Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomic DNA Extraction and WGS
2.2. Serogroup and ST Determination
2.3. Prediction of Stress-Related Genes, Biofilm-Forming Related Genes and Disinfectant Resistant Genes
2.4. Prediction of Prophages and Plasmids
2.5. Biofilm-Forming Test
2.6. Disinfectant Tolerance
2.7. Statistical Analysis
3. Results
3.1. STs Distribution of L. monocytogenes Strains from RTE Food Processing Plants
3.2. Genetic Features of Four STs L. monocytogenes in the Two RTE Meat Food Processing Plants
3.3. Biofilm-Forming Associated Genes and the Biofilm-Forming Ability of Four L. monocytogenes STs Isolates
3.4. Disinfection Efficiencies of Chlorine-Containing Disinfectant against Different ST Biofilm Cells and Disinfectant-Resistant Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lianou, A.; Sofos, J.N. A review of the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food service environments. J. Food Prot. 2007, 70, 2172–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Que, F.X.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes contamination in a ready-to-eat meat processing plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef]
- Orsi, R.H.; Borowsky, M.L.; Lauer, P.; Young, S.K.; Nusbaum, C.; Galagan, J.E.; Birren, B.W.; Ivy, R.A.; Sun, Q.; Graves, L.M.; et al. Short-term genome evolution of Listeria monocytogenes in a non-controlled environment. BMC Genom. 2008, 9, 539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Wang, J.; Chang, Z.Y.; Liu, X.; Chen, W.; Yu, Y.; Wang, X.; Dong, Q.; Ye, Y.; Zhang, X. Listeria monocytogenes contamination characterization in two ready-to-eat meat plants from 2019 to 2020 in Shanghai. Front. Microbiol. 2021, 12, 729114. [Google Scholar] [CrossRef]
- Gandhi, M.; Chikindas, M.L. Listeria: A foodborne pathogen that knows how to survive. Int. J. Food Microbiol. 2007, 113, 1–15. [Google Scholar] [CrossRef]
- Kim, J.W.; Kathariou, S. Temperature-dependent phage resistance of Listeria monocytogenes epidemic clone II. Appl. Environ. Microbiol. 2009, 75, 2433–2438. [Google Scholar] [CrossRef] [Green Version]
- Fox, E.M.; Allnutt, T.; Bradbury, M.I.; Fanning, S.; Chandry, P.S. Comparative genomics of the Listeria monocytogenes ST204 subgrou. Front. Microbiol. 2016, 7, 2057. [Google Scholar] [CrossRef] [Green Version]
- Knudsen, G.M.; Nielsen, J.B.; Marvig, R.L.; Ng, Y.; Worning, P.; Westh, H.; Gram, L. Genome-wide-analyses of Listeria monocytogenes from food-processing plants reveals clonal diversity and dates the emergence of persisting sequence types. Environ. Microbiol. Rep. 2017, 9, 428–440. [Google Scholar] [CrossRef] [Green Version]
- Maury, M.M.; Tsai, Y.; Charlier, C.; Touchon, M.; Chenal-Francisque, V.; Leclercq, A.; Criscuolo, A.; Gaultier, C.; Roussel, S.; Brisabois, A.; et al. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat. Genet. 2016, 48, 308–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, A.; Criscuolo, A.; Pouseele, H.; Maury, M.M.; Leclercq, A.; Tarr, C.; Björkman, J.T.; Dallman, T.; Reimer, A.; Enouf, V.; et al. Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat. Microbiol. 2016, 2, 16185. [Google Scholar] [CrossRef] [PubMed]
- Annabel, L.N.; Monika, D.; Martin, W.; Schmitz-Esser, S. Plasmids contribute to food processing environment-associated stress survival in three Listeria monocytogenes ST121, ST8, and ST5 strains. Int. J. Food Microbiol. 2019, 299, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Muhterem-Uyar, M.; Ciolacu, L.; Wagner, K.H.; Wagner, M.; Schmitz-Esser, S.; Stessl, B. New Aspects on Listeria monocytogenes ST5-ECVI Predominance in a Heavily Contaminated Cheese Processing Environment. Front. Microbiol. 2018, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Qian, W.; Zhang, X.; Wang, H.; Ye, K.; Bai, Y.; Zhou, G. Prevalence, genetic diversity and antimicrobial resistance of Listeria monocytogenes isolated from ready-to-eat meat products in Nanjing, China. Food Control. 2015, 50, 202–208. [Google Scholar] [CrossRef]
- Jacquet, C.; Doumith, M.; Glaser, P.; Martin, P.; Buchrieser, C. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [Green Version]
- Doumith, M.; Cazalet, C.; Simoes, N.; Frangeul, L.; Jacquet, C.; Kunst, F.; Martin, P.; Cossart, P.; Glaser, P.; Buchrieser, C. New aspects regarding evolution and virulence of Listeria monocytogenes revealed by comparative genomics and DNA arrays. Infect. Immun. 2004, 72, 1072–1083. [Google Scholar] [CrossRef] [Green Version]
- Nelson, K.E.; Fouts, D.E.; Mongodin, E.F.; Ravel, J.; DeBoy, R.T.; Kolonay, J.F.; Rasko, D.A.; Angiuoli, S.V.; Gill, S.R.; Paulsen, I.T.; et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 2004, 2, 2386–2395. [Google Scholar] [CrossRef] [Green Version]
- Gray, M.J.; Zadoks, R.N.; Fortes, E.D.; Dogan, B.; Cai, S.; Chen, Y.; Scott, V.N.; Gombas, D.E.; Boor, K.J.; Wiedmann, M. Listeria monocytogenes isolates from foods and humans form distinct but overlapping populations. Appl. Environ. Microbiol. 2004, 70, 5833–5841. [Google Scholar] [CrossRef] [Green Version]
- Barbour, A.H.; Rampling, A.; Hormaeche, C.E. Variation in the infectivity of Listeria monocytogenes isolates following intragastric inoculation of mice. Infect. Immun. 2001, 69, 4657–4660. [Google Scholar] [CrossRef] [Green Version]
- Ryan, S.; Begley, M.; Hill, C.; Gahan, C.G. A five-gene stress survival islet (SSI-1) that contributes to the growth of Listeria monocytogenes in suboptimal conditions. J. Appl. Microbiol. 2010, 109, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Sela, S.; Frank, S.; Belausov, E.; Pinto, R. A mutation in the luxS gene influences Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 2006, 72, 5653–5658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burall, L.S.; Grim, C.J.; Mammel, M.K.; Datta, A.R. Whole genome sequence analysis using JSpecies tool establishes clonal relationships between Listeria monocytogenes strains from epidemiologically unrelated listeriosis outbreaks. PLoS ONE 2015, 11, e0150797. [Google Scholar] [CrossRef] [PubMed]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Le Monnier, A.; Brisse, S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef] [Green Version]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [Green Version]
- Schmita-Esser, S.; Muller, A.; Stessl, B.; Wagner, M. Genomes of sequence type 121 Listeria monocytogenes strains harbor highly conserved plasmids and prophages. Front. Microbiol. 2015, 6, 380. [Google Scholar] [CrossRef] [Green Version]
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common plant-derived terpenoids present increased anti-biofilm potential against Staphylococcus bacteria composed to a quaternary ammonium biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef]
- Buchrieser, C.; Rusniok, C.; Listeria, C.T.; Kunst, F.; Cossart, P.; Glaser, P. Comparison of the genome sequences of Listeria monocytogenes and Listeria innocua: Clues for evolution and pathogenicity. FEMS Immunol. Med. Microbiol. 2003, 35, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, H.; Takakura, K.; Sone, Y.; Itano, Y.; Nishikawa, Y. Biofilm formation and resistance to benzalkonium chloride in Listeria monocytogenes isolated from a fish processing plant. J. Food Prot. 2013, 76, 1179–1186. [Google Scholar] [CrossRef]
- Wang, J.; Ray, A.J.; Hammons, S.R.; Oliver, H.F. Persistent and transient Listeria monocytogenes strains from retail deli environments vary in their ability to adhere and form biofilms and rarely have inlA premature stop codons. Foodborne Pathog. Dis. 2015, 12, 151–158. [Google Scholar] [CrossRef]
- Müller, A.; Rychli, K.; Muhterem-Uyar, M.; Zaiser, A.; Stessl, B.; Guinane, C.M.; Cotter, P.D.; Wagner, M.; Schmitz-Esser, S. Tn6188-a novel transposon in Listeria monocytogenes responsible for tolerance to benzalkonium chloride. PLoS ONE 2013, 8, e76835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, F.M.; Leonard, N.; Jordan, K. Physiological and transcriptional characterization of persistent and nonpersistent Listeria monocytogenes isolates. Appl. Environ. Microbiol. 2011, 77, 6559–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrita, P.; Trigo, M.J.; Ferreira, R.B.; Brito, L. Differences in the expression of cold stress-related genes in the swarming motility among persistent and sporadic strains of Listeria monocytogenes. Foodborne Pathog. Dis. 2015, 12, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Wiktorczyk-Kapischke, N.; Skowron, K.; Grudlewska-Buda, K.; Wałecka-Zacharska, E.; Korkus, J.; Gospodarek-Komkowska, E. Adaptive Response of Listeria monocytogenes adaptive response to the stress factors in the food processing environment. Front. Microbiol. 2021, 12, 710085. [Google Scholar] [CrossRef] [PubMed]
- Haubert, L.; Zehetmeyr, M.L.; da Silva, W.P. Resistance to benzalkonium chloride and cadmium chloride in Listeria monocytogenes isolates from food and food processing environments in southern Brazil. Can. J. Microbiol. 2019, 65, 429–435. [Google Scholar] [CrossRef]
- Mafuna, T.; Matle, I.; Magwedere, K.; Pierneef, R.E.; Reva, O.N. Whole genome-based characterization of Listeria monocytogenes isolates recovered from the food chain in South Africa. Front. Microbiol. 2021, 12, 669287. [Google Scholar] [CrossRef]
- Pasquali, F.; Palma, F.; Guillier, L.; Lucchi, A.; Lucchi, A.; De Cesare, A.; Manfreda, G. Listeria monocytogenes sequence types 121 and 14 repeatedly isolated within one year of sampling in a rabbit meat processing plant: Persistence and ecophysiology. Front. Microbiol. 2018, 29, 596. [Google Scholar] [CrossRef] [PubMed]
- James, P.F.; Gregory, R.S.; Joseph, F.F. Formation of biofilm at different nutrient levels by various genotypes of Listeria monocytogenes. J. Food Protect. 2006, 69, 826–834. [Google Scholar] [CrossRef]
- Olier, M.; Pierre, F.; Rousseaux, S.; Lamaitre, J.P.; Rousset, A.; Piveteau, P.; Guzzo, J. Expression of truncated internalin A is invovled in impaired internalization of some Listeria monocytogenes isolates carried asymptomatically by humans. Infect. Immun. 2003, 71, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Kuenne, C.; Billion, A.; Mraheil, M.A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Barbuddhe, S.; et al. Reassessment of the Listeria monocytogenes pan-genome reveals dynamic integration hotspots and mobile genetic elements as major components of the accessory genome. BMC Genom. 2013, 14, 47. [Google Scholar] [CrossRef] [Green Version]
- Suokko, A.; Savijoki, K.; Malinen, E.; Palva, A.; Varmanen, P. Characterization of a mobile clpL gene from Lactobacillus rhamnosus. Appl. Environ. Microbiol. 2005, 71, 2061–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajfasz, J.K.; Martínez, A.R.; Rivera-Ramos, I.; Abranches, J.; Koo, H.; Quivey, R.G., Jr.; Lemos, J.A. Role of Clp proteins in expression of virulence properties of Streptococcus mutans. J. Bacteriol. 2009, 191, 2060–2068. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.D.; Kwon, H.Y.; Kim, E.H.; Kim, K.W.; Briles, D.E.; Pyo, S.; Rhee, D.K. Decrease in penicillin susceptibility due to heat shock protein ClpL in Streptococcus pneumoniae. Antimicrob Agents Chemother. 2011, 55, 2714–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harter, E.; Wagner, E.M.; Zaiser, A.; Halecker, S.; Wagner, M.; Rychli, K. Stress survival islet 2, predominantly present in Listeria monocytogenes strains of sequence type 121, is involved in the alkaline and oxidative stress responses. Appl. Environ. Microbiol. 2017, 83, e00827-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, I.; Klinger, S.; Dooms, M.; Flekna, G.; Stessl, B.; Leclercq, A.; Hill, C.; Allerberger, F.; Wagner, M.J.A.; Microbiology, E. Stress survival islet (SSI-1) survey in Listeria monocytogenes reveals an insert common to Listeria innocua in sequence type 121 L. monocytogenes strains. Appl. Environ. Microbiol. 2011, 77, 2169–2173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Suárez, J.V.; Ortiza, S.; López-Alonso, V. Potential impact of the resistance to quanternary ammonium disinfectants on the persistence of Listeria monocytogenes in food processing environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Biswas, I. ClpL is required for folding of CtsR in Streptococcus mutans. J. Bacteriol. 2013, 195, 576–584. [Google Scholar] [CrossRef]
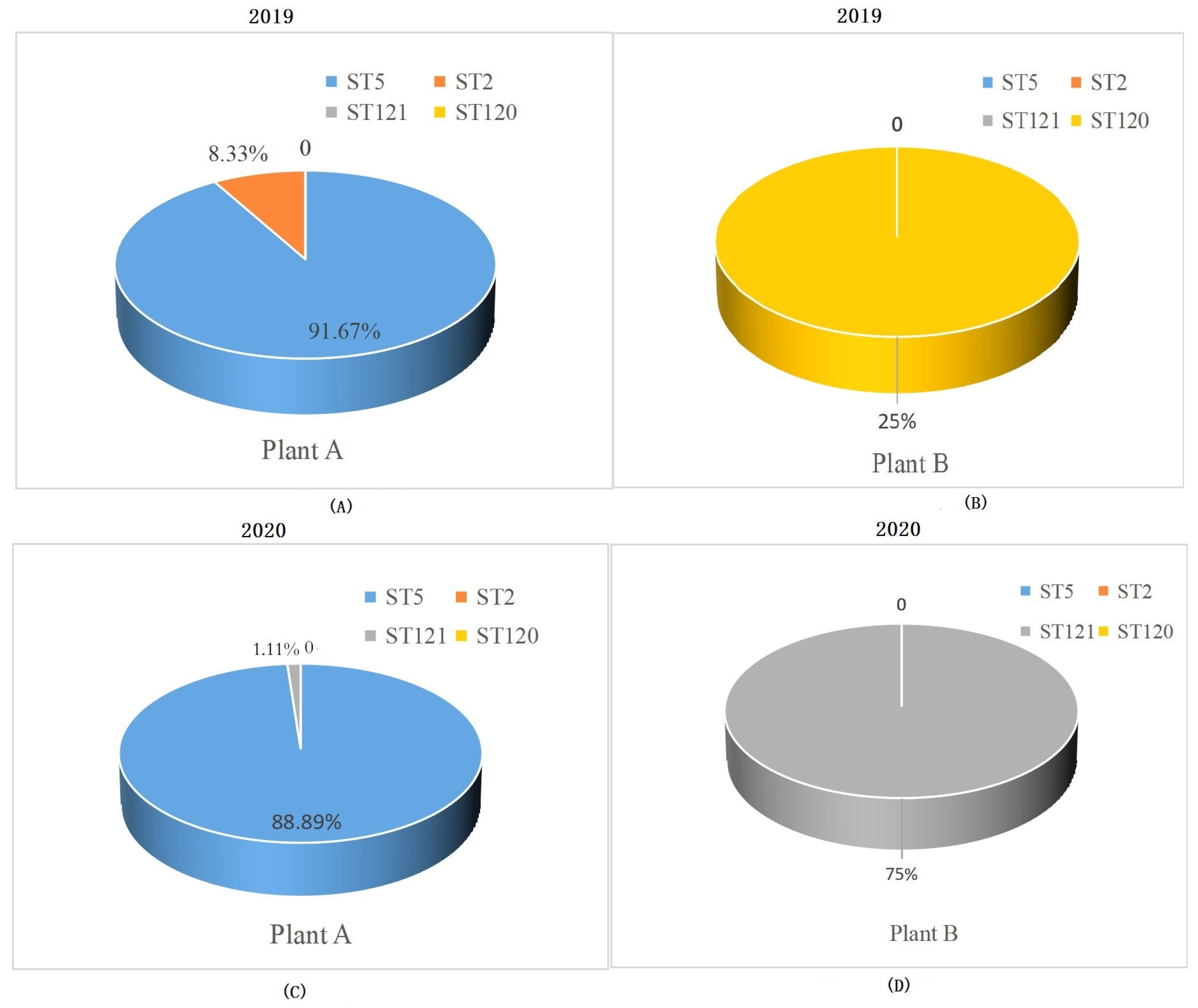
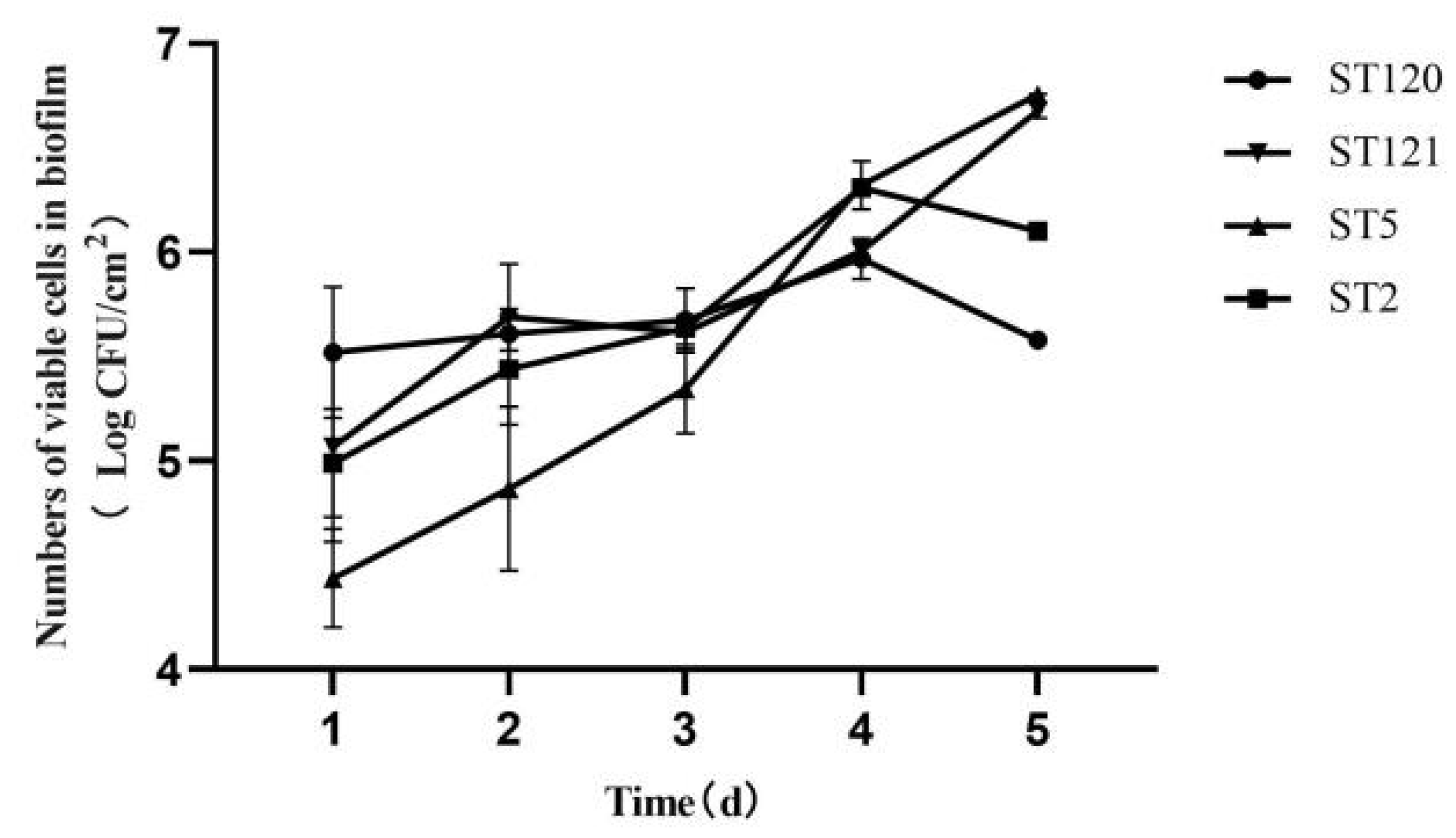
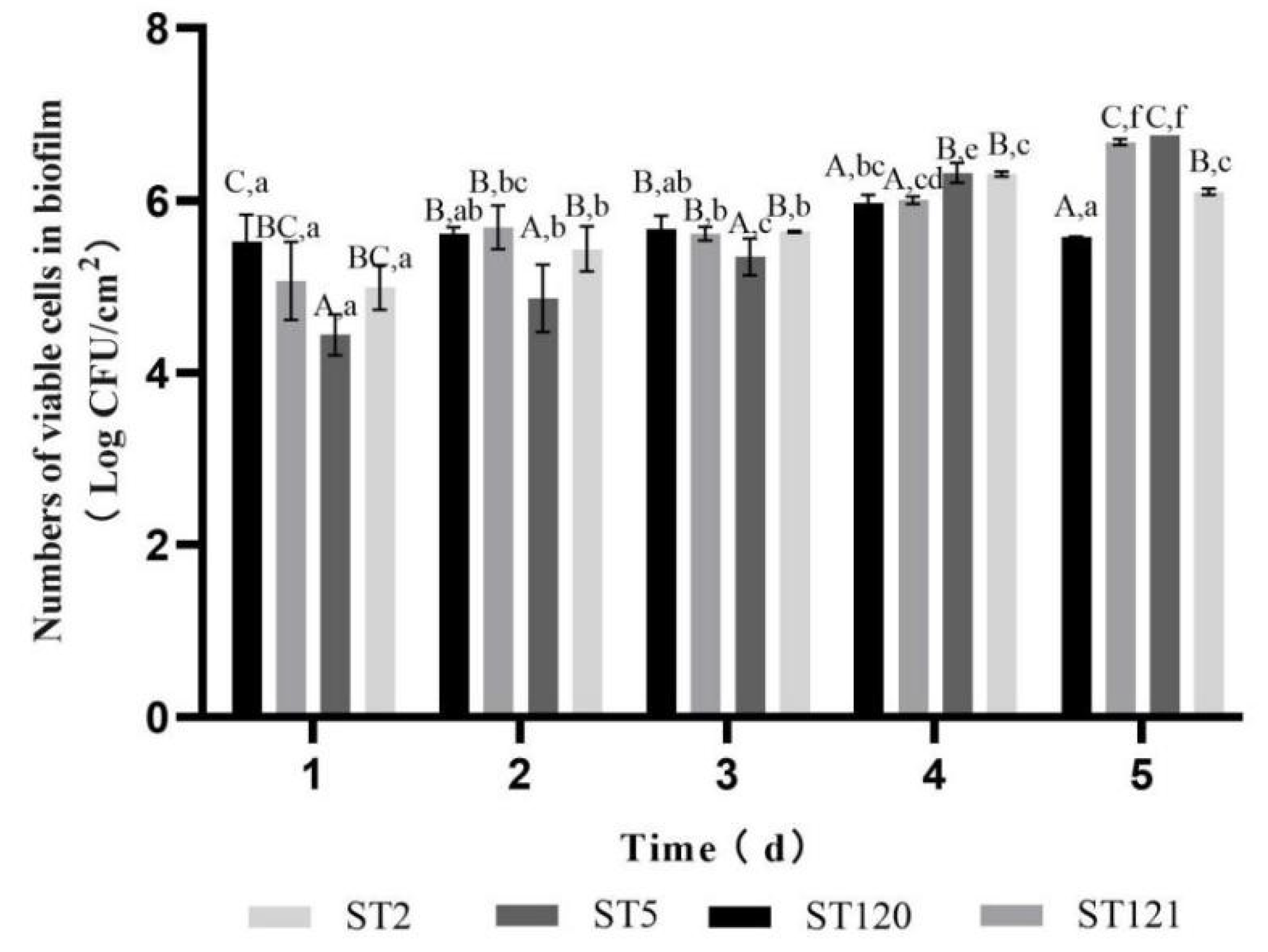
| ST | Serogroup | Disinfectant Resistant Genes | Stress Survival Ilset | inlA | qacH, ermE, ermC | Plasmids | Intact Prophages | Biofilm Formation-Related Genes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| actA | prfA | lmo0673 | recO | lmo2504 | luxS | ||||||||
| 2 | IVb | mdrL, lde | - | truncated inlA | - | pLM33 pLM5578 | B025_NC_ 009812 | − | + | + | + | − | − |
| 5 | IIb | clpL, mdrL, lde | SSI-1 | inlA | - | pLM33 | B025_NC_ 009812 | − | + | + | + | + | + |
| 120 | IIa | mdrL, lde | SSI-1 | inlA | - | - | - | + | + | + | + | + | + |
| 121 | IIa | clpL, mdrL, lde | SSI-1, SSI-2 | inlA | - | pLM5578 | - | − | − | + | + | + | + |
| Strains | Disinfectant Concentration (mg/L) | Log Reduction Values (LogCFU/cm2) and Disinfection Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Disinfectant Treatment Time (s) | ||||||
| 0 | 30 | 60 | ||||
| ST120 | 125 | 6.08 ± 0.06 A | 3.17 ± 0.04 Ba | 52.04% | 6.08 ± 0.06 Ba | 100.00% |
| 250 | 4.01 ± 0.04 Bb | 65.94% | 6.08 ± 0.06 Aa | 100.00% | ||
| 500 | 6.08 ± 0.06 Ac | 100.00% | 6.08 ± 0.06 Aa | 100.00% | ||
| ST121 | 125 | 6.31 ± 0.23 A | 3.13 ± 0.14 Ba | 49.69% | 4.94 ± 0.97 ABa | 78.27% |
| 250 | 3.36 ± 0.04 Ab | 57.40% | 5.84 ± 0.71 Aa | 92.63% | ||
| 500 | 6.31 ± 0.23 Ac | 100.00% | 6.31 ± 0.23 Aa | 100.00% | ||
| ST5 | 125 | 6.32 ± 0.03 A | 2.84 ± 0.09 Aa | 44.93% | 3.34 ± 0.62 Aa | 52.85% |
| 250 | 3.83 ± 0.12 ABb | 60.63% | 4.82 ± 1.28 Abc | 76.17% | ||
| 500 | 6.32 ± 0.03 Ac | 100.00% | 6.32 ± 0.03 Ac | 100.00% | ||
| ST2 | 125 | 6.36 ± 0.03 A | 3.64 ± 0.12 Ca | 57.27% | 5.54 ± 1.40 ABa | 87.04% |
| 250 | 6.36 ± 0.03 Cb | 100.00% | 6.36 ± 0.03 Aa | 100.00% | ||
| 500 | 6.36 ± 0.03 Ab | 100.00% | 6.36 ± 0.03 Aa | 100.00% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Chen, W.; Fang, Z.; Yu, Y.; Bi, J.; Wang, J.; Dong, Q.; Zhang, H. Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment. Foods 2022, 11, 2561. https://doi.org/10.3390/foods11172561
Liu X, Chen W, Fang Z, Yu Y, Bi J, Wang J, Dong Q, Zhang H. Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment. Foods. 2022; 11(17):2561. https://doi.org/10.3390/foods11172561
Chicago/Turabian StyleLiu, Xin, Wenjie Chen, Zhixin Fang, Ying Yu, Jing Bi, Jing Wang, Qingli Dong, and Hongzhi Zhang. 2022. "Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment" Foods 11, no. 17: 2561. https://doi.org/10.3390/foods11172561
APA StyleLiu, X., Chen, W., Fang, Z., Yu, Y., Bi, J., Wang, J., Dong, Q., & Zhang, H. (2022). Persistence of Listeria monocytogenes ST5 in Ready-to-Eat Food Processing Environment. Foods, 11(17), 2561. https://doi.org/10.3390/foods11172561








