Abstract
Sea buckthorn berries have been reported to have beneficial effects on plasma lipid profile and cardiovascular health. This study aimed to investigate the impact of intervention with sea buckthorn berry puree on plasma metabolomics profile and gut microbiota in hypercholesterolemic subjects. A total of 56 subjects with hypercholesterolemia consumed 90 g of sea buckthorn berry puree daily for 90 days, and plasma metabolomic profile was studied at 0 (baseline), 45, and 90 days of intervention by using proton nuclear magnetic resonance spectroscopy (1H NMR). Gut microbiota composition was analyzed at the baseline and after 90 days of supplementation by using high-throughput sequencing. The plasma metabolic profile was significantly altered after 45 days of intervention as compared to the baseline (day 0). A clear trend of returning to the baseline metabolomic profile was observed in plasma when the intervention extended from 45 days to 90 days. Despite this, the levels of several key plasma metabolites such as glucose, lactate, and creatine were lowered at day 90 compared to the baseline levels, suggesting an improved energy metabolism in those patients. In addition, intervention with sea buckthorn puree enriched butyrate-producing bacteria and other gut microbes linked to lipid metabolisms such as Prevotella and Faecalibacterium while depleting Parasutterella associated with increased risks of cardiovascular disease. These findings indicate that sea buckthorn berries have potential in modulating energy metabolism and the gut microbiota composition in hypercholesterolemic patients.
1. Introduction
Hypercholesterolemia is one of the most important risk factors for cardiovascular disease (CVD) which is the leading cause of death in global diseases [1]. Diet and genetic background as well as diseases such as obesity and type 2 diabetes contribute to elevated levels of cholesterols in the blood [2]. Healthy food choices have been considered an important approach to reducing the risk of CVD. Natural products and phytochemicals with beneficial effects on CVD, such as phenolic compounds from fruits and vegetables, have increasingly drawn attention. Epidemiological and interventional studies have indicated that vegetables and fruits, especially berry meals, have the potential to promote cardiovascular health [3,4,5].
Sea buckthorn (Hippophaë rhamnoides) berries are rich in bioactive compounds such as flavonoids, vitamins, phenolic acids, and carotenoids [6]. Feeding sea buckthorn berries or polyphenols extract have shown the beneficial modulatoy effects on carbohydrate and lipid metabolisms. Feeding daily 7–28 mg/kg body weight polyphenols extract from sea buckthorn berries to rats with hyperlipidemia for 5 weeks has reduced serum lipids and inflammatory markers as well as alleviated vascular impairment by decreasing the expression of eNOS, ICAM-1, and LOX-1 in aortas [7]. Feeding 25–100 mg/kg body weight sea buckthorn berries per day for 10 days decreased blood glucose level and insulin resistance in db/db mice by improving pancreas and revering of insulin resistance and quebrachitol present in sea buckthorn might have contributed to the effects [8]. Intake of sea buckthorn freeze-dried powder (400 mg/kg body weight per day) for 10 weeks improved lipid metabolism in obese mice via downregulating the expression of lipogenic genes including SREBP-1c, PPAR-γ, ACC, and SCD1, and upregulating gene expression of enzymes of fatty acid β-oxidation pathways including HSL, CPT-1, and ACOX [9]. A review has summarised that the mechanisms of the effect of sea buckthorn berries on cardiovascular health can be classified as regulating lipid metabolism and decreasing platelet aggregation and inflammation [10]. However, there were contradictory results obtained from some clinical studies; for example, 229 healthy participants consuming daily 28 g of sea buckthorn berries for 3 months did not show altered levels of serum total, HDL (high-density lipoproteins), LDL (low-density lipoproteins) cholesterol, or changes in the serum triacylglycerol concentrations [11]. In another study, consumption of sea buckthorn berries for 33–35 days showed little effects on plasma cholesterol and triacylglycerol levels in 80 overweight women [12].
Characteristic fecal bacterial signature has been found in patients with hypercholesterolemia in comparison to healthy subjects, such as Anaeroplasma and Haemophilus being negatively correlated to total cholesterol and triglyceride levels and positively to HDL particle size [13]. Sea buckthorn berries abundant in polyphenols are a good source of prebiotic substrate. Due to the low absorption and bioavailability of polyphenols in the small intestine, a significant part of dietary polyphenols reaches the colon and is metabolized by gut microbiota [14]. An in vitro study has shown regulating the effect of sea buckthorn berries on gut microbiota by increasing the abundances of Bacteroides and Prevotella as well as lactic acid bacteria [15]. Furthermore, protein extracted from sea buckthorn seed has also shown prebiotic properties by increasing the abundances of Clostridiumcoccoides, Bifidobacterium, and Lactobacillus in diabetic mice [16].
However, the gut microbiota-regulatory effect of sea buckthorn berries on humans has not been studied. Due to the contradictory and inconclusive results from the interventions of sea buckthorn berries in humans, the plasma metabolomics and sequencing technics capable of revealing changes in small organic molecules and gut microbiota provide different perspectives to investigate the effect of sea buckthorn berries. To the best knowledge of the authors, this is the first study to explore the effects of sea buckthorn berries on plasma metabolomic profile and gut microbiota composition in hypercholesterolemic populations.
2. Materials and Methods
2.1. Ethics
This study protocol was registered as ChiCTR1800014406 at www.chictr.org.cn (accessed on 16 August 2022) and approved by Peking University Institution Review Board (Ethical approval number: IRB00001052-17052). Written informed consent was obtained from all subjects.
2.2. Study Participants and Intervention
A total of 56 participants were recruited in this interventional trial through online advertising and by handing out leaflets in the residential areas nearby Peking University Health Center. The details of inclusion and exclusion criteria were listed in our previous publication [17]. Briefly, males and postmenopausal females aged 50–70 years old with (1) serum total cholesterol between 5.2–7.2 mmol/L; (2) insulin less than 25 mU/L; (3) blood pressure less than 160/99 mmHg; (4) hemoglobin less than 120 g/L; (5) thyroid-stimulating hormone between 0.3–4.2 mU/L; (6) alanine aminotransferase less than 60 U/L; and (7) creatinine was less than 115 mmol/L. The exclusion criteria included the diagnosis of diabetes, thyroid diseases, renal diseases, hematological diseases, hepatic dysfunction or myocardial infarction, as well as use of cardiovascular medication, lipid-lowering drugs or dietary supplements, people with persistent inflammatory disease or mental illness, people who cannot participate in this study independently.
All participants were asked to take a bottle of of sea buckthorn berries (Hippophae rhamnoides L. subsp. Sinensis) puree (30 g) three times a day for 90 days. They were told to keep the same previous dietary habits and physical activities. However, the participants were advised to avoid taking other dietary supplements during the study. Fasting blood samples were collected by an experienced technician from 8:00 to 10:00 AM at day 0, 45, and 90, and then were centrifuged at 3000× g for 15 min and stored at 80 °C until use.
2.3. Biochemical Parameters
Plasma ApoA1 and ApoB were measured by using a Hitachi 7170A/7180 Biochemical Analyzer (Hitachi, Japan). The plasma IL-6 and TNF-a level was determined with an ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Malondialdehyde (MDA) was measured by a determination kit (Wanleibio, Shenyang, China).
2.4. 1H NMR Metabolomic Analysis
Proton nuclear magnetic resonance spectroscopy (1H NMR) metabolomics analysis was performed from plasma by following the protocol described previously [18]. The samples were prepared and analysed in a randomized order. Briefly, an aliquot of 220 µL plasma was mixed with 440 µL phosphate buffer (90 mmol/L NaH2PO4, pH = 7.4) containing 15% D2O. After centrifuging, 600 µL of the resulting supernatants were transferred into 5-mm NMR tubes. The NMR experiments were performed at 298 K on a 600 MHz Bruker Avance-III NMR spectrometer (Bruker BioSpin AG, Fällanden, Switzerland) equipped with a Prodigy TCI cryoprobe and a precooled SampleJet sample changer. Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence was used. Metabolites were identified based on 1D CPMG NMR chemical shifts reported in Chenomx NMR Suite 7.5 software (Chenomx Inc., Edmonton, AB, Canada), 2D NMR (1H−13C heteronuclear single-quantum correlation spectroscopy (HSQC) and 1H−1H correlation spectroscopy (COSY)), and the metabolite database Human Metabolome Database (HMDB, http://www.hmdb.ca, accessed on 16 August 2022). The Representative CPMG spectrum of plasma samples is shown in Figure S1. Altogether, 22 metabolites were identified using Chenomx and 2D NMR (Figures S2 and S3), and their chemical shifts and peak multiplicity are summarized in Table S1.
2.5. Collection of Fecal Samples and Analysis of Gut Microbiota
Fecal samples were collected at the day 0 and day 90. 24-hour feces was collected and pooled from 48 participants at each time pointsAround 500 mg of fecal samples from each participant were collected by using disposable collectors and tubes. DNA was extracted from fecal samples of subjects, and 16sRNA Illumina Genome Analyzer was performed using MiSeq platform. The genomic DNA extraction from feces, PCR amplification, and sequencing were conducted by the Institute of Microbiology, Chinese Academy of Science, Beijing, China, and followed the previous protocol described [19].
2.6. Statistical Analysis
The values were defined as the mean ± standard deviation (SD). The differences between three-time points were analyzed by One-way ANOVA and followed by the post hoc Bonferroni test when the data is normally distributed and variances were homogeneous or else the Kruskal–Wallis test and the post hoc Tamhane test were applied, The significance of differences was described as * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 between groups. Linear discriminant analysis (LDA) effect size (LEfSe) was used to discriminate differentially abundant bacterial taxa, bacterial taxa with LDA absolute value >2 were considered differentially abundant bacterial.
3. Results
3.1. Effects of Sea Buckthorn Puree on Lipid Biomarkers, Lipid Peroxidation Product, and Inflammatory Markers
In the current study, apolipoprotein (ApoA-I and ApoB), a lipid peroxidation product (malondialdehyde), and inflammatory markers (TNF-α and IL-6) in plasma were studied at baseline and after 45 days and 90 days of intervention. ApoA-I and ApoB remained stable from day 0 to day 45 and significantly decreased at day 90 compared to day 45 (Figure 1A,B). No significant changes were seen in the ratio of ApoB/ApoA-I (Figure 1C). The lipid peroxidation product malondialdehyde (MDA) was significantly decreased at day 90 compared to that at day 0 (Figure 1D). Plasma TNF-α and IL-6 levels were not altered (Figure 1E,F).
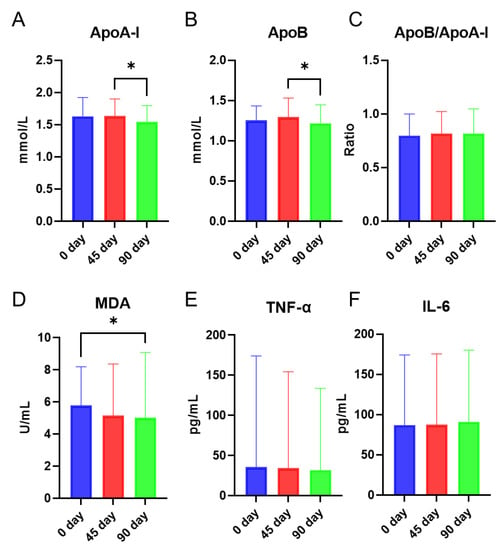
Figure 1.
Effects of intervention with sea buckthorn puree on ApoA-I (A), ApoB (B), Ratio of ApoB/ApoA-I (C), malondialdehyde (MDA) (D), TNF-α (E), and IL-6 (F). n = 56, each group. Note: * p < 0.05.
3.2. Effects of Sea Buckthorn Berries Puree on the Plasma Metabolites
Twenty-two metabolites including amino acids (branched-chain amino acids, aromatic amino acids, histidine, etc.), compounds related to glucose metabolism (pyruvate, citrate, lactate, etc.), and as well as other metabolites (creatine, creatinine, and acetate, etc.) were identified from plasma 1H NMR spectra.
Principal component analysis (PCA) was performed based on the plasma metabolic profile to investigate the metabolite alterations associated with the sea buckthorn puree intervention. PCA score plot (Figure 2A) showed a metabolic separation between day 45 and day 0/90 by the first principal component (27.3%), contributed mainly by the altered levels of lipids (resonance generated from CH3 protons of fatty acyl chain or fatty acid chain), serine, alanine, threonine, glycine, tyrosine, acetate, leucine, and isoleucine shown in the PCA loading plot (Figure 2B). A slight separation of plasma metabolic profile was found between day 0 and day 90 by the second principal component (14.3%) (Figure 2A), mainly contributed by unsaturated lipid (resonance generated from CH protons of unsaturated fatty acyl chain or fatty acid chain) and 3-hydroxybutyrate (Figure 2B). Overall, plasma metabolic profile was significantly changed from day 0 to day 45, and returned to similar levels as the baseline when the intervention proceeded to day 90.
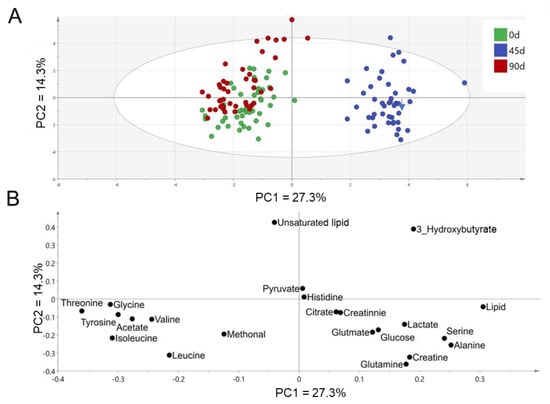
Figure 2.
PCA score plot (A) and loading plot (B) based on metabolites identified from plasma 1NMR spectra. n = 44, each group.
After multivariate analysis, histograms are made to show changes in each metabolite at three different time points. Changes of metabolites related to glycolysis and tricarboxylic acid (TCA) cycle were shown in Figure 3A. Glucose level was decreased after 90 days of intervention compared to the level at day 0/45, a similar change was also observed in lactate levels. Valine was decreased at day 45 and then increased at day 90. The lipid level was slightly increased from day 0 to day 45 and then dropped to near the baseline at day 90. Unsaturated lipid level was increased from day 0/45 to day 90 (Figure 3B).
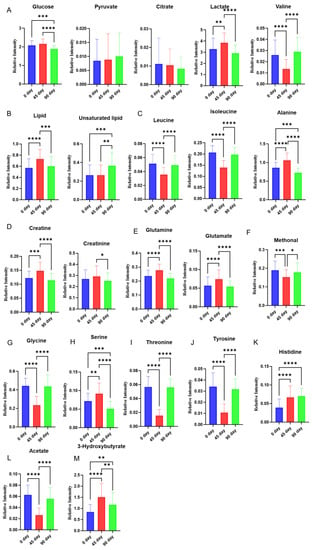
Figure 3.
Effect of sea buckthorn puree on plasma metabolites in hypercholesterolemia population. Metabolites involved in glycolysis and TCA cycle (glucose, lactate, alanine, citrate, and pyruvate) (A), Lipids (B), Branched-chain amino acids (leucine, isoleucine, and valine) (C), Creatine and creatinine (D), Glutamine and glutamate (E), Methanol (F), Glycine (G), Serine (H), Threonine (I), tyrosine (J), Histidine (K), Acetate (L), 3-hydroxybutyrate (M). n = 44, each group. Note: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
Branched-chain amino acids (leucine, isoleucine, and valine) were also altered during the intervention. Isoleucine and leucine were decreased during the time point from day 0 to day 45 and then increased to the baseline level at day 90. Alanine was increased at 45 compared to the baseline and then decreased at day 90 to a lower than the baseline (Figure 3C).
The levels of creatine, creatinine, glutamate, glutamine, serine, and 3-hydroxybutyrate shared similar trajectories, those metabolites were firstly increased at day 45 compared to day 0, and then decreased at day 90 compared to the level at day 45. The levels of creatine, glutamine, serine, and 3-hydroxybutyrate at day 90 were even lower than the baseline (Figure 3D,E,H,M). Methonal, glycine, threonine, tyrosine, and acetate have reversed trajectories compared to the metabolites mentioned above, they were decreased from the baseline to day 45 and then increased back to the level close to the baseline (Figure 3F,G,I,J,L).
3.3. Effects of Sea Buckthorn Puree on Gut Microbiota
To analyze the altered gut microbiota composition in the hypercholesterolemia population by the intervention of sea buckthorn puree, 16s RNA sequencing was performed on bacteria in feces collected at day 0 and day 90. The richness and diversity of gut microbiota composition were shown in Figure 4A. The richness of gut microbiota measured as the total number of genera and gut microbial diversity represented as Chao1 and Shannon indexes were not changed after 90 days of intervention of sea buckthorn puree. Cladogram and histogram of LDA score (Figure 4B and Figure 5) generated from LEfSe (LDA Effect Size) analysis showed the different abundance of taxa after treating sea buckthorn berries puree for 90 days.
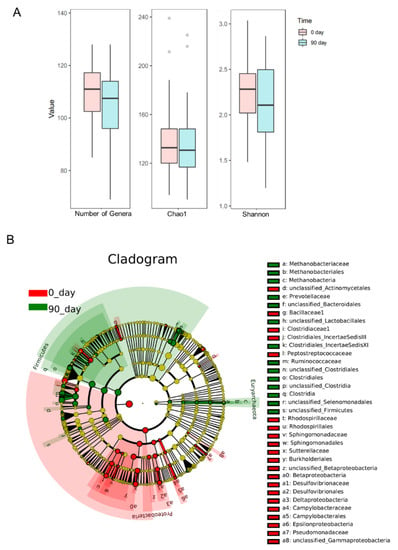
Figure 4.
The richness (number of genera) and diversity (Chao1 and Shannon indexes) of gut microbiota (A). The structure of the microbiota is presented in the cladograms (B), the circle radiated inside-out represents the classification of taxa level from phylum to family. Each small circle at different classification levels represents a taxon and the diameter of the small circle is proportional to the relative abundance. The taxa not with significant differences were colored by yellow and significantly different taxa were colored by different groups, the taxa marked with red color were enriched in the baseline. n = 48, each group.
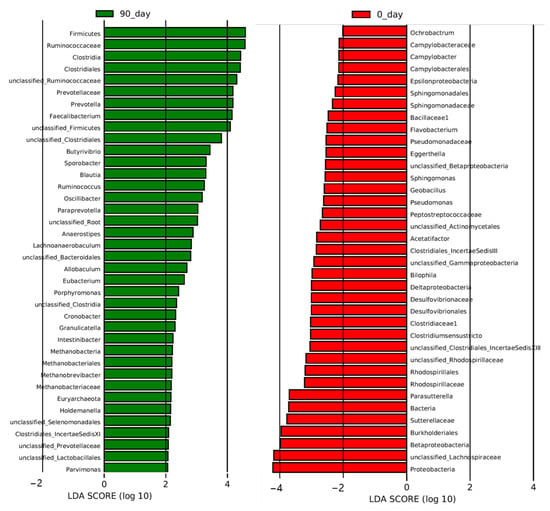
Figure 5.
Effect of sea buckthorn berries puree on plasma metabolites in hypercholesterolemia population. From phylum to Genus. The results of LEfSe (LDA Effect Size) analysis level at week 8. The histogram of LDA score generated from LEfSe analysis showed the differentially abundant species (the absolute value of LDA greater than 2) after the treatment of sea buckthorn berries puree. The length of the bars (LDA Score) represents the influencing degree of the species. The species with positive or negative LDA score have higher or lower abundance after the treatment. n = 48, each group.
To determine the most altered taxa, the histogram of LDA score showed that the top five most upregulated gut microbiota taxa were phylum Firmicutes, family Ruminococcaceae, class Clostridia, order Clostridiales, and a genus from the family Ruminococcaceae (unclassified_Ruminococcaceae) (Figure 5). Phylum Proteobacteria, a genus from the family Lachnospiraceae (unclassified_Lachnospiraceae), class Betaproteobacteria, class Burkholderiales, and family Sutterellaceae were the top five most downregulated gut microbiota taxa by the intervention (Figure 5).
Disbiome database was firstly published in 2018 to provide associations between microorganisms and diseases classified in the Medical Dictionary for Regulatory Activities system. Table 1 summarizes the characteristics of those enriched or depleted genera affected by the intake of sea buckthorn puree [20]. We found that 9 out of 21 enriched genera were butyrate producers which all belonged to the class Clostridiales (Anaerostipes, Ruminococcus, Oscillibacter, Butyrivibrio, Blautia, Eubacterium, Faecalibacterium, unclassified_Ruminococcaceae, and Sporobacter). Only three genera characterized as butyrate producers were depleted after the intervention (Clostridiumsensustricto, unclassified_Lachnospiraceae, and Unclassified_Clostridiales_IncertaeSedis XI). In addition, Prevotella and another genus from Prevotellaceae (unclassified_Prevotellaceae), both of which were high-fiber diet-related probiotics, were also enriched by the treatment. Although two potential pathogens related to metabolic diseases (Holdemanella and Allobaculum) were enriched by the intervention [21], several potential pathogens were depleted after the treatment, such as Parasutterella related to the development of kidney stone and hypertension [22], Acetatifactor related to the pathogenesis of obesity and lipid acid metabolism [23], Eggerthella associated with coronary heart disease and chronic kidney disease [24], and Bilophila related to obesity [25].

Table 1.
Altered genera affected by sea buckthorn puree and their characteristics generated from Disbiome [20].
4. Discussion
This is the first study investigating the impact of sea buckthorn puree on plasma metabolic profile and the composition of gut microbiota in the hypercholesterolemic population. Differences in plasma metabolic profiles and gut microbiota were observed before and after the treatment.
Previously we reported the baseline demographic and clinical characteristics of the patients [17]; the total cholesterol was slightly increased from day 0 to day 45 and then decreased to the level close to that of the baseline at the end of the intervention. Soluble vascular cell adhesion molecule-1 was decreased at day 45 compared to level at the baseline and then increased at day 90 [17]. There were no changes in triglycerides, systolic blood pressure, diastolic blood pressure, HDL, LDL, BMI, or high-sensitivity C-reactive protein by the intervention throughout 90 days [17].
ApoB is abundant in very low-density lipoproteins (VLDL) and LDL which are atherogenic particles, leading to the entrapment of these lipoproteins in the arterial wall [26]. ApoA-I is mainly present in HDL particles, which transfer excess cholesterol from peripheral cells to the liver. In addition, ApoA-I exerts anti-inflammatory and antioxidant effects [27]. The ratio of ApoB/ApoA-I has been recognized as a marker for cardiovascular disease, the lower ApoB/ApoA-I ratio is associated with lower risk of cardiovascular diseases [26]. In this study, both ApoB and ApoA-I were decreased from day 0/45 to the end of the intervention, but the ratio of ApoB/ApoA-I was not changed throughout the intervention. Malondialdehyde (MDA) is one of the final products of polyunsaturated fatty acids peroxidation initiated by free radicals [28]. MDA was decreased by the intervention at day 90 compared to the baseline, indicating a possible radical scavenging property of sea buckthorn puree [6].
Plasma 1H NMR metabolomics revealed that glucose and lactate were increased first from the baseline to day 45, and thereafter their levels dropped all the way till the end of the intervention to the level lower than the baseline. This changing pattern was in line with those of the total cholesterol [17] as well as some detected gluconeogenic amino acids such as alanine, glutamine, and serine found in this study. We speculated that the extra intake of sugar in the sea buckthorn puree and/or increased gluconeogenic amino acids might cause an increase in glucose and its non-oxidative glycolysis product lactate at day 45, nevertheless, the glucose-lowering effect of sea buckthorn puree appeared at the end of the intervention. An increase of lactate is partly contributed by dysregulated pyruvate metabolism, in which pyruvate is converted to lactate rather than acetyl-CoA [29]. Moreover, hypoxia and oxidative stress in skeletal muscle and adipose tissue caused by misfunctioning lactate transporter monocarboxylate transport protein 1 in hypercholesterolemia could also lead to increased lactate [30,31,32]. Decreased glucose and lactate levels at day 90 compared to the baseline might indicate improved glycolysis and oxidative stress by the intervention of sea buckthorn puree, in which polyphenols present in sea buckthorn berries might have played a role [8]. Although the alteration of lipid (resonance generated from CH3 protons of fatty acyl chain or fatty acid chain) was also consistent with the changes in the total cholesterol [17], the unsaturated lipid level (resonance generated from CH protons of unsaturated fatty acyl chain or fatty acid chain) behaved differently and was not altered from the baseline to day 45; however, extension of intervention to 90 days resulted in increase in the level of unsaturated lipids.
The catabolism of branched-chain amino acids (BCAAs, leucine, isoleucine, and valine) is controlled by a common enzyme branched-chain ketoacid dehydrogenase kinase in skeletal muscle, however, the trajectories of these metabolites changes were not consistent, which might be due to the differential uptake of these amino acids to skeletal muscle [33]. The circulating levels of BCAAs have been reported to be positively correlated with insulin resistance in type 2 diabetes [33]. However, in this clinical study, participants with insulin resistance and type 2 diabetes were excluded. Interestingly, in subjects or animal models without insulin resistance, circulating BCAAs, particularly leucine and isoleucine, might play an important role in regulating glucose metabolism. For example, leucine has been shown to stimulate insulin secretion by deactivating adrenergic α2A receptor via mTOR (Mammalian Target of Rapamycin) pathway in healthy SD rats [34]; Isoleucine has been recognized as a blood glucose-lowering amino acid by increasing glucose uptake in skeletal muscle in healthy Wistar rats [35]. In this study isoleucine and leucine levels were increased from day 45 to day 90, which might partly contribute to lowered plasma glucose from day 45 to day 90 in the population with hypercholesterolemia.
A follow-up cohort study including 4,483 men has shown that men with elevated plasma creatinine level had higher cholesterol and lower HDL levels and were more likely to have cardiovascular disease [36]. In addition, elevated plasma creatinine along with increased plasma cholesterol has been recognized as an indication of kidney malfunction [36]. In this study, the changes of creatinine level were also in line with cholesterol level [17], indicating creatinine can be a potential biomarker for hypercholesterolemia and kidney malfunction. The lowered level of creatinine at day 90 than those at day 0 and day 45 might suggest an improvement of hypercholesterolemia and kidney function by sea buckthorn puree. The relationship between plasma creatinine and hypercholesterolemia deserves further study.
Dietary supplementation of creatine has been reported to be beneficial to lipid metabolism [37]. For example, creatine supplementation (20 g/day for 5 days, followed by 10 g/day for 51 days) decreased the plasma total cholesterol and triacylglycerols without changing plasma LDL and HDL levels huamn [38]. However, both plasma creatine shown in this study and total cholesterol as reported in previous study [17] were found at the highest level at day 45. These results of previous research and the current study indicated that the dietary creatine and endogenous creatine might behave differently in patients with hypercholesterolemia. When the intervention time proceeded to day 90, the plasma creatine decreased to levels below that at the baseline (day 0).
Inhibition of the initiation and progression of atherosclerotic lesions in low-density lipoprotein receptor (LDLR−/−) knock-out mice was associated with the alteration of amino acid metabolism, which was characterized by increased glycine, valine, and glutamine [39]. These results indicate that glycine, valine, and glutamine are involved in the initiation and progression of atherosclerotic lesions. In this study, the levels of glycine and valine decreased at day 45 of the intervention and then increased to the baseline level at the end of the 90-day intervention; however, the glutamine level was first increased and then decreased back to the baseline level. Further research is needed to confirm the possible clinical relevance of such biochemical changes in metabolomic profile.
Circulating acetate level has been reported to be negatively associated with the levels of blood lipid biomarkers including total cholesterol in 60 hypercholesterolemic adults [40]. Acetate may play a crucial role in brown adipocyte differentiation through the induction of mitochondrial biogenesis, leading to an increased oxygen consumption rate in white and brown adipocytes of mice [41]. An increased oxygen consumption rate was associated with a reduction of reactive oxygen species in hyperlipidemia [42]. Acetate was decreased at day 45 of the intervention period, indicating a possible increase of reactive oxygen species due to the sugar intake [43] via intervention with sea buckthorn puree. As the intervention proceeded to the end of 90-day period, the acetate level fell back to the baseline, suggesting that the functional component in the sea buckthorn berries such as polyphenols possibly decreased reactive oxygen species production [44].
Due to the low absorption, a large proportion of dietary phenolic compounds reaches the colon, where they are metabolized by gut microbiota [14,45]. Polyphenols-gut microbe interactions follow a two-way relationship, with polyphenols shaping the gut microbiota which in turn convert polyphenols into simpler metabolites with increased bioavailability [14,45]. Sea buckthorn berries have been found to increase the abundance of Bacteroides and Prevotella bacteria in vitro [15]. Intake of freeze-dried sea buckthorn berry powder increased Akkermansia and Bacteroides in the gut of obese mice [9]. Furthermore, feeding diabetic mice with proteins extracted from sea buckthorn seed increased the abundance of Clostridiumcoccoides, Bifidobacterium, and Lactobacillus [16]. Our present study is the first one to reveal the effect of sea buckthorn puree on the gut microbiota in humans.
In the present research, we found that intervention with sea buckthorn puree led to alterations in 11 genera of butyrate producers as identified by Disbiome database [20], of which 9 genera were enriched and only 3 genera were depleted by the intervention. Butyrate production in the gut has been reported to be associated with increased gut barrier integrity and improved gut immune responses [46]. Our results showed that supplementation with sea buckthorn puree has the potential to increase the abundance of those butyrate producers (Anaerostipes, Ruminococcus, Oscillibacter, Butyrivibrio, Blautia, Eubacterium, Faecalibacterium, unclassified_Ruminococcaceae, and Sporobacter), which might improve the barrier integrity and immune responses of the gut.
In this study, the abundance of Prevotella and Faecalibacterium was increased by sea buckthorn puree in a hypercholesterolemic population. Intake of phenolics has been reported to increase the abundance of Prevotella and to enhance the gut barrier integrity in mice [47]. Sea buckthorn berries also increased the abundance of Prevotella in vitro [15]. Ruminococcus was reported to be associated with glucose and lipid metabolism in postmenopausal women with obesity [48]. In addition, Prevotella and Faecalibacterium were found to be linked to higher levels of circulating lipids, specifically lysophosphatidylethanolamine and phosphatidylglycerols, which are important intermediates for lipid metabolism and metabolic signaling [49]. These results indicated a possible improvement in the gut barrier as well as glucose and lipid metabolism by sea buckthorn puree in the population with hypercholesterolemia. Parasutterella has been reported to be positively associated with BMI, type 2 diabetes, and low-grade inflammation in obesity revealed by a cohort study [50]; however, although Parasutterella abundance was deceased by the sea buckthorn puree, the plasma inflammatory markers TNF-α and IL-6 were not altered in population with hypercholesterolemia. High intakes of α-linolenic acid (ALA) were linked to a reduced abundance of Parasutterella [50]. Dietary intake of ALA may have beneficial effects on cardiovascular health. The negative association between Parasutterella and ALA indicates that regulation of gut microbiota might be a mechanism involved in the positive cardiovascular effects of ALA [51]. The depletion of Parasutterella by intervention with sea buckthorn puree indicated the potential benefits to the cardiovascular system in the population with hypercholesterolemia. Acetatifactor has been frequently demonstrated to be associated with bile acid metabolism in metabolic diseases [23,52,53]. Acetatifactor has been found to be correlated positively with the concentration of secondary bile acids such as lithocholic acid and ursodeoxycholic acid, which can regulate bile acid metabolism and glucose and lipid metabolisms by activating the nuclear farnesoid X receptor and the G protein-coupled membrane receptor 5 [23]. However, in our present study Acetatifactor was depleted by the intervention with sea buckthorn puree, of which the mechanism and clinical relevance need more research.
This study revealed the modulatory effects of sea buckthorn puree on plasma metabolomic profile and gut microbiota composition in the hypercholesterolemia population. 1H NMR metabolomics was applied to study the plasma samples collected at the baseline as well as after 45 days and 90 days of intervention. The plasma metabolic profile was significantly altered at day 45 compared to the baseline (day 0), as characterized by increased levels of glucose, lipid, lactate, creatine, glutamine, glutamate, serine, and 3-hydroxybutyrate and decreased content of valine, leucine, isoleucine, glycine, threonine, tyrosine, and acetate. As the intervention proceeded to day 90, the general metabolic profile returned to the level similar to the baseline. However, several metabolites remained at different levels from the baseline; for example, the levels of glucose, lactate, creatine, glutamine, and serine remained lower till the end of the 90-day intervention. The extra intake of sugar as part of the sea buckthorn puree could have played a role in influencing energy metabolism in the hypercholesterolemia population leading to an increase in plasma levels of glucose, lactate, and lipids at day 45 of the intervention period, compromising the beneficial effects of sea buckthorn on sugar and lipid metabolism. Nevertheless, the glucose and lactate levels were decreased to the levels lower than the baseline, and lipid levels to the baseline at day 90, which might have been contributed by the action of the bioactive components in the sea buckthorn puree such as phenolic compounds.
Sea buckthorn puree intervention also modulated gut microbes, such as enrichment of butyrate-producing bacteria and other gut microbes associated with lipid metabolism (Prevotella and Faecalibacterium) as well as depletion of Parasutterella potentially harmful to cardiovascular health.
Overall, this is the first study investigating the effects of dietary intervention with sea buckthorn puree on plasma metabolomic profile and gut microbiota composition in the hypercholesterolemia population. The findings of the study provide valuable information for further research on the health-promoting effects of sea buckthorn berries. All the participants, whose data were included in the analysis, confirmed that they have consumed the sea buckthorn puree as instructed. However, consumption of sea buckthorn three times a day for 90 days can not be verified for each participant; therefore it can not be excluded that a decreased compliance of the participants during the later phase of the intervention might have caused the revert of the metabolomic profiles towards the baseline. The lack of a control group without intervention is a limitation of this study. Further research using a placebo-controlled study design needs to be conducted to confirm the findings observed in the current study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods11162481/s1, Figure S1: Representative CPMG spectrum of plasma samples; Figure S2: 2D COSY spectrum; Figure S3: 2D HSQC spectrum; Table S1: Chemical shifts and peak multiplicity of metabolites.
Author Contributions
Conceptualization, B.Y., Y.Z.; Methodology-Conducted the human intervention, F.Z., J.Z. and P.L.; Formal analysis, Investigation, Data curation, Visualization, K.C., F.Z., B.Y.; Methodology-metabolomics study, K.C.; Methodology-data of sequencing, F.Z.; Funding and resources, B.Y., K.C., Y.Z.; Supervison and project administration, B.Y., Y.Z.; Writing- Original draft preparation, K.C.; Validation, Writing-Reviewing and Editing, B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by competitive funding to strengthen universities’ research profiles from the Academy of Finland (decision no. 318894), by the National Natural Science Foundation of China (No. 81472969), the China Scholarship Council, and the Finland-China Network in Food and Health as a pilot of the global program for research and innovation funded by the Ministry of Education and Culture of Finland.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Peking University Institution Review Board (Ethical approval number: IRB00001052-17052, 11 January 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the Raghunath Parijani and members of the Instrument Center of the University of Turku for their helpful training in using NMR.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steinberg, D. Atherogenesis in Perspective: Hypercholesterolemia and Inflammation as Partners in Crime. Nat. Med. 2002, 8, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P. Dyslipidaemia. Lancet 2003, 362, 717–731. [Google Scholar] [CrossRef]
- Larmo, P.S.; Kangas, A.J.; Soininen, P.; Lehtonen, H.M.; Suomela, J.P.; Yang, B.; Viikari, J.; Ala-Korpela, M.; Kallio, H.P. Effects of Sea Buckthorn and Bilberry on Serum Metabolites Differ According to Baseline Metabolic Profiles in Overweight Women: A Randomized Crossover Trial. Am. J. Clin. Nutr. 2013, 98, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, H.M.; Suomela, J.P.; Tahvonen, R.; Vaarno, J.; Venojärvi, M.; Viikari, J.; Kallio, H. Berry Meals and Risk Factors Associated with Metabolic Syndrome. Eur. J. Clin. Nutr. 2010, 64, 614–621. [Google Scholar] [CrossRef]
- Chong, M.F.F.; MacDonald, R.; Lovegrove, J.A. Fruit Polyphenols and CVD Risk: A Review of Human Intervention Studies. Br. J. Nutr. 2010, 104 (Suppl. 3), S28–S39. [Google Scholar] [CrossRef]
- Xu, Y.J.; Kaur, M.; Dhillon, R.S.; Tappia, P.S.; Dhalla, N.S. Health Benefits of Sea Buckthorn for the Prevention of Cardiovascular Diseases. J. Funct. Foods 2011, 3, 2–12. [Google Scholar] [CrossRef]
- Yang, F.; Suo, Y.; Chen, D.; Tong, L. Protection against Vascular Endothelial Dysfunction by Polyphenols in Sea Buckthorn Berries in Rats with Hyperlipidemia. Biosci. Trends 2016, 10, 188–196. [Google Scholar] [CrossRef]
- Xue, Y.; Miao, Q.; Zhao, A.; Zheng, Y.; Zhang, Y.; Wang, P.; Kallio, H.; Yang, B. Effects of Sea Buckthorn (Hippophaë Rhamnoides) Juice and L-Quebrachitol on Type 2 Diabetes Mellitus in Db/Db Mice. J. Funct. Foods 2015, 16, 223–233. [Google Scholar] [CrossRef]
- Guo, C.; Han, L.; Li, M.; Yu, L. Seabuckthorn (Hippophaë Rhamnoides) Freeze-Dried Powder Protects against High-Fat Diet-Induced Obesity, Lipid Metabolism Disorders by Modulating the Gut Microbiota of Mice. Nutrients 2020, 12, 265. [Google Scholar] [CrossRef]
- Sayegh, M.; Miglio, C.; Ray, S. Potential Cardiovascular Implications of Sea Buckthorn Berry Consumption in Humans. Int. J. Food Sci. Nutr. 2014, 65, 521–528. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, B.; Yang, Y.; Pham, T.X.; Park, Y.-K.; Manatou, J.; Koo, S.I.; Chun, O.K.; Lee, J.-Y. Berry Anthocyanins Suppress the Expression and Secretion of Proinflammatory Mediators in Macrophages by Inhibiting Nuclear Translocation of NF-ΚB Independent of NRF2-Mediated Mechanism. J. Nutr. Biochem. 2014, 25, 404–411. [Google Scholar] [CrossRef]
- Lehtonen, H.M.; Suomela, J.P.; Tahvonen, R.; Yang, B.; Venojärvi, M.; Viikari, J.; Kallio, H. Different Berries and Berry Fractions Have Various but Slightly Positive Effects on the Associated Variables of Metabolic Diseases on Overweight and Obese Women. Eur. J. Clin. Nutr. 2011, 65, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J.C.E. Faecal Bacterial and Short-Chain Fatty Acids Signature in Hypercholesterolemia. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Attri, S.; Sharma, K.; Raigond, P.; Goel, G. Colonic Fermentation of Polyphenolics from Sea Buckthorn (Hippophae rhamnoides) Berries: Assessment of Effects on Microbial Diversity by Principal Component Analysis. Food Res. Int. 2018, 105, 324–332. [Google Scholar] [CrossRef]
- Yuan, H.; Shi, F.; Meng, L.; Wang, W. Effect of Sea Buckthorn Protein on the Intestinal Microbial Community in Streptozotocin-Induced Diabetic Mice. Int. J. Biol. Macromol. 2018, 107, 1168–1174. [Google Scholar] [CrossRef]
- Nissen, S.E.; Tsunoda, T.; Tuzcu, E.M.; Schoenhagen, P.; Cooper, C.J.; Yasin, M.; Eaton, G.M.; Lauer, M.A.; Sheldon, W.S.; Grines, C.L.; et al. Effect of Recombinant ApoA-I Milano on Coronary Atherosclerosis in Patients with Acute Coronary Syndromes: A Randomized Controlled Trial. JAMA 2003, 290, 2292–2300. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of Anthocyanin Extracts from Bilberry (Vaccinium myrtillus L.) and Purple Potato (Solanum tuberosum L. Var. ’Synkeä Sakari’) on the Plasma Metabolomic Profile of Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Kortesniemi, M.; Pariyani, R.; Zhang, Y.; Yang, B. Effects of Acylated and Nonacylated Anthocyanins Extracts on Gut Metabolites and Microbiota in Diabetic Zucker Rats: A Metabolomic and Metagenomic Study. Food Res. Int. 2022, 153, 110978. [Google Scholar] [CrossRef]
- Janssens, Y.; Nielandt, J.; Bronselaer, A.; Debunne, N.; Verbeke, F.; Wynendaele, E.; Van Immerseel, F.; Vandewynckel, Y.P.; De Tré, G.; De Spiegeleer, B. Disbiome Database: Linking the Microbiome to Disease. BMC Microbiol. 2018, 18. [Google Scholar] [CrossRef]
- Tang, W.; Yao, X.; Xia, F.; Yang, M.; Chen, Z.; Zhou, B.; Liu, Q. Modulation of the Gut Microbiota in Rats by Hugan Qingzhi Tablets during the Treatment of High-Fat-Diet-Induced Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Jiang, Y.; Tan, A.; Ye, J.; Xian, X.; Xie, Y.; Wang, Q.; Yao, Z.; Mo, Z. 16S RRNA Gene Sequencing Reveals Altered Composition of Gut Microbiota in Individuals with Kidney Stones. Urolithiasis 2018, 46, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zhang, A.H.; Miao, J.H.; Sun, H.; Yan, G.L.; Wu, F.F.; Wang, X.J. Gut Microbiota as Important Modulator of Metabolism in Health and Disease. RSC Adv. 2018, 8, 42380–42389. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The Gut Microbiome in Atherosclerotic Cardiovascular Disease. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Maya-Lucas, O.; Murugesan, S.; Nirmalkar, K.; Alcaraz, L.D.; Hoyo-Vadillo, C.; Pizano-Zárate, M.L.; García-Mena, J. The Gut Microbiome of Mexican Children Affected by Obesity. Anaerobe 2019, 55, 11–23. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. The ApoB/ApoA-I Ratio: A Strong, New Risk Factor for Cardiovascular Disease and a Target for Lipid-Lowering Therapy--a Review of the Evidence. J. Intern. Med. 2006, 259, 493–519. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A. The Rationale for Using ApoA-I as a Clinical Marker of Cardiovascular Risk. J. Intern. Med. 2006, 259, 447–454. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef]
- Shulman, G.I. Unraveling the Cellular Mechanism of Insulin Resistance in Humans: New Insights from Magnetic Resonance Spectroscopy. Physiology 2004, 19, 183–190. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Liu, H.; Zhang, X.; Ding, X.; Liu, S.; Han, B.; Wang, H.; Duan, X.; Sun, T. Low Lactic Acid and Hypercholesterolemia Reduce 90-Day Mortality in Patients Suffering From Septic Shock According to the Sepsis-3 Definition. 2021. Available online: https://www.researchsquare.com/article/rs-380037/v1 (accessed on 16 August 2022).
- Crawford, S.O.; Hoogeveen, R.C.; Brancati, F.L.; Astor, B.C.; Ballantyne, C.M.; Schmidt, M.I.; Young, J.H. Association of Blood Lactate with Type 2 Diabetes: The Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 2010, 39, 1647–1655. [Google Scholar] [CrossRef]
- Hegazy, A.M.; Azeem, A.A.; Shahy, E.M.; El-Sayed, E.M. Comparative Study of Cholinergic and Oxidative Stress Biomarkers in Brains of Diabetic and Hypercholesterolemic Rats. Hum. Exp. Toxicol. 2016, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Denis McGarry, J. Dysregulation of Fatty Acid Metabolism in the Etiology of Type 2 Diabetes. Diabetes 2002, 51, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dolinger, M.; Ritaccio, G.; Mazurkiewicz, J.; Conti, D.; Zhu, X.; Huang, Y. Leucine Stimulates Insulin Secretion via Down-Regulation of Surface Expression of Adrenergic A2A Receptor through the MTOR (Mammalian Target of Rapamycin) Pathway: IMPLICATION IN NEW-ONSET DIABETES IN RENAL TRANSPLANTATION*. J. Biol. Chem. 2012, 287, 24795. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Yamaoka, I.; Nakayama, M.; Mochizuki, S.; Sugahara, K.; Yoshizawa, F. Isoleucine, a Blood Glucose-Lowering Amino Acid, Increases Glucose Uptake in Rat Skeletal Muscle in the Absence of Increases in AMP-Activated Protein Kinase Activity. J. Nutr. 2005, 135, 2103–2108. [Google Scholar] [CrossRef] [PubMed]
- Schaeffner, E.S.; Kurth, T.; Curhan, G.C.; Glynn, R.J.; Rexrode, K.M.; Baigent, C.; Buring, J.E.; Gaziano, J.M. Cholesterol and the Risk of Renal Dysfunction in Apparently Healthy Men. J. Am. Soc. Nephrol. 2003, 14, 2084–2091. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Earnest, C.P.; Almada, A.L.; Mitchell, T.L. High-Performance Capillary Electrophoresis-Pure Creatine Monohydrate Reduces Blood Lipids in Men and Women. Clin. Sci. 1996, 91, 113–118. [Google Scholar] [CrossRef]
- Li, B.; Lu, X.; Wang, J.; He, X.; Gu, Q.; Wang, L.; Yang, Y. The Metabonomics Study of P-Selectin Glycoprotein Ligand-1 (PSGL-1) Deficiency Inhibiting the Progression of Atherosclerosis in LDLR-/- Mice. Int. J. Biol. Sci. 2018, 14, 36. [Google Scholar] [CrossRef]
- Fechner, A.; Kiehntopf, M.; Jahreis, G. The Formation of Short-Chain Fatty Acids Is Positively Associated with the Blood Lipid-Lowering Effect of Lupin Kernel Fiber in Moderately Hypercholesterolemic Adults. J. Nutr. 2014, 144, 599–607. [Google Scholar] [CrossRef]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-Chain Fatty Acid Acetate Stimulates Adipogenesis and Mitochondrial Biogenesis via GPR43 in Brown Adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef]
- Amiya, E. Interaction of Hyperlipidemia and Reactive Oxygen Species: Insights from the Lipid-Raft Platform. World J. Cardiol. 2016, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Ghanim, H.; Ravishankar, S.; Sia, C.L.; Viswanathan, P.; Mohanty, P.; Dandona, P. Prolonged Reactive Oxygen Species Generation and Nuclear Factor-ΚB Activation after a High-Fat, High-Carbohydrate Meal in the Obese. J. Clin. Endocrinol. Metab. 2007, 92, 4476–4479. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of Gut Microbiota with Dietary Polyphenols and Consequences to Human Health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Su, H.; Xie, L.; Xu, Y.; Ke, H.; Bao, T.; Li, Y.; Chen, W. Pelargonidin-3- O-Glucoside Derived from Wild Raspberry Exerts Antihyperglycemic Effect by Inducing Autophagy and Modulating Gut Microbiota. J. Agric. Food Chem. 2020, 68, 13025–13037. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.D.; Larsen, L.H. Specific Gut Microbiota Features and Metabolic Markers in Postmenopausal Women with Obesity. Nutr. Diabetes 2015, 5, e159. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Henneke, L.; Schlicht, K.; Andreani, N.A.; Hollstein, T.; Demetrowitsch, T.; Knappe, C.; Hartmann, K.; Jensen-Kroll, J.; Rohmann, N.; Pohlschneider, D.; et al. A Dietary Carbohydrate–Gut Parasutterella–Human Fatty Acid Biosynthesis Metabolic Axis in Obesity and Type 2 Diabetes. Gut Microbes 2022, 14, 2057778. [Google Scholar] [CrossRef]
- Yamada, T.; Takahashi, D.; Hase, K. The Diet-Microbiota-Metabolite Axis Regulates the Host Physiology. J. Biochem. 2016, 160, 1–10. [Google Scholar] [CrossRef]
- Wang, X.; Xia, J.; Jiang, C. Role of Gut Microbiota in the Development of Non-Alcoholic Fatty Liver Disease. Liver Res. 2019, 3, 25–30. [Google Scholar] [CrossRef]
- Kübeck, R.; Bonet-Ripoll, C.; Hoffmann, C.; Walker, A.; Müller, V.M.; Schüppel, V.L.; Lagkouvardos, I.; Scholz, B.; Engel, K.H.; Daniel, H.; et al. Dietary Fat and Gut Microbiota Interactions Determine Diet-Induced Obesity in Mice. Mol. Metab. 2016, 5, 1162. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).