Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Cold Plasma Treatment
2.3. Microbiological Analysis
2.4. pH Analysis
2.5. Color Analysis
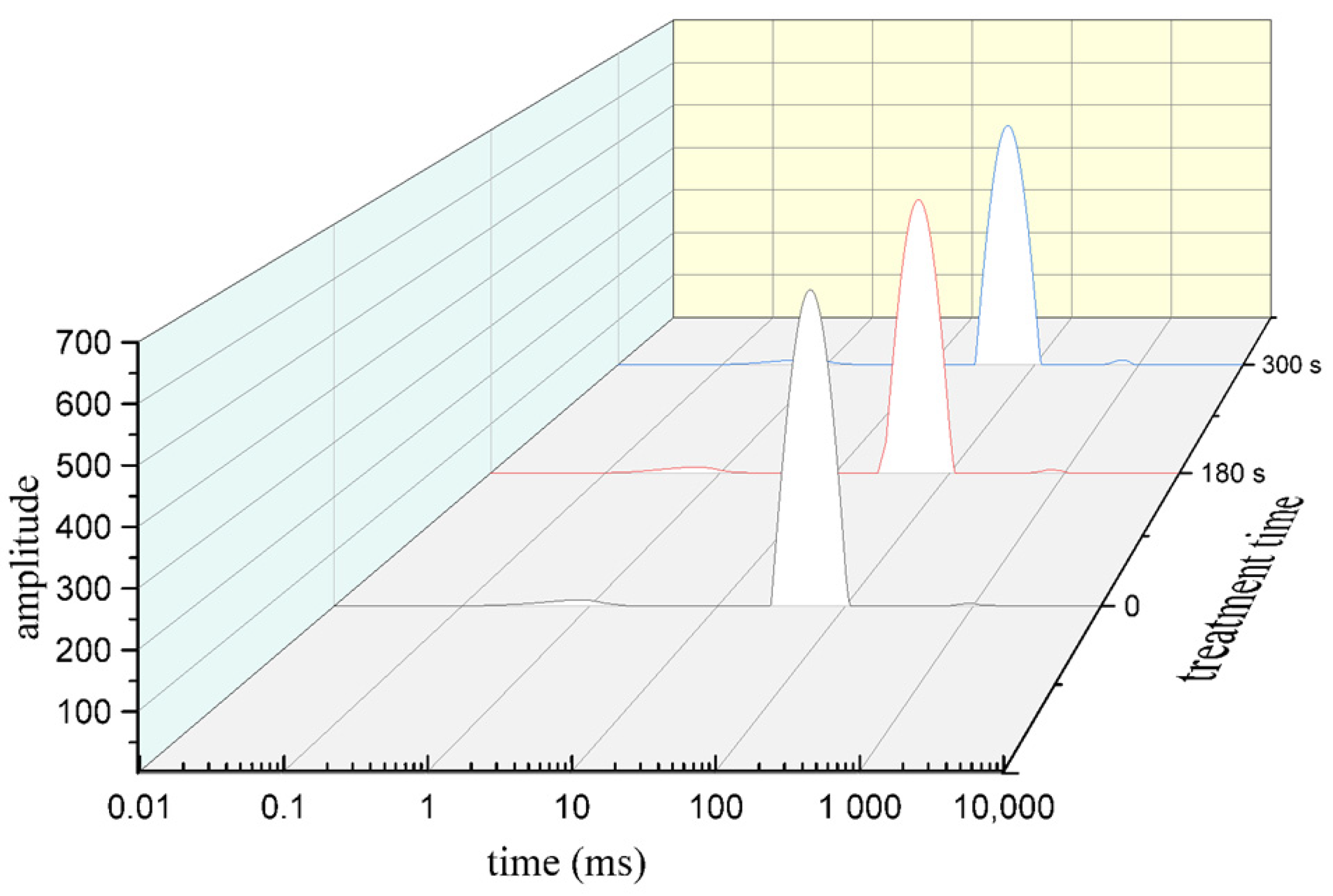
2.6. Low Field NMR (Nuclear Magnetic Resonance) Analysis
2.7. Texture Profile Analysis (TPA)
2.8. Thiobarbituric Acid Reactive Substances (TBARS) Analysis
2.9. Protein Carbonyls and Sulfhydryls Analysis
2.10. Total Volatile Basic Nitrogen (TVB-N) Analysis
2.11. Statistical Analysis
3. Results and Discussion
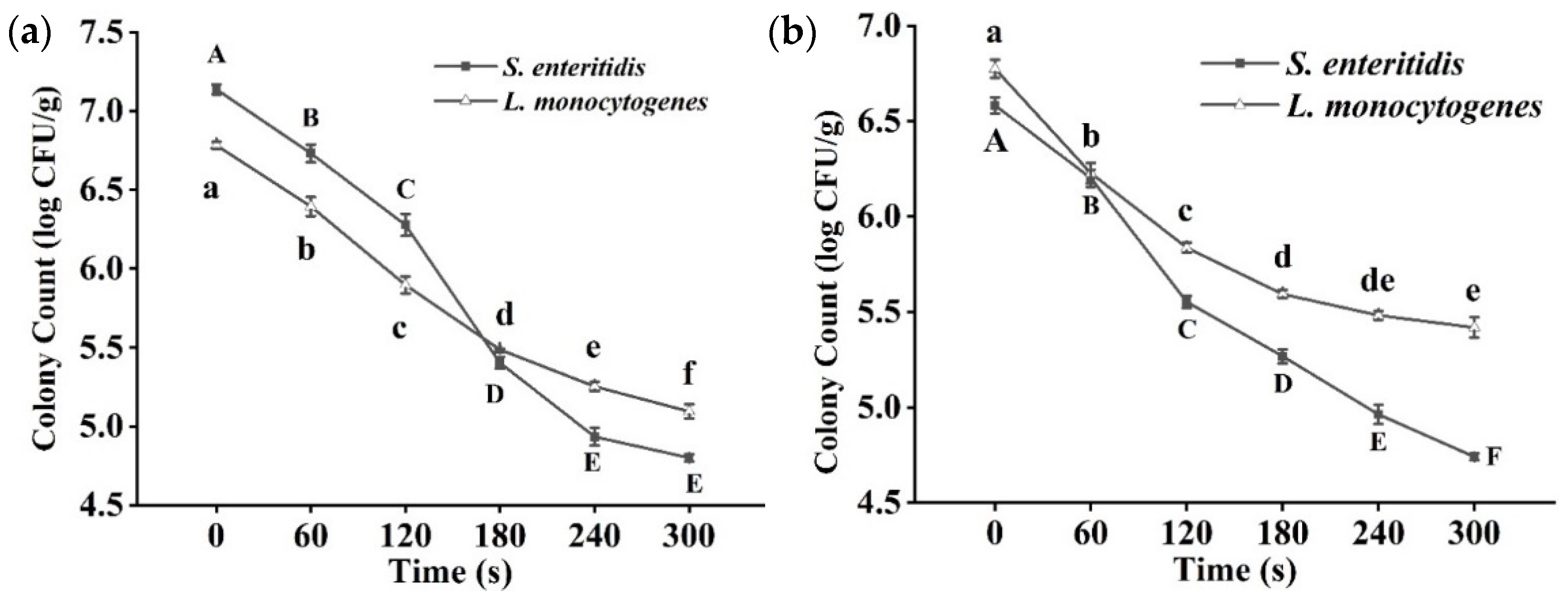
3.1. Bacterial Analysis
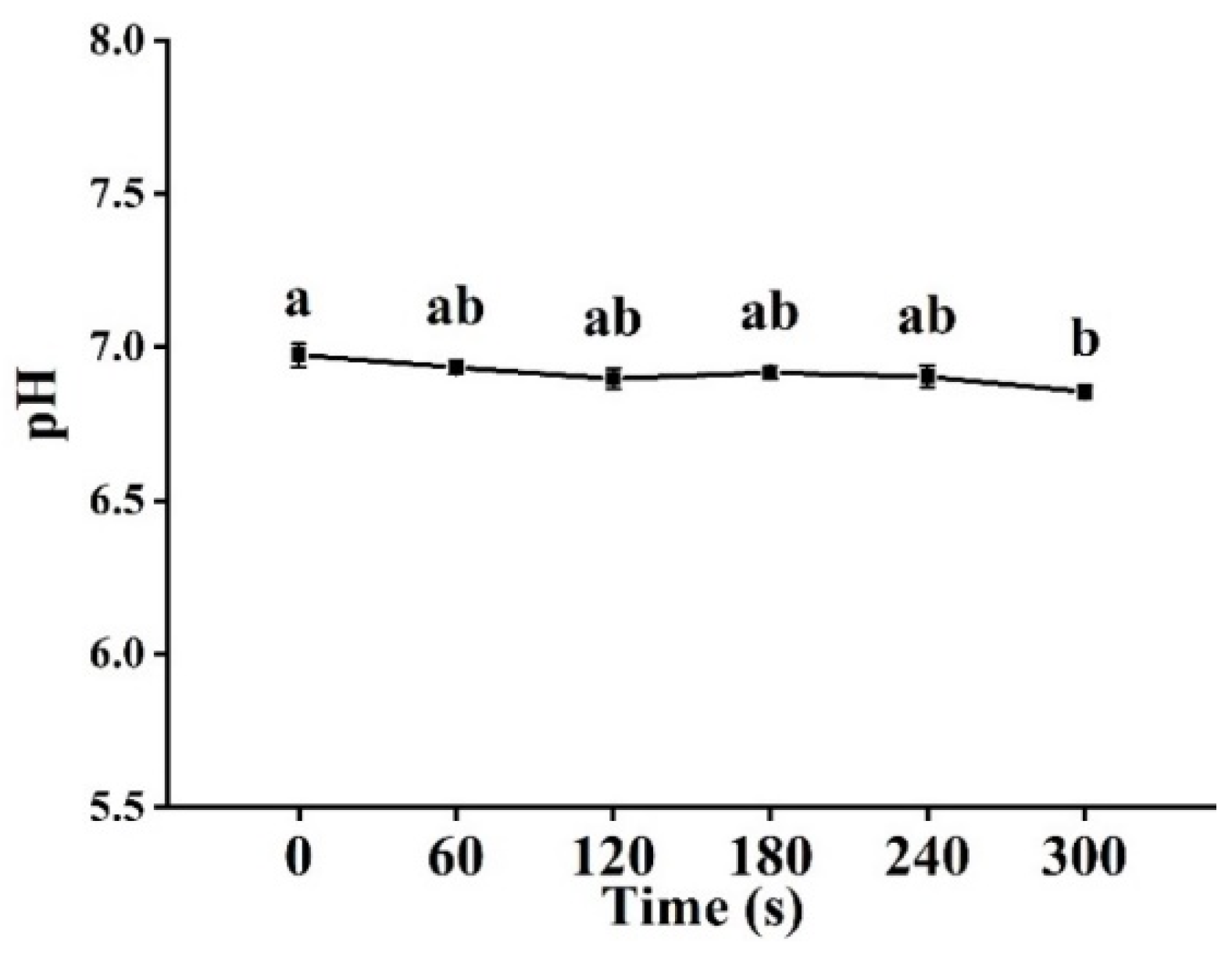
3.2. pH Analysis
3.3. Color Analysis
3.4. Low Field NMR Analysis
3.5. Texture Analysis
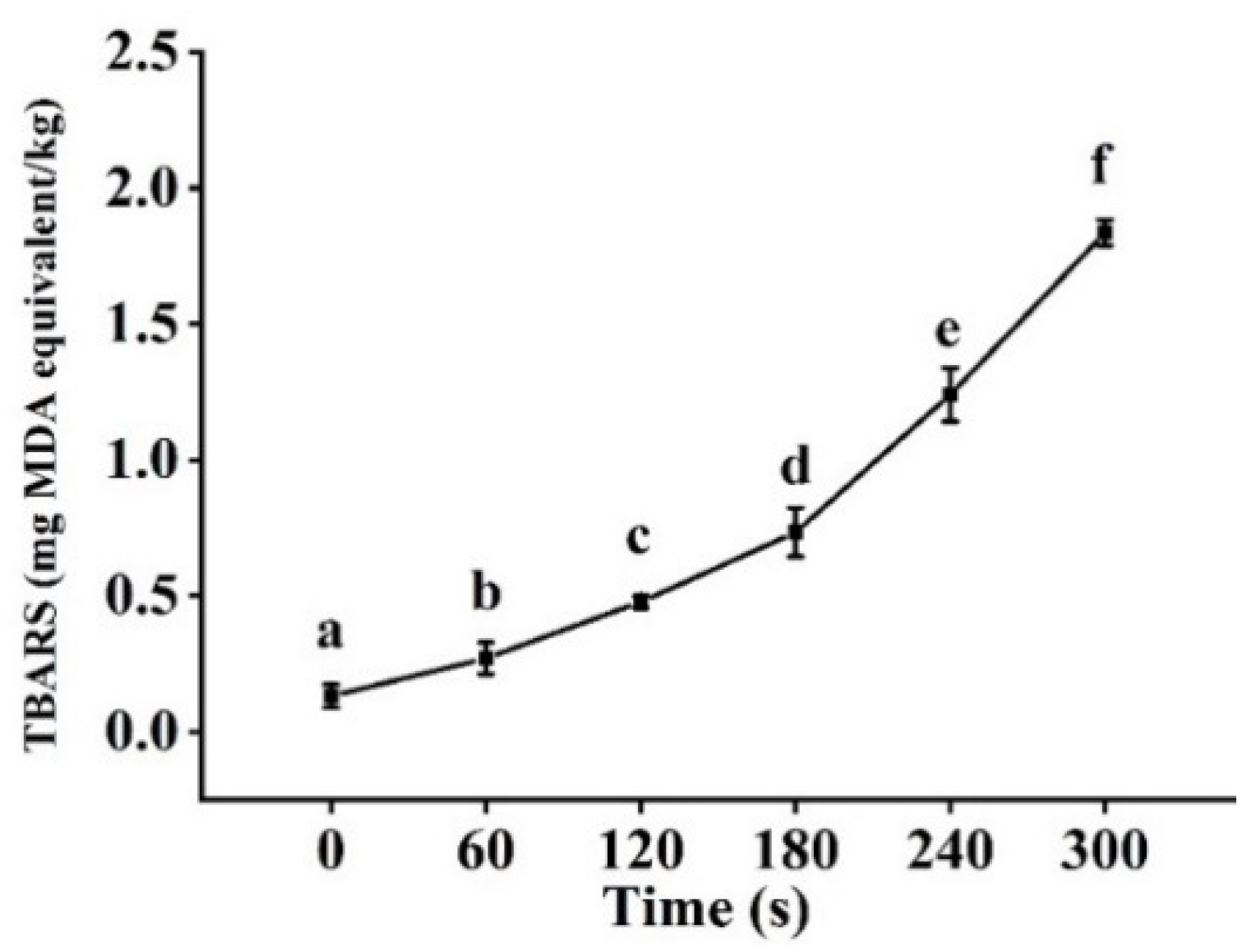
3.6. TBARS Analysis
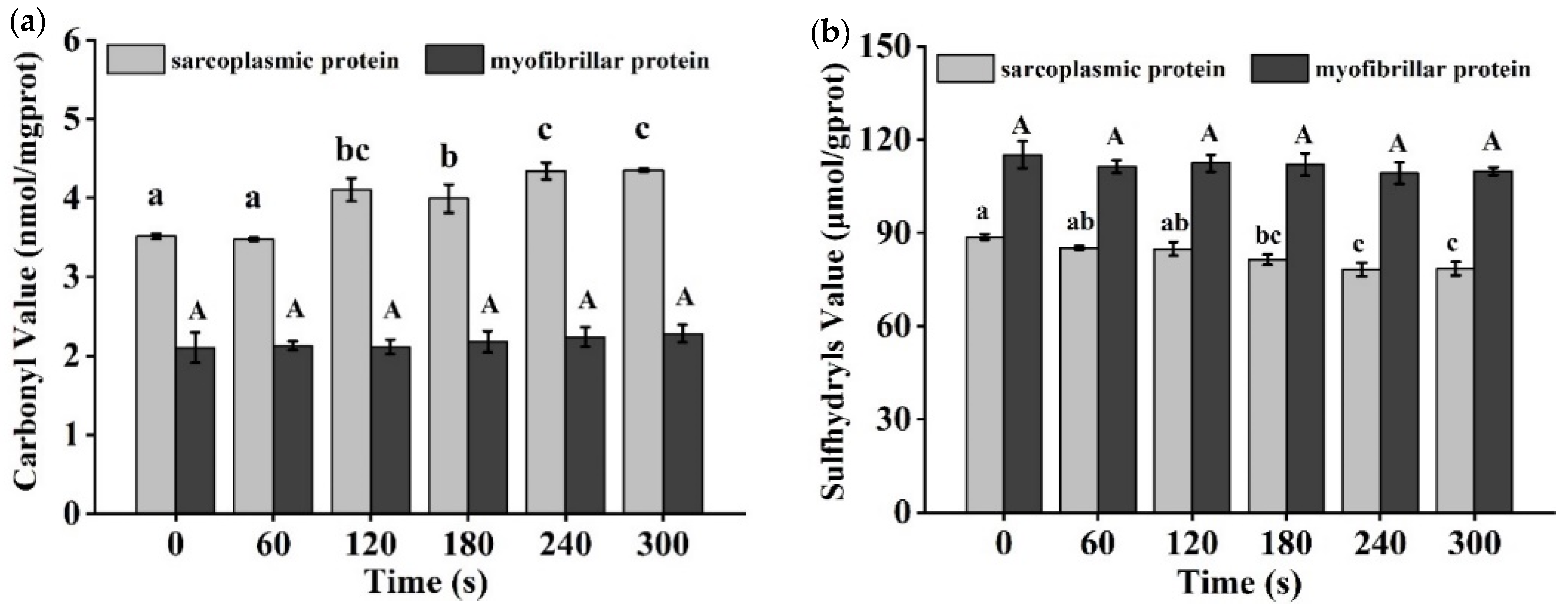
3.7. Carbonyl and Sulfhydryl Level Analysis
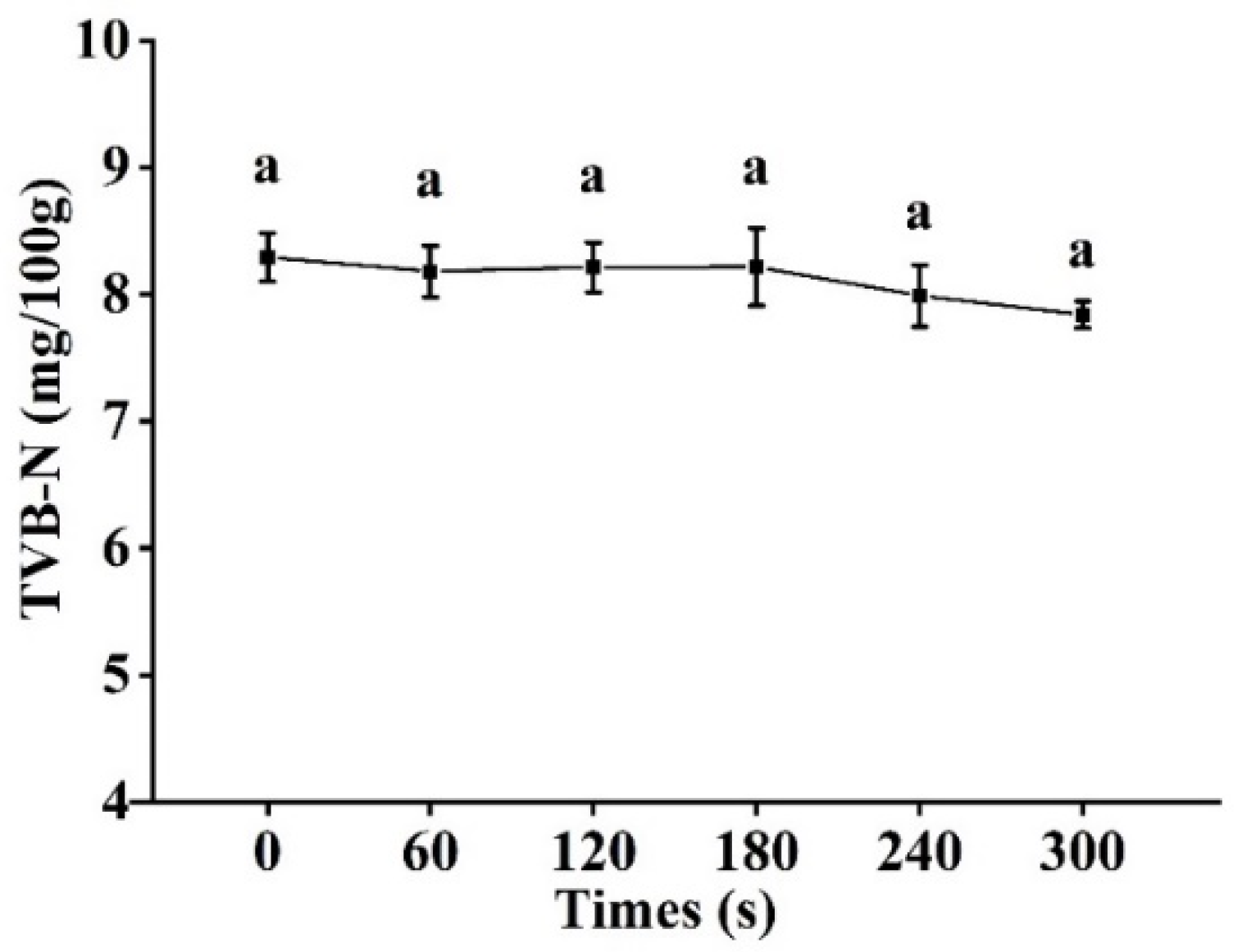
3.8. TVB-N Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samples, S. The effects of storage and preservation technologies on the quality of fish products: A review. J. Food Process. Preserv. 2015, 39, 1206–1215. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery prod-ucts—Significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Vázquez, M.; Torres, J.A.; Gallardo, J.M.; Saraiva, J.; Aubourg, S.P. Lipid hydrolysis and oxidation development in frozen mackerel (Scomber scombrus): Effect of a high hydrostatic pressure pre-treatment. Innov. Food Sci. Emerg. Technol. 2013, 18, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lo-renzo, J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Wang, W.; Qi, W.; Yue, L.; Ye, Q. Effect of 10 MeV E-beam irradiation combined with vacuum-packaging on the shelf life of Atlantic salmon fillets during storage at 4 °C. Food Chem. 2014, 145, 535–541. [Google Scholar] [CrossRef]
- Li, X.; Farid, M. A review on recent development in non-conventional food sterilization technologies. J. Food Eng. 2016, 182, 33–45. [Google Scholar] [CrossRef]
- Ronit, M.; Anika, S.; Pratap, S.A. Recent developments in cold plasma decontamination technology in the food industry. Trends Food Sci. Technol. 2018, 80, 93–103. [Google Scholar] [CrossRef]
- Gordillo-Vázquez, F.J. Air plasma kinetics under the influence of sprites. J. Phys. D Appl. Phys. 2008, 41, 234016. [Google Scholar] [CrossRef] [Green Version]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmos-pheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Zhuang, H.; Zhao, J.; Wang, J.; Yan, W.; Zhang, J. Differences in cellular damage induced by dielectric barrier discharge plasma between Salmonella Typhimurium and Staphylococcus aureus. Bioelectrochemistry 2020, 132, 107445. [Google Scholar] [CrossRef] [PubMed]
- Albertos, I.; Martin-Diana, A.B.; Cullen, P.J.; Tiwari, B.K.; Ojha, K.S.; Bourke, P.; Rico, D. Shelf-life extension of herring (Clu-pea harengus) using in-package atmospheric plasma technology. Innov. Food Sci. Emerg. Technol. 2019, 53, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Albertos, I.; Martín-Diana, A.; Cullen, P.J.; Tiwari, B.K.; Ojha, S.K.; Bourke, P.; Álvarez, C.; Rico, D. Effects of dielectric barri-er discharge (DBD) generated plasma on microbial reduction and quality parameters of fresh mackerel (Scomber scombrus) fillets. Innov. Food Sci. Emerg. Technol. 2017, 44, 117–122. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Shelf-life of refrigerated Asian sea bass slices treated with cold plasma as affected by gas composition in packaging. Int. J. Food Microbiol. 2020, 324, 108612. [Google Scholar] [CrossRef]
- Puligundla, P.; Choi, S.; Mok, C. Microbial decontamination of Gwamegi (semi-dried pacific saury) using corona discharge plasma jet, including physicochemical and sensory evaluation. J. Aquat. Food Prod. Technol. 2018, 27, 274–283. [Google Scholar] [CrossRef]
- Pérez-Andrés, J.M.; de Alba, M.; Harrison, S.M.; Brunton, N.P.; Cullen, P.J.; Tiwari, B.K. Effects of cold atmospheric plasma on mackerel lipid and protein oxidation during storage. LWT-Food Sci. Technol. 2020, 118, 108697. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.Z.; Chen, J.Y.; Chen, D.Z.; Deng, S.G.; Xu, B. Effect of cold plasma on maintaining the quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. J. Sci. Food Agric. 2019, 99, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Zouelm, F.; Abhari, K.; Hosseini, H.; Khani, M. The effects of cold plasma application on quality and chemical spoilage of Pacific White Shrimp (Litopenaeus vannamei) during refrigerated storage. J. Aquat. Food Prod. Technol. 2019, 28, 624–636. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhuang, H.; Lawrence, K.; Zhang, J.H. Disinfection of chicken fillets in packages with atmospheric cold plasma: Effects of treatment voltage and time. J. Appl. Microbiol. 2018, 124, 1212–1219. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Zhuang, H.; Yan, W.; Zhang, J. Effect of in-package high voltage dielectric barrier discharge on micro-biological, color and oxidation properties of pork in modified atmosphere packaging during storage. Meat Sci. 2019, 149, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F.; Rico, E.; Flores, J. Cathepsin B, D, H and L activities in the processing of dry-cured ham. J. Sci. Food Agric. 1993, 62, 157–161. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spec-trophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Hultin, H.O. Chemical, physical, and functional properties of cod proteins modified by a nonenzymic free-radical-generating system. J. Agric. Food Chem. 1997, 45, 310–320. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Ma, T.; Bao, K.; Yu, X.; Duan, Z.; Shen, X.; Li, C. Effect of EGCG-gelatin biofilm on the quality and micro-bial composition of tilapia fillets during chilled storage. Food Chem. 2020, 305, 125454. [Google Scholar] [CrossRef] [PubMed]
- Gök, V.; Aktop, S.; Özkan, M.; Tomar, O. The effects of atmospheric cold plasma on inactivation of Listeria monocytogenes and Staphylococcus aureus and some quality characteristics of pastırma—A dry-cured beef product. Innov. Food Sci. Emerg. Technol. 2019, 56, 102188. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [Green Version]
- Qian, J.; Ma, L.; Yan, W.; Zhuang, H.; Huang, M.; Zhang, J.; Wang, J. Inactivation kinetics and cell envelope damages of foodborne pathogens Listeria monocytogenes and Salmonella Enteritidis treated with cold plasma. Food Microbiol. 2022, 101, 103891. [Google Scholar] [CrossRef]
- Misra, N.N.; Yepez, X.; Xu, L.; Keener, K. In-package cold plasma technologies. J. Food Eng. 2019, 244, 21–31. [Google Scholar] [CrossRef]
- Kim, H.J.; Yong, H.I.; Park, S.; Choe, W.; Jo, C. Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr. Appl. Phys. 2013, 13, 1420–1425. [Google Scholar] [CrossRef]
- Girard, F.; Badets, V.; Blanc, S.; Gazeli, K.; Marlin, L.; Authier, L.; Svarnas, P.; Sojic, N.; Clement, F.; Arbault, S. Formation of reactive nitrogen species including peroxynitrite in physiological buffer exposed to cold atmospheric plasma. RSC Adv. 2016, 6, 78457–78467. [Google Scholar] [CrossRef]
- Lee, H.; Yong, H.I.; Kim, H.-J.; Choe, W.; Yoo, S.J.; Jang, E.J.; Jo, C. Evaluation of the microbiological safety, quality changes, and genotoxicity of chicken breast treated with flexible thin-layer dielectric barrier discharge plasma. Food Sci. Biotechnol. 2016, 25, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, H.; Hinton, A., Jr.; Zhang, J. Influence of in-package cold plasma treatment on microbiological shelf life and appearance of fresh chicken breast fillets. Food Microbiol. 2016, 60, 142–146. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Brychcy, E.; Jarmoluk, A. Effect of low-pressure cold plasma on surface microflora of meat and quality attributes. J. Food Sci. Technol. 2015, 52, 1228–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plas-ma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Compr. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef]
- Moutiq, R.; Misra, N.N.; Mendonca, A.; Keener, K. In-package decontamination of chicken breast using cold plasma tech-nology: Microbial, quality and storage studies. Meat Sci. 2020, 159, 107942. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. High voltage cold atmospheric plasma: Antibacterial properties and its effect on quality of Asian sea bass slices. Innov. Food Sci. Emerg. Technol. 2019, 52, 305–312. [Google Scholar] [CrossRef]
- Okpala, C.O.R.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of Pacific white shrimp (Litopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Sci. Technol. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Zhao, Y.; Lan, W.; Shen, J.; Xu, Z.; Xie, J. Combining ozone and slurry ice treatment to prolong the shelf-life and quality of large yellow croaker (Pseudosciaena crocea). LWT-Food Sci. Technol. 2022, 154, 112615. [Google Scholar] [CrossRef]
- Bae, S.C.; Park, S.Y.; Choe, W.; Ha, S.D. Inactivation of murine norovirus-1 and hepatitis A virus on fresh meats by atmos-pheric pressure plasma jets. Food Res. Int. 2015, 76, 342–347. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, H.; Zhang, J. Inactivation of spoilage bacteria in package by dielectric barrier discharge atmospheric cold plasma—Treatment time effects. Food Bioprocess Technol. 2016, 9, 1648–1652. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Cold plasma combined with liposomal ethanolic coconut husk extract: A po-tential hurdle technology for shelf-life extension of Asian sea bass slices packaged under modified atmosphere. Innov. Food Sci. Emerg. Technol. 2020, 65, 102448. [Google Scholar] [CrossRef]
- Estevez, M. Protein carbonyls in meat systems: A review. Meat Sci. 2011, 89, 259–279. [Google Scholar] [CrossRef]
- Bahrami, N.; Bayliss, D.; Chope, G.; Penson, S.; Perehinec, T.; Fisk, I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chem. 2016, 202, 247–253. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase. Food Bioprod. Process. 2016, 98, 181–188. [Google Scholar] [CrossRef]
- Koddy, J.K.; Miao, W.; Hatab, S.; Tang, L.; Deng, S. Understanding the role of atmospheric cold plasma (ACP) in maintain-ing the quality of hairtail (Trichiurus Lepturus). Food Chem. 2020, 343, 128418. [Google Scholar] [CrossRef]
- Miao, W.; Nyaisaba, B.M.; Koddy, J.K.; Chen, M.; Hatab, S.; Deng, S. Effect of cold atmospheric plasma on the physicochem-ical and functional properties of myofibrillar protein from Alaska pollock (Theragra chalcogramma). Int. J. Food Sci. Technol. 2020, 55, 517–525. [Google Scholar] [CrossRef]
- Liao, X.; Su, Y.; Liu, D.; Chen, S.; Hu, Y.; Ye, X.; Wang, J.; Ding, T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis). Food Control 2018, 94, 307–314. [Google Scholar] [CrossRef]
- Tolouie, H.; Mohammadifar, M.A.; Ghomi, H.; Hashemi, M. Cold atmospheric plasma manipulation of proteins in food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 2583–2597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Time (s) | ΔL | Δa | Δb | ΔE | ΔC |
|---|---|---|---|---|---|
| 60 | 2.02 ± 0.43 ab | −1.03 ± 0.15 b | 0.07 ± 0.18 ab | 2.30 ± 0.41 a | 1.06 ± 0.16 a |
| 120 | 2.13 ± 0.57 b | −1.93 ± 0.09 bc | 0.90 ± 0.14 ab | 3.08 ± 0.37 a | 2.14 ± 0.14 ab |
| 180 | 3.85 ± 0.09 c | −3.07 ± 0.53 c | 0.88 ± 0.60 ab | 5.08 ± 0.47 b | 3.25 ± 0.67 bc |
| 240 | 5.07 ± 0.20 d | −3.86 ± 0.56 d | 0.34 ± 0.18 b | 6.38 ± 0.17 c | 3.87 ± 0.56 c |
| 300 | 5.37 ± 0.27 d | −4.04 ± 0.32 d | 0.11 ± 0.22 ab | 6.72 ± 0.41 c | 4.04 ± 0.32 c |
| Time (s) | T2b | T21 | T22 |
|---|---|---|---|
| 0 | 62.95 ± 3.42 a | 2002.15 ± 27.43 a | 8.47 ± 1.97 a |
| 180 | 64.55 ± 3.88 a | 1946.55 ± 18.97 ab | 11.72 ± 1.39 a |
| 300 | 64.26 ± 4.28 a | 1925.85 ± 10.41 b | 15.23 ± 1.84 b |
| Time (s) | Hardness (g) | Elasticity (mm) | Viscous (g) | Chewiness (mJ) | Cohesion |
|---|---|---|---|---|---|
| 0 | 594.50 ± 21.50 a | 2.96 ± 0.09 a | 2.14 ± 0.34 a | 6.77 ± 0.41 a | 0.44 ± 0.02 a |
| 60 | 373.80 ± 19.70 ab | 2.89 ± 0.05 a | 2.43 ± 0.37 a | 5.08 ± 0.27 b | 0.47 ± 0.01 ab |
| 120 | 327.80 ± 18.90 b | 2.96 ± 0.05 a | 2.57 ± 0.29 ab | 4.87 ± 0.19 b | 0.49 ± 0.02 ab |
| 180 | 283.70 ± 10.50 b | 2.93 ± 0.02 a | 2.71 ± 0.30 ab | 4.75 ± 0.46 b | 0.49 ± 0.01 ab |
| 240 | 257.20 ± 13.40 c | 2.89 ± 0.05 a | 3.14 ± 0.51 ab | 4.25 ± 0.44 b | 0.51 ± 0.02 b |
| 300 | 224.20 ± 10.20 d | 2.99 ± 0.08 a | 3.86 ± 0.59 b | 4.13 ± 0.31 b | 0.51 ± 0.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Fu, T.; Wang, Y.; Zhang, J. Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets. Foods 2022, 11, 2398. https://doi.org/10.3390/foods11162398
Wang J, Fu T, Wang Y, Zhang J. Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets. Foods. 2022; 11(16):2398. https://doi.org/10.3390/foods11162398
Chicago/Turabian StyleWang, Jiamei, Tengfei Fu, Yuanyuan Wang, and Jianhao Zhang. 2022. "Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets" Foods 11, no. 16: 2398. https://doi.org/10.3390/foods11162398
APA StyleWang, J., Fu, T., Wang, Y., & Zhang, J. (2022). Effects of High-Voltage Atmospheric Cold Plasma Treatment on Microbiological and Quality Characters of Tilapia Fillets. Foods, 11(16), 2398. https://doi.org/10.3390/foods11162398





