 2019, 54, 2288–2299. [Google Scholar] [CrossRef]
- Alam, M.; Kaur, J.; Khaira, H.; Gupta, K. Extrusion and Extruded Products: Changes in Quality Attributes as Affected by Extrusion Process Parameters: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 445–473. [Google Scholar] [CrossRef]
- Félix-Medina, J.V.; Montes-Ávila, J.; Reyes-Moreno, C.; Perales-Sánchez, J.X.K.; Gómez-Favela, M.A.; Aguilar-Palazuelos, E.; Gutiérrez-Dorado, R. Second-Generation Snacks with High Nutritional and Antioxidant Value Produced by an Optimized Extrusion Process from Corn/Common Bean Flours Mixtures. LWT 2020, 124, 109172. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, S.; Isono, N.; Noda, T. Genotypic Diversity in Physico-Chemical, Pasting and Gel Textural Properties of Chickpea (Cicer arietinum L.). Food Chem. 2010, 122, 65–73. [Google Scholar] [CrossRef]
- Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Utilisation of Chickpea Protein Isolates for Production of Peptides with Angiotensin I-Converting Enzyme (ACE)-Inhibitory Activity. J. Sci. Food Agric. 2002, 82, 960–965. [Google Scholar] [CrossRef]
- Henda, Y.B.; Labidi, A.; Arnaudin, I.; Bridiau, N.; Delatouche, R.; Maugard, T.; Piot, J.-M.; Sannier, F.; Thiéry, V.; Bordenave-Juchereau, S. Measuring Angiotensin-I Converting Enzyme Inhibitory Activity by Micro Plate Assays: Comparison Using Marine Cryptides and Tentative Threshold Determinations with Captopril and Losartan. J. Agric. Food Chem. 2013, 61, 10685–10690. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Vecchi, B.; Rinaldi, G.; Añón, M.C. Amaranth Seed Protein Hydrolysates Have in Vivo and in Vitro Antihypertensive Activity. Food Chem. 2011, 126, 878–884. [Google Scholar] [CrossRef]
- Valdez-Flores, M.; Germán-Báez, L.J.; Gutiérrez-Dorado, R.; Medina-Godoy, S.; Norzagaray-Valenzuela, C.; Hernández-Verdugo, S.; Reyes-Moreno, C.; Valdez-Ortiz, A. Improving Bioactivities of Jatropha curcas Protein Hydrolysates by Optimizing with Response Surface Methodology the Extrusion Cooking Process. Ind. Crops Prod. 2016, 85, 353–360. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Girgih, A.T.; Malomo, S.A.; Onuh, J.O.; Aluko, R.E. Thermoase-Derived Flaxseed Protein Hydrolysates and Membrane Ultrafiltration Peptide Fractions Have Systolic Blood Pressure-Lowering Effects in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2014, 15, 18131–18147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciau-Solís, N.A.; Acevedo-Fernández, J.J.; Betancur-Ancona, D. In Vitro Renin–Angiotensin System Inhibition and in Vivo Antihypertensive Activity of Peptide Fractions from Lima Bean (Phaseolus lunatus L.). J. Sci. Food Agric. 2018, 98, 781–786. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Galicia-Martínez, S.; Acevedo-Fernández, J.J.; Santaolalla-Tapia, J.; Betancur-Ancona, D. Evaluation of Hypotensive and Antihypertensive Effects of Velvet Bean (Mucuna pruriens L.) Hydrolysates. J. Med. Food 2017, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A Novel Antioxidant and ACE Inhibitory Peptide from Rice Bran Protein: Biochemical Characterization and Molecular Docking Study. LWT 2017, 75, 93–99. [Google Scholar] [CrossRef]
- Olagunju, A.I.; Omoba, O.S.; Enujiugha, V.N.; Alashi, A.M.; Aluko, R.E. Antioxidant Properties, ACE/Renin Inhibitory Activities of Pigeon Pea Hydrolysates and Effects on Systolic Blood Pressure of Spontaneously Hypertensive Rats. Food Sci. Nutr. 2018, 6, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Rodríguez, A.; Milán-Carrillo, J.; Reyes-Moreno, C.; González de Mejía, E. Characterization of Peptides Found in Unprocessed and Extruded Amaranth (Amaranthus hypochondriacus) Pepsin/Pancreatin Hydrolysates. Int. J. Mol. Sci. 2015, 16, 8536–8554. [Google Scholar] [CrossRef] [Green Version]
- Yanqing, L.; Yuyang, H.; Xiaoqi, D.; Zhimin, L.; Wentao, L.; Guang, Z.; Zhu, Y.; Zhu, X. Effect of Enzymatic Hydrolysis Followed after Extrusion Pretreatment on the Structure and Emulsibility of Soybean Protein. Process Biochem. 2022, 116, 173–184. [Google Scholar] [CrossRef]
- Guzmán-Ortiz, F.A.; Hernández-Sánchez, H.; Yee-Madeira, H.; Martín-Martínez, S.; Robles-Ramírez MD, C.; Rojas-López, M.; De JBerríos, J.; Mora-Escobedo, R. Physico-Chemical, Nutritional and Infrared Spectroscopy Evaluation of an Optimized Soybean/Corn Flour Extrudate. J. Food Sci. Technol. 2015, 52, 4066–4077. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Xu, Y.; Liu, Z.; Sui, Z.; Corke, H. Removal of Starch Granule-Associated Proteins Promotes α-Amylase Hydrolysis of Rice Starch Granule. Food Chem. 2020, 330, 127313. [Google Scholar] [CrossRef]
- Jogi, N.; Yathisha, U.G.; Bhat, I.; Mamatha, B.S. Antihypertensive Activity of Orally Consumed ACE-I Inhibitory Peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, A.L.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. The Vasoactive Mas Receptor in Essential Hypertension. J. Clin. Med. 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Yin, Y.; Zhao, W.; Chen, F.; Liu, J. Antihypertensive Effect of Angiotensin-Converting Enzyme Inhibitory Peptide RVPSL on Spontaneously Hypertensive Rats by Regulating Gene Expression of the Renin–Angiotensin System. J. Agric. Food Chem. 2014, 62, 912–917. [Google Scholar] [CrossRef] [PubMed]

| Chickpea Flour | Protein (g/100 g) | Fat (g/100 g) | Ash (g/100 g) | Carbohydrates (g/100 g) |
|---|---|---|---|---|
| Unextruded | 24.04 ± 0.25 a | 3.60 ± 0.11 a | 3.36 ± 0.03 a | 69.00 ± 0.17 a |
| Extruded | 23.92 ± 0.18 a | 4.73 ± 0.49 b | 3.63 ± 0.03 b | 67.72 ± 0.68 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez-Ontiveros, J.; Reyes-Moreno, C.; Ramírez-Torres, G.I.; Figueroa-Salcido, O.G.; Arámburo-Gálvez, J.G.; Montoya-Rodríguez, A.; Ontiveros, N.; Cuevas-Rodríguez, E.O. Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods 2022, 11, 2562. https://doi.org/10.3390/foods11172562
Chávez-Ontiveros J, Reyes-Moreno C, Ramírez-Torres GI, Figueroa-Salcido OG, Arámburo-Gálvez JG, Montoya-Rodríguez A, Ontiveros N, Cuevas-Rodríguez EO. Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods. 2022; 11(17):2562. https://doi.org/10.3390/foods11172562
Chicago/Turabian StyleChávez-Ontiveros, Jeanett, Cuauhtémoc Reyes-Moreno, Giovanni Isaí Ramírez-Torres, Oscar Gerardo Figueroa-Salcido, Jesús Gilberto Arámburo-Gálvez, Alvaro Montoya-Rodríguez, Noé Ontiveros, and Edith Oliva Cuevas-Rodríguez. 2022. "Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate" Foods 11, no. 17: 2562. https://doi.org/10.3390/foods11172562
APA StyleChávez-Ontiveros, J., Reyes-Moreno, C., Ramírez-Torres, G. I., Figueroa-Salcido, O. G., Arámburo-Gálvez, J. G., Montoya-Rodríguez, A., Ontiveros, N., & Cuevas-Rodríguez, E. O. (2022). Extrusion Improves the Antihypertensive Potential of a Kabuli Chickpea (Cicer arietinum L.) Protein Hydrolysate. Foods, 11(17), 2562. https://doi.org/10.3390/foods11172562








