Abstract
The analysis of mycotoxins in food and feed using liquid chromatography coupled with mass spectrometry is considered advantageous because the hyphenated technology enables simultaneous determination of multiple mycotoxins. Multi-mycotoxin analysis requires special consideration of quality control parameters to ensure proper evaluation of data quality for all target mycotoxins in method development and routine sample analysis. Mycotoxin matrix reference materials, especially certified reference materials, are stable and homogeneous matrices with certified traceability, concentrations, and uncertainty for mycotoxin(s) of interest. The use of these reference materials for single mycotoxin analysis has been a well-accepted practice and should be extended to multi-mycotoxin analysis. This opinion piece discusses the following essential metrological and operational components to improve data quality: (1) purposes of multi-mycotoxin reference materials; (2) comparison of reference materials, certified reference materials, and in-house quality control materials; (3) advantages of using reference materials for multi-mycotoxin analysis; (4) current trends and challenges of multi-mycotoxin reference materials. Potential applications of reference materials discussed here can improve routine mycotoxin determination and will lead to better accuracy and consistency of results. Quality control processes that incorporate reference materials in the field of mycotoxin analysis ensure successful development and implementation of liquid chromatography mass spectrometry-based multi-mycotoxin methods.
1. Introduction
Though the history of mycotoxin research is long [1], mass spectrometry (MS)-based mycotoxin analysis was used in the 1980s and 1990s primarily as a complementary tool to mycotoxin quantitation or confirmation using thin-layer chromatography (TLC), enzyme-linked immunoassay (ELISA), and liquid chromatography-fluorescence/UV detector (LC-FLD/UV) [2,3]. Since the early 2000s, liquid chromatography coupled with mass spectrometry (LC–MS) has been increasingly used by mycotoxin testing laboratories [4,5]. Compared to other existing analytical technologies, modern LC–MS offers superior data acquisition speed, sensitivity, and specificity. Multiple mycotoxins can be identified and quantified in one analysis, simplifying sample preparation and increasing throughput. It is not surprising that in this short time, LC–MS has become an indispensable tool for mycotoxin analysis [6,7,8]. While LC–MS has considerable application potential, monitoring and demonstrating the performance of multi-mycotoxin methods in a practical manner is a challenging quality assurance (QA) and quality control (QC) issue.
2. Purpose of Multi-Mycotoxin Reference Materials
Traditionally, to ensure data quality, laboratories have used reference materials (RMs), certified reference materials (CRMs), and/or in-house materials that contain a single mycotoxin or class of mycotoxins as an important QC component during method development and routine sample analysis [9]. Figure 1 illustrates the role of these QC samples in LC–MS-based mycotoxin analysis. Based on pre-designed quality system protocols, suitable QC samples are selected prior to the analysis, and then analyzed with calibration standards and samples using the same analytical methods. The assessment of the control sample data is then used to support decision-making based on data quality (e.g., traceability, accuracy, and precision). If the QC samples are consistently measured within a defined timeframe, the results could be used for short- and long-term statistical assessments (e.g., control chart) [10,11]. Limits of acceptable, warning, and unacceptable values could be statistically established and used for real-time assessment of method performance, which would provide key information to decision makers to accept or reject generated data and take corrective actions to address systemic bias or random errors. Furthermore, as mycotoxin analysis continues to capture global interest [12,13], the establishment of traceability using appropriate QC samples can serve as a metrological component that contributes to international confidence, comparability, and acceptance of measurements [14,15].
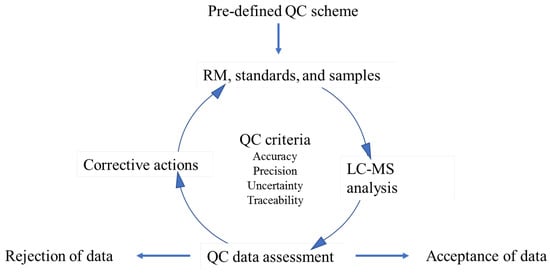
Figure 1.
The role of QC samples in LC–MS-based mycotoxin analysis.
3. Comparison of Reference Materials, Certified Reference Materials, and in-House Quality Control Materials
To fulfill QC requirements, it is imperative for laboratories to select and analyze suitable control samples, providing actionable information to support pre-defined QC schemes, post-analysis assessment, and the decision-making process. Materials prepared in-house (e.g., contaminated test batches and target analytes spiked into blank matrices) are often used as QC samples. While such practice is flexible, convenient, and economic, uncontrollable errors could be introduced that compromise the quality of QC samples if in-house materials are insufficiently characterized. Finding a balance between operational costs and characterization of in-house prepared samples without compromising accuracy or traceability is challenging. To prepare and characterize multi-mycotoxin QC samples, laboratories also need to pay careful attention to technical challenges in the material planning stage. For example, to save time and control operating cost, labs are rarely willing to establish traceability or characterize sample homogeneity for in-house quality control materials. Instead, spike samples are often used. Spiking target mycotoxin(s) onto a matrix as a positive control seems practical for single mycotoxin analysis but the selection of the matrix and spiking concentrations is not easily extended to multiple mycotoxins. Many food and feed matrices are prone to multi-mycotoxin contamination, making “blank” matrices difficult to find. Spiking-incurred matrices require that the matrix be screened for target mycotoxins first; otherwise, appropriate spiking concentrations for all target mycotoxins cannot be calculated. Even if the concentration of each mycotoxin could be appropriately determined in a selected matrix, individual spiking of multiple mycotoxins would be a tedious operation. Furthermore, each spiking step could introduce unique errors, making the uncertainty related to the QC sample uncontrollable and impossible to estimate. Using multiple single-mycotoxin QC samples might circumvent the above technical barriers, but this approach will slow down operation and, more importantly, for multi-mycotoxin analysis, lead to evaluation of target mycotoxin concentrations under different conditions than in the real samples. Frequently, when multiple single mycotoxin samples are used, the matrices that are available do not match up well with those being analyzed in the study. One should not expect that a corn RM would give much indication of method performance in a fatty matrix such as milk or peanut butter. Problems also arise that even by selecting multiple single mycotoxin samples, there are still mycotoxins that will not be covered, and assumptions should not be made regarding their performance with the method. ISO has established protocols [16,17] regarding characterization of candidate materials that could be used for QC, but following these protocols on a regular basis would significantly increase operational costs. In general, the primary concern regarding in-house-prepared QC samples has long been that the characterization of individual properties is not fully conducted and independently confirmed in terms of traceability, uncertainty, long-term stability, availability, and analyte profile.
When results of poorly characterized QC samples fall out of acceptable ranges, the root cause of errors is difficult to identify because the QC sample itself could be a major source of error. Studies have demonstrated that when using in-house materials, laboratories had difficulty generating consistent results or explaining the inconsistent observations among laboratories [18,19,20,21,22]. Large variability in analytical results offers little confidence to users who rely on the data to make important decisions based on the results. This suggests that either in-house QC samples must be fully characterized prior to their use or reference materials should be used for multi-mycotoxin analysis.
For single-mycotoxin analysis, RMs, especially CRMs, have proven to be an appealing and risk-reducing tool [23,24,25,26,27]. The long and successful history of use of CRMs to ensure measurement accuracy suggests the potential benefit for multi-mycotoxin analysis. In accordance with ISO requirements [16,17,28], an RM must be homogenous, stable, and suitable for its intended purpose. Mycotoxin RMs that have been characterized for one or more properties (e.g., target mycotoxin concentration) could be used for quality control, proficiency testing, and/or method validation. A certified mycotoxin reference material is accompanied by a certificate that documents how the concentration of the mycotoxin(s) is certified with established traceability to the International System of Units (SI) as well as the uncertainty with an estimated level of confidence.
A CRM prepared and released by the National Institute of Standards and Technology (NIST) is referred to as a standard reference material (SRM), which was developed following NIST-specified certification criteria [10,29,30]. To determine the identity and assign a concentration of mycotoxin(s) with the highest confidence and metrological traceability in an SRM, a definitive method (e.g., stable isotope dilution-LC–MS) with primary standards was used. Included in the certificate are details of how the mycotoxin SRM was characterized such as certifying laboratories; methods used for certification measurement; how materials were collected, prepared, and homogenized; how results were statistically evaluated; appropriate uses of the SRMs [31,32]. Figure 2 and Figure 3 illustrate how NIST SRM 1565 was developed and the assigned values of the 12 mycotoxins. Apart from these extremely strict requirements and time-consuming preparation and certification, the multi-mycotoxin SRM offers many benefits that in-house materials lack.
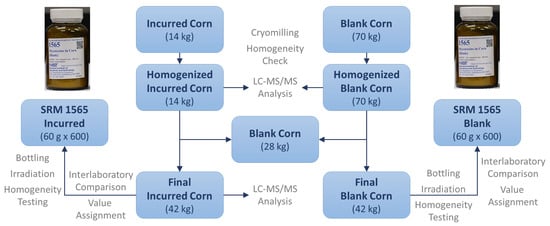
Figure 2.
Development of SRM 1565.
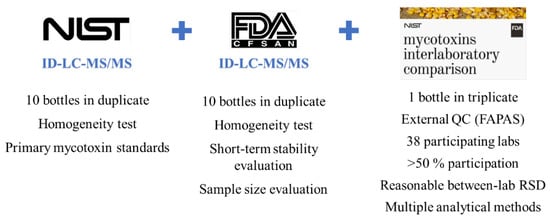
Figure 3.
Value assignment of SRM 1565.
4. Advantages of Using Reference Materials for Multi-Mycotoxin Analysis
A major benefit of multi-mycotoxin RMs and CRMs is their ease of use. With certified values and uncertainties, CRMs can serve as a real-time indicator for the performance of measurements of multiple mycotoxins in well-characterized and representative matrices. A comparison of results generated by the users to certified values and uncertainties would indicate possible qualitative or quantitative issues and help users to identify the cause of their issues quickly. For example, SRM 1565 was analyzed to assess the performance of an LC–MS-based multi-mycotoxin analysis at FDA/CFSAN (Table 1). The absolute difference between the measured averages and the reference values were calculated and compared to the certified uncertainty (confidence level = 95%, k = 2). For ten out of the twelve mycotoxins, the difference between the respective measurements and reference values was less than the uncertainty defined in the Certificate of Analysis, which suggested satisfactory quantitation in terms of accuracy. For HT-2 toxin, the differences were greater than the defined uncertainty, indicating to the analyst a potential need for corrective action. A common pitfall in quantitative measurements is to ignore the propagation of uncertainty associated with the analytical method and associated reference materials. The uncertainty of the analytical method, while not always available or systemically estimated, could be roughly estimated using the standard deviation of measurements [33]. After factoring in the uncertainty of HT-2 toxin associated with the analytical method, the combined uncertainty (confidence level = 95%; k = 2) was 7.6 ng/g, which was larger than the difference between the certified and the measured values (6.5 ng/g). The measured average of 31.7 ng/g of HT-2 toxin was, therefore, not significantly different from the certified value of 38.2 ng/g. Such analyte-dependent assessment cannot be performed when in-house QC samples are used, due to insufficient uncertainty information. Instead, an arbitrary accuracy or precision threshold is chosen and applied to all target analytes, ignoring the fact that the uncertainty associated with individual analytes varies. It is worth noting that uncertainty varies with the measurement of different mycotoxins in different samples. Applying a pre-defined acceptable threshold to analytical results of in-house materials often creates misleading information regarding method performance of individual mycotoxins. Without a thorough evaluation of uncertainty, or when applying an inappropriate threshold, the threshold could be set too strictly, triggering rejection of good data or unnecessary corrective actions. Conversely, if the threshold is too high, it could result in acceptance of poor data. On the contrary, multi-mycotoxin CRMs provide individual uncertainty of target mycotoxins so that analyte-dependent evaluation can be performed as was demonstrated.

Table 1.
LC–MS analysis of three replicates of SRM 1565.
Another benefit of using CRMs is to establish traceability of analytical results to the SI. Traceability is an important requirement for methods used with ISO standards and the use of CRMs can help labs more easily establish traceability. Established traceability of mycotoxin measurements is important to demonstrate the level of competence of testing laboratories [34] and to dictate acceptance of mycotoxin measurements. Levels of mycotoxins detected play an important role in the quality and price of agricultural commodities that are prone to mycotoxin contamination for domestic and international trade. To achieve international acceptance of mycotoxin measurements, traceability of mycotoxin measurements used for both buyers and sellers is recommended [35].
5. Current Trends and Challenges
For method development and validation studies, evaluation of accuracy is one of the most important but difficult components. The analysis of RMs and CRMs can demonstrate whether a candidate method could provide accurate quantitation and a related level of confidence. When validation involves multiple laboratories, RMs and CRMs ensure comparability of data and eliminate analytical bias and variability introduced by participating laboratories using individually prepared in-house samples [36,37,38]. One concern with regular use of RMs, especially CRMs, is their cost. Purchasing multiple single-mycotoxin RMs can quickly affect operational costs. Using a multi-mycotoxin RM or CRM is much more cost-effective as one material could provide important QC data for multiple mycotoxins, resulting in a better economy of scale.
The use of multi-mycotoxin RMs and CRMs relieves laboratories from the burden of developing and characterizing in-house QC samples and provides an effective and reliable tool to evaluate mycotoxin measurements in terms of accuracy, precision, and traceability. ISO documents emphasize the importance of RMs, and the prospects for development of new RMs are enormous. However, the application of multi-mycotoxin RMs, especially CRMs, has been stymied by the issue of availability. Though a handful of single-mycotoxin RMs are available, using several single-mycotoxin RMs to evaluate multi-mycotoxin measurements is, as with using single-mycotoxin methods to screen for multiple mycotoxins, an inefficient practice. Thus far, only a few multi-mycotoxin CRMs have been developed due to their lengthy production cycles. To keep up with the increasing need for multi-mycotoxin RMs, international efforts and collaboration to develop various mycotoxin RMs are continuous and ongoing [39]. In recent years, government agencies in the US, EU, Canada, and Brazil have taken the lead in fulfilling the constant need for mycotoxin RMs, especially multi-mycotoxin RMs [40,41,42,43]. The development of multi-mycotoxin RMs involves many target mycotoxins, a long production cycle, and a diversity of candidate matrices. These issues suggest that careful attention must be paid at the planning stage to such matters as the selection of representative matrices and mycotoxins of regulatory and health significance. The development of multi-mycotoxin RMs needs a tremendous amount of resources that no single institute could afford; therefore, a long-term mutualistic collaboration between suppliers and users of RMs should be established to meet existing needs (e.g., multi-mycotoxin RM in animal feeds) and emerging needs (e.g., multi-mycotoxin RM in cannabis) in the foreseeable future.
Author Contributions
Conceptualization, K.Z. and M.P.; methodology, K.Z. and M.P.; investigation, K.Z. and M.P.; resources, K.Z. and M.P.; data curation, K.Z. and M.P.; writing—original draft preparation, K.Z. and M.P.; writing—review and editing, K.Z. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study has received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request following Public Access to Results of FDA-Funded Scientific Research.
Acknowledgments
The authors thank Joanne Berger (FDA Library) and Christine H. Parker (FDA/Center for Food Safety and Applied Nutrition) for editing the manuscript and providing helpful comments.
Conflicts of Interest
The authors declare no conflict of interest.
Disclaimer
Certain commercial equipment, instruments, or materials may be identified in this paper to adequately specify the experimental procedure. Such identification is not intended to imply recommendation or endorsement by the FDA or NIST, nor is it intended to imply that the materials or equipment identified are necessarily the best available for the purpose.
References
- Pitt, J.I.; Miller, J.D. A Concise History of Mycotoxin Research. J. Agric. Food Chem. 2017, 65, 7021–7033. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M.; Lau, P.Y.; Kanhere, S.R. Gas chromatography with electron capture and mass spectrometric detection of deoxynivalenol in wheat and other grains. J. Assoc. Off. Anal. Chem. 1981, 64, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.A.; White, K.D.; Trucksess, M.W.; Thomas, F.S. Capillary gas chromatography/mass spectrometry with chemical ionization and negative ion detection for confirmation of identity of patulin in apple juice. J. AOAC. Int. 2000, 83, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sforza, S.; Dall’asta, C.; Marchelli, R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom. Rev. 2006, 25, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S.; Berthiller, F.; Burdaspal, P.; Crews, C.; Jonker, M.A.; Krska, R.; MacDonald, S.; Malone, B.; Maragos, C.; Sabino, M.; et al. Developments in mycotoxin analysis: An update for 2009–2010. World Mycotoxin J. 2011, 4, 3–28. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Hu, X.; Zhang, Q. Advanced hyphenated chromatographic-mass spectrometry in mycotoxin determination: Current status and prospects. Mass. Spectrom. Rev. 2013, 32, 420–452. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Cramer, B.; Dall’Asta, C.; Iha, M.H.; Lattanzio, V.M.T.; Maragos, C.; Solfrizzo, M.; Stranska, M.; Stroka, J.; Sumarah, M. Developments in mycotoxin analysis: An update for 2018–2019. World Mycotoxin J. 2020, 13, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wong, J.W.; Krynitsky, A.J.; Trucksess, M.W. Perspective on Advancing FDA Regulatory Monitoring for Mycotoxins in Foods using Liquid Chromatography and Mass Spectrometry (Review). J. AOAC Int. 2016, 99, 90–894. [Google Scholar] [CrossRef]
- van Egmond, H.P. Mycotoxins: Regulations, quality assurance and reference materials. Food Addit. Contam. 1995, 12, 321–330. [Google Scholar] [CrossRef]
- Taylor, J.K. Handbook for SRM Users. Gaithersburg; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1993.
- ISO 7870-1:2019; Control Charts—Part 1 General Guidelines. International Organization for Standardization (ISO): Geneva, Switzerland, 2019.
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 8137–8142. [Google Scholar] [CrossRef]
- Taniwaki, M.H.; Pitt, J.I.; Copetti, M.V.; Teixeira, A.A.; Iamanaka, B.T. Understanding Mycotoxin Contamination Across the Food Chain in Brazil: Challenges and Opportunities. Toxins 2019, 11, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Li, X.; Li, H. Certified Reference Materials and Metrological Traceability for Mycotoxin Analysis. J. AOAC Int. 2019, 102, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- De Bièvre, P. Making measurement results metrologically traceable to SI units requires more than just expressing them in SI units. Accredit. Qual. Assur. 2010, 15, 267–268. [Google Scholar] [CrossRef] [Green Version]
- ISO Guide 33:2015; Reference Materials—Good Practice. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- ISO-35:2017; Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Friesen, M.D. Report on the Statistical Analysis of Results Obtained for the Analysis of Ochratoxin in Wheat Flour; Report EC/92/15–2; MYCOTOXIN Sample Survey Programme, International Agency for Research on Cancer: Lyon, France, 1989.
- Pettersson, H.; Langseth, W. Intercomparison of Trichothecene Analysis and Feasibility to Produce Certified Calibrants and Reference Material. Final Report, I. Method Studies; BCR Information, Project Report EUR 20285/1 EN; European Communities: Brussels, Belgium, 2002; pp. 1–82. [Google Scholar]
- Pettersson, H.; Langseth, W. Intercomparison of Trichothecene Analysis and Feasibility to Produce Certified Calibrants and Reference Material. Final Report, I. Homogeneity and Stability Studies, Final Intercomparison; BCR Information, EU Project Report EUR 20285/2 EN; European Communities: Brussels, Belgium, 2002; pp. 1–145. [Google Scholar]
- Schuhmacher, R.; Krska, R.; Grasserbauer, M. Interlaboratory comparison test for the determination of the Fusarium mycotoxins deoxynivalenol in wheat and zearalenone in maize. Fresenius’ J. Anal. Chem. 1997, 359, 510–515. [Google Scholar] [CrossRef]
- Krska, R.; Welzig, E.; Berthiller, F.; Molinelli, A.; Mizaikoff, B. Advances in the analysis of mycotoxins and its quality assurance. Food Addit. Contam. 2005, 22, 345–353. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Wagstaffe, P.J. Aflatoxin M1 in whole milk-powder reference materials. Food Addit. Contam. 1988, 5, 315–319. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Patel, S.; Paulsch, W.E.; Sizoo, E.A.; Tuinstra, L.G.; Wood, G.; Boenke, A.; Schurer, B.; Wagstaffe, P.J. Food additives and contaminants: The development of five animal feed reference materials, certified for their aflatoxin B1 content. Food Addit. Contam. 1994, 11, 449–477. [Google Scholar] [CrossRef]
- Gilbert, J.; Sharman, M.; Wood, G.M.; Boenke, A.; Wagstaffe, P.J. The preparation, validation and certification of the aflatoxin content of two peanut butter reference materials. Food Addit. Contam. 1991, 8, 305–320. [Google Scholar] [CrossRef]
- Gilbert, A. BCR-and M&T-activities in the area of mycotoxin analysis in food and feedstuffs. Nat. Toxins. 1995, 3, 243–247. [Google Scholar]
- Sharpless, K.E.; Phinney, C.S.; Wood, L.J.; Yen, J.H.; Howell, D.W. Value assignment of nutrient and aflatoxin concentrations in standard reference material 2387 peanut butter. J. Agric. Food Chem. 2003, 51, 6745–6751. [Google Scholar] [CrossRef]
- ISO Guide 30:2015; Terms and Definitions Used in Connection with RMs. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Phillips, M.; Sharpless, K.E.; Wise, J. Standard reference materials for food analysis. Anal. Bioanal Chem. 2013, 405, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- May, W.; Parris, R.; Beck, C.; Fassett, J.; Greenberg, R.; Guenther, F.; Kramer, G.; Wise, S.; Gills, T.; Colbert, J.; et al. NIST Special Publication 260-136; Standard Reference Materials: Definitions of Terms and Modes Used at NIST for Value-Assignment of Reference Materials for Chemical Measurements; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2000. Available online: https://www.nist.gov/system/files/documents/srm/SP260-136.PDF (accessed on 12 August 2022).
- NIST Certificate for SRM 2387. Available online: https://www-s.nist.gov/srmors/certificates/2387.pdf (accessed on 12 August 2022).
- NIST Certificate for SRM 1565. Available online: https://www-s.nist.gov/srmors/certificates/1565.pdf (accessed on 12 August 2022).
- ERM Application Note 1. Available online: https://ec.europa.eu/jrc/en/reference-materials/application-notes (accessed on 12 August 2022).
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO): Geneva, Switzerland, 2005.
- Joint BIPM, OIML, ILAC, and ISO Declaration on Metrological Traceability. Available online: https://www.bipm.org/utils/common/pdf/BIPM-OIML-ILAC-ISO_joint_declaration_2018.pdf (accessed on 12 August 2022).
- Ciasca, B.; Pascale, M.; Altieri, V.G.; Longobardi, F.; Suman, M.; Catellani, D.; Lattanzio, V.M.T. In-house validation and small-scale collaborative study to evaluate analytical performances of multimycotoxin screening methods based on liquid chromatography-high-resolution mass spectrometry: Case study on Fusarium toxins in wheat. J. Mass. Spectrom. 2018, 53, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Schaab, M.R.; Southwood, G.; Tor, E.R.; Aston, L.S.; Song, W.; Eitzer, B.; Majumdar, S.; Lapainis, T.; Mai, H.; et al. A Collaborative Study: Determination of Mycotoxins in Corn, Peanut Butter, and Wheat Flour Using Stable Isotope Dilution Assay (Sida) And Liquid Chromatography—Tandem Mass Spectrometry (Lc-Ms/MS). J. Agric. Food Chem. 2017, 65, 7138–7152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liao, C.D.; Prakash, S.; Conway, M.; Cheng, H.F. Interlaboratory Validation of a Stable Isotope Dilution and Liquid Chromatography Tandem Mass Spectrometry Method for the Determination of Aflatoxins in Milk, Milk-Based Infant Formula, and Feed. J. AOAC Int. 2018, 101, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Josephs, R.D.; Li, X.; Li, X.; Guo, Z.; Garrido, B.; Un, I.; Daireaux, A.; Choteau, T.; Martos, G.; Westwood, S.; et al. The BIPM Mycotoxin Metrology Capacity Building and Knowledge Transfer Program: Accurate Characterization of a Pure Aflatoxin B1 Material to Avoid Calibration Errors. J. AOAC Int. 2019, 102, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Tangni, E.K.; Debongnie, P.; Huybrechts, B.; Van Hove, F.; Callebaut, A. Towards the development of innovative multi-mycotoxin reference materials as promising metrological tool for emerging and regulated mycotoxin analyses. Mycotoxin Res. 2017, 33, 15–24. [Google Scholar] [CrossRef]
- Bates, J.; Bahadoor, A.; Cui, Y.; Meija, J.; Windust, A.; Melanson, J.E. Certification of Ochratoxin A Reference Materials: Calibration Solutions OTAN-1 and OTAL-1 and a Mycotoxin-Contaminated Rye Flour MYCO-1. J. AOAC Int. 2019, 102, 1756–1766. [Google Scholar] [CrossRef]
- do Rego, E.C.P.; Leal, R.V.P.; Bandeira, R.D.C.C.; da Silva, M.R.; Campos, E.G.; Petronilho, C.F.; Rodrigues, J.M. Challenges on Production of a Certified Reference Material of Ochratoxin A in Roasted Coffee: A Brazilian Experience. J. AOAC Int. 2019, 102, 1725–1731. [Google Scholar] [CrossRef]
- Phillips, M.M.; Seal, T.M.L.; Ness, J.M.; Zhang, K.J. Development and Characterization of a Multimycotoxin Reference Material. AOAC Int. 2019, 102, 1642–1650. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).