Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of SPPHs and Determination of Free Amino Acids
2.2.1. Preparation of SPPHs
2.2.2. Determination of Free Amino Acids
2.3. Cell Culture
2.4. Proliferation Inhibition of MGC-803 Cells
- As: Treated samples groups (culture medium, cells, CCK-8, SPPHs).
- Ac: Blank control groups (culture medium, cells, CCK-8).
- Ab: Background groups (culture medium, CCK-8).
2.5. Morphologic Observation of MGC-803 Cells
2.5.1. General Microscope
2.5.2. Fluorescence Microscope
2.5.3. Laser Scanning Confocal Microscopy (LSCM)
2.6. Apoptosis
2.7. Cell Cycle Assay
2.8. Statistical Analysis
3. Results
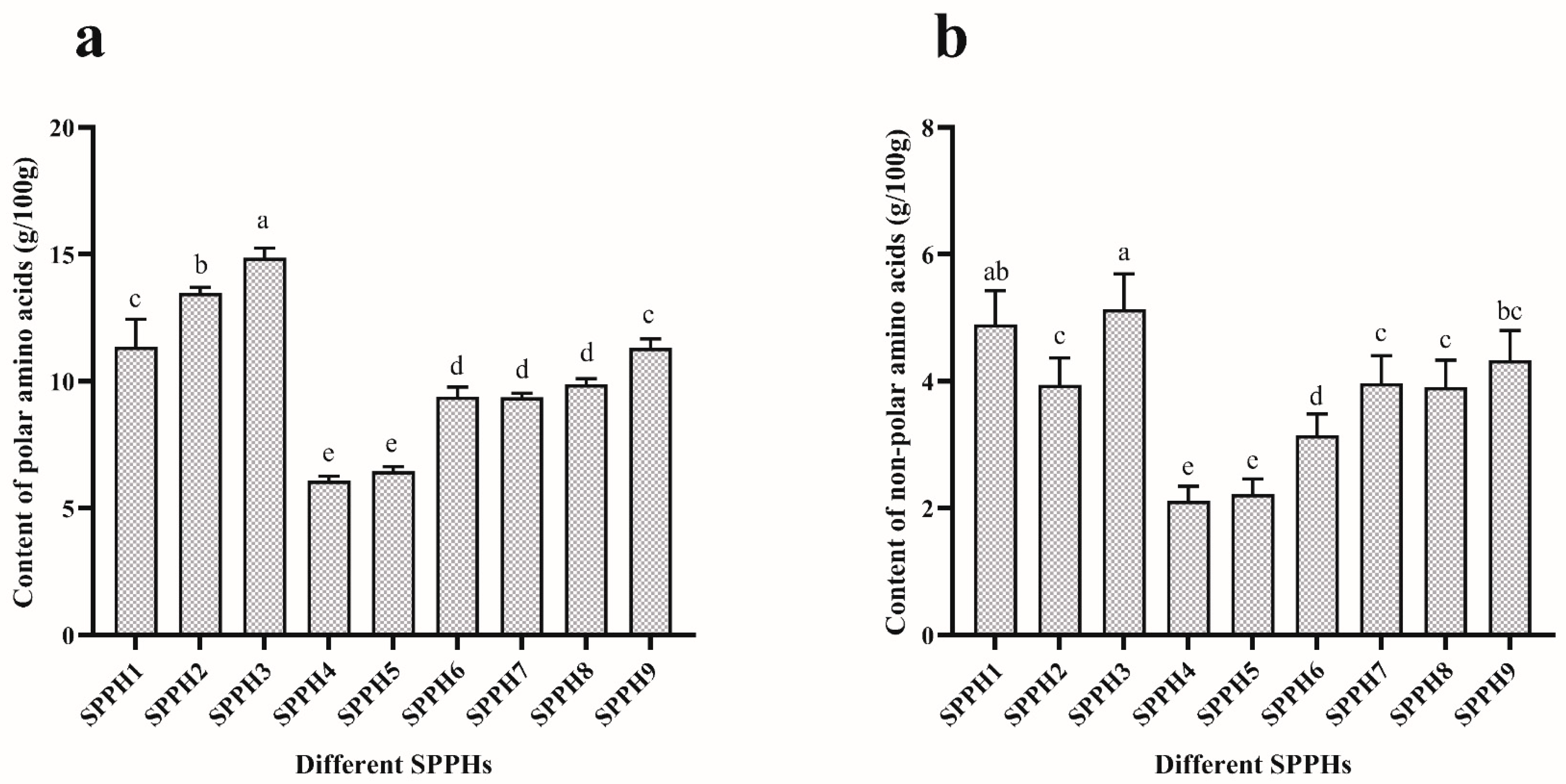
3.1. Content of Free Amino Acids
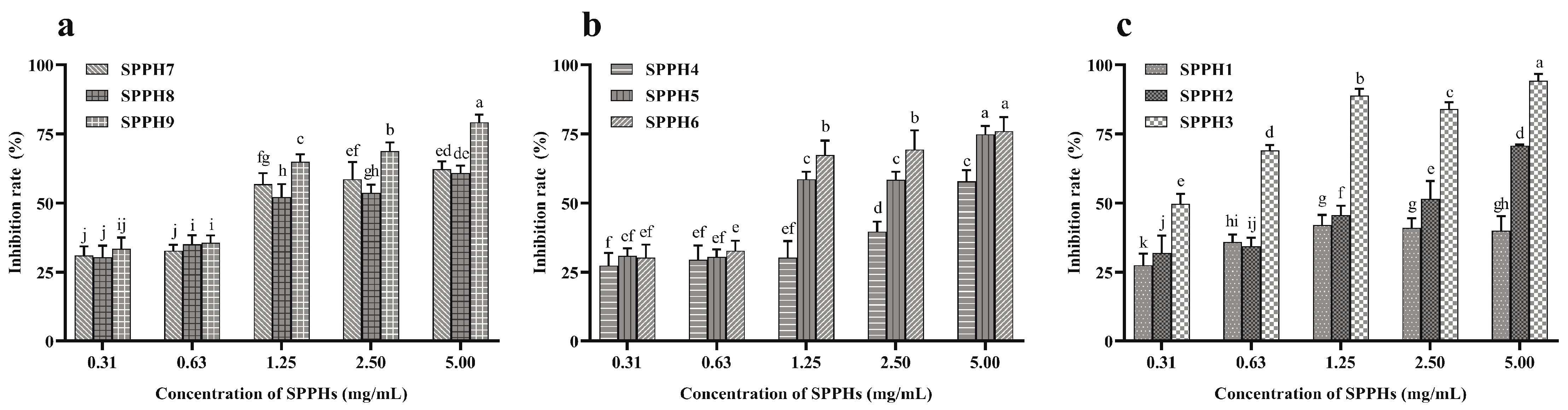
3.2. Proliferation Inhibition Effect of SPPHs on MGC-803 Cells
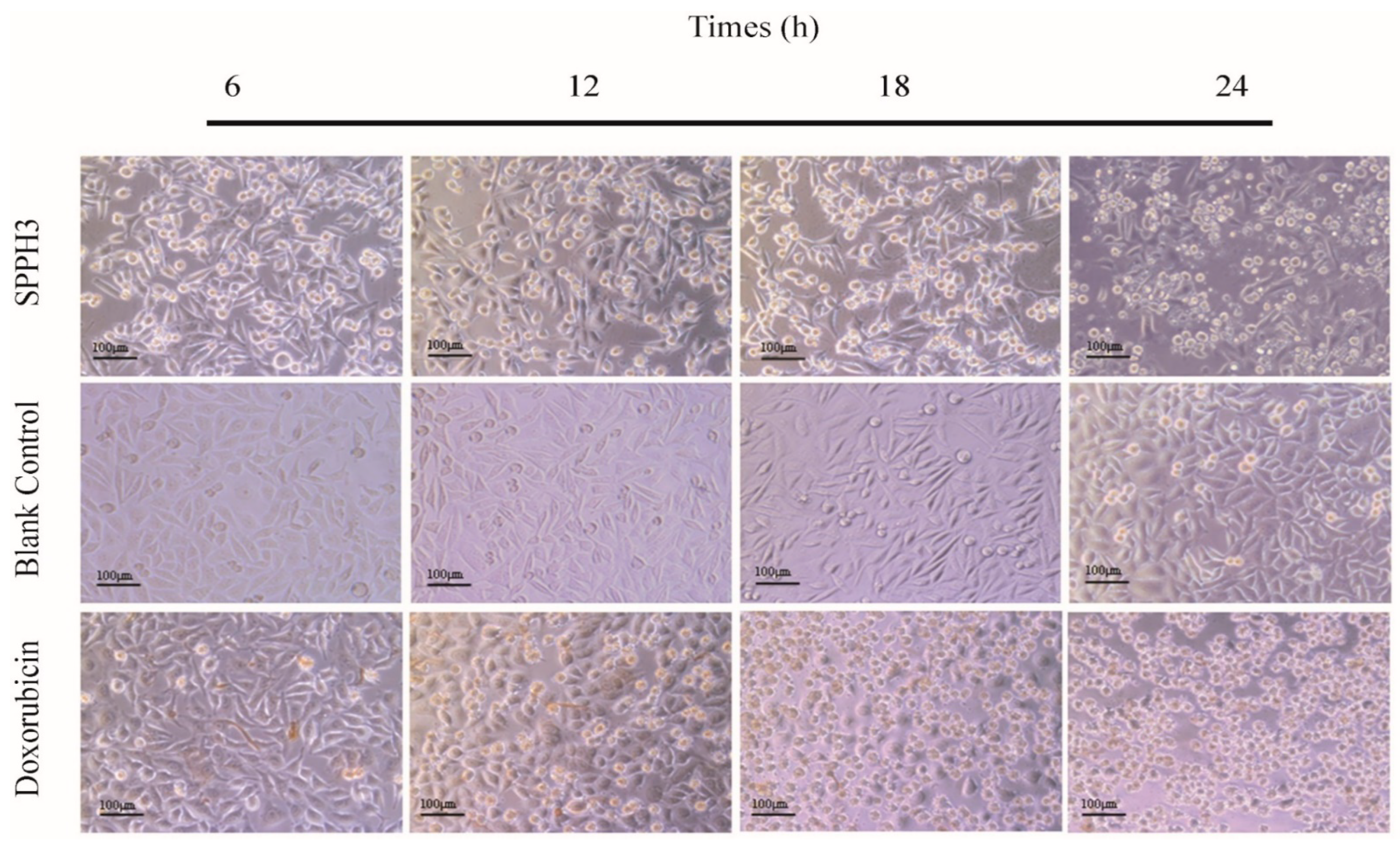
3.3. Effect of SPPHs on the Morphology of MGC-803 Cells
3.3.1. Inverted Microscope Observation Results
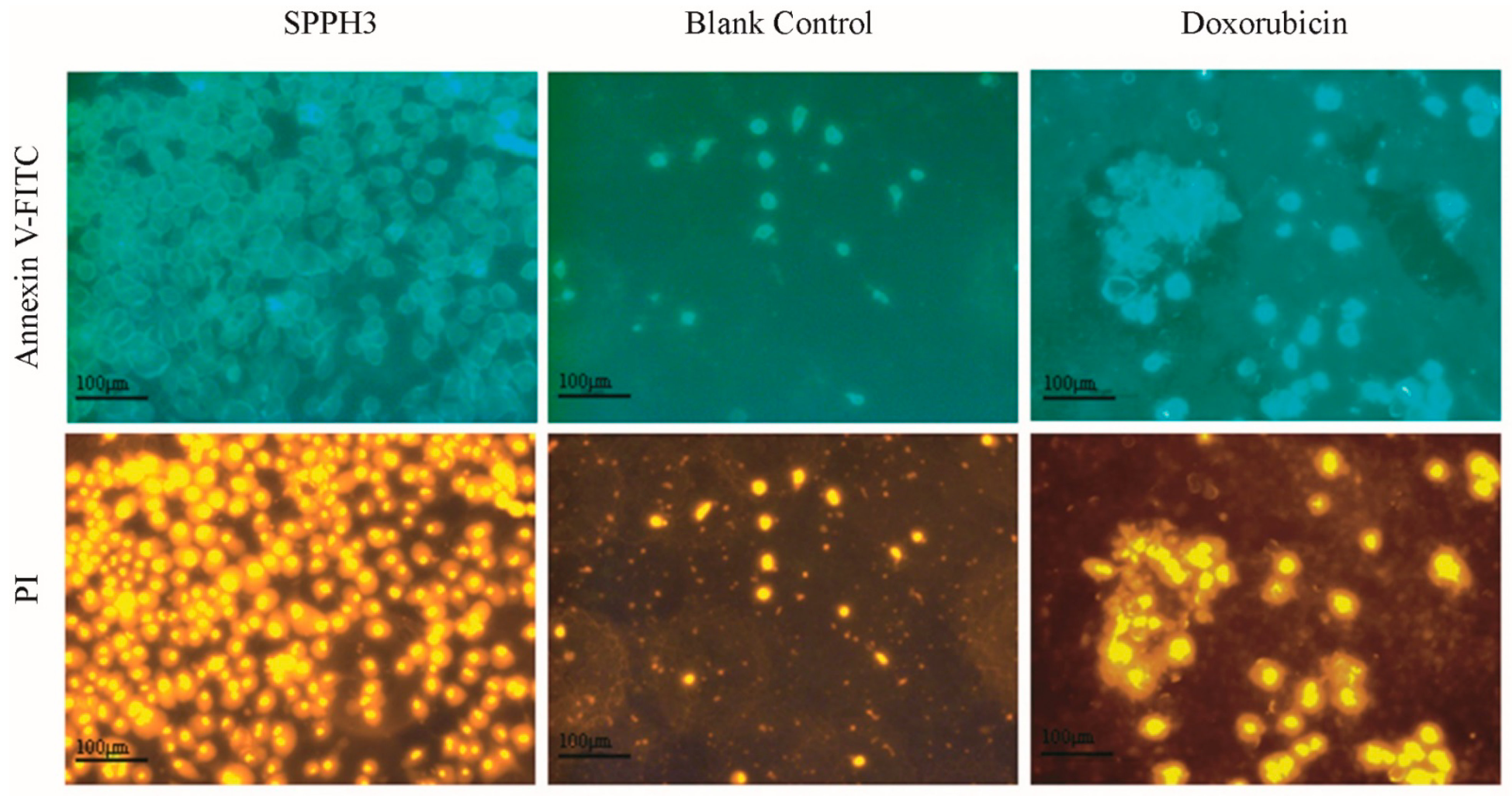
3.3.2. Fluorescence Microscope Observation Results
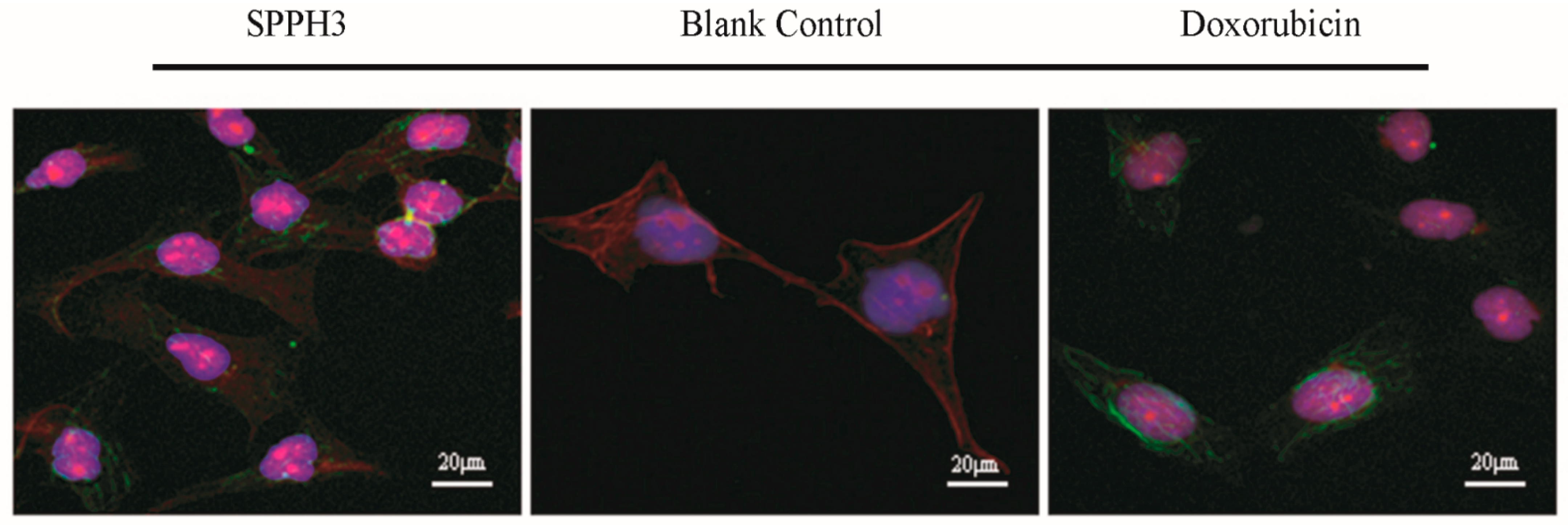
3.3.3. LSCM Observation Results
3.4. Apoptosis and Cycle of MGC-803 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weixin, L.; Lixia, M.; Leiyan, W.; Yuxiao, Z.; Haifeng, Z.; Sentai, L. Effects of silkworm pupa protein hydrolysates on mitochondrial substructure and metabolism in gastric cancer cells. J. Asia-Pac. Entomol. 2019, 22, 387–392. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Ma, A.; Feng, D.; He, Y.; Yan, W. Effects of silkworm pupa protein on apoptosis and energy metabolism in human colon cancer DLD-1 cells. Food Sci. Hum. Wellness 2022, 11, 1171–1176. [Google Scholar] [CrossRef]
- Sadat, A.; Biswas, T.; Cardoso, M.H.; Mondal, R.; Ghosh, A.; Dam, P.; Nesa, J.; Chakraborty, J.; Bhattacharjya, D.; Franco, O.L.; et al. Silkworm pupae as a future food with nutritional and medicinal benefits. Curr. Opin. Food Sci. 2022, 44, 100818. [Google Scholar] [CrossRef]
- Deori, M.; Boruah, D.C.; Devi, D.; Devi, R. Antioxidant and antigenotoxic effects of pupae of the muga silkworm Antheraea assamensis. Food Biosci. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhao, J.-G.; Wei, Z.-G.; Zhang, Y.-Q. The renal protection of flavonoid-rich ethanolic extract from silkworm green cocoon involves in inhibiting TNF-α-p38 MAP kinase signalling pathway in type 2 diabetic mice. Biomed. Pharmacother. 2019, 118, 109379. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Masuda, K.; Ida, S.; Tada, H.; Bando, M.; Abe, K.; Tatematsu, K.-I.; Sezutsu, H.; Oyama, T.; Chikamatsu, K.; et al. In vitro assessment of antitumor immune responses using tumor antigen proteins produced by transgenic silkworms. J. Mater. Sci. Mater. Med. 2021, 32, 58. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Siriwong, S.; Thumanu, K. Pupae protein extracts exert anticancer effects by downregulating the expression of IL-6, IL-1β and TNF-α through biomolecular changes in human breast cancer cells. Biomed. Pharmacother. 2020, 128, 110278. [Google Scholar] [CrossRef]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Li, X.T.; Xie, H.Q.; Chen, Y.J.; Lang, M.Z.; Chen, Y.Y.; Shi, L.G. Silkworm Pupa Protein Hydrolysate Induces Mitochondria-Dependent Apoptosis and S Phase Cell Cycle Arrest in Human Gastric Cancer SGC-7901 Cells. Int. J. Mol. Sci. 2018, 19, 1013. [Google Scholar] [CrossRef]
- Xie, S. Antioxidant and Tumor Growth Inhibition Activity of Peptides Prepared from Silkworm Pupae Protein. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2015. [Google Scholar]
- Li, W. Effect of active peptide components of silkworm pupa on mitochondrial apoptosis pathway in MGC-803 cells. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2018. [Google Scholar]
- Pfeuti, G.; Brown, L.S.; Longstaffe, J.G.; Peyronel, F.; Bureau, D.P.; Kiarie, E.G. Predicting the standardized ileal digestibility of crude protein in feather meal fed to broiler chickens using a pH-stat and a FT-Raman method. Anim. Feed Sci. Technol. 2020, 261, 114340. [Google Scholar] [CrossRef]
- Ozawa, S.; Miyano, H.; Ito, M. Advances in amino acid analysis and Amino Acid Analyzer L-8900. Hitachi Sci. Instrum. News 2015, 6, 33–43. [Google Scholar]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.E.; Samarasekera, C.; Swedlow, J.R. Chapter 14—Quantitative Analysis of Digital Microscope Images. In Methods in Cell Biology; Sluder, G., Wolf, D.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 114, pp. 337–367. [Google Scholar]
- Augustine, D.; Rao, R.S.; Anbu, J.; Chidambara Murthy, K.N. In vitro cytotoxic and apoptotic induction effect of earthworm coelomic fluid of Eudrilus eugeniae, Eisenia foetida, and Perionyx excavatus on human oral squamous cell carcinoma-9 cell line. Toxicol. Rep. 2019, 6, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xin, Z.; Zhu, Y.; Wang, J. Mesoporous carbon nanospheres featured multifunctional fluorescent nanoprobe: Simultaneous activation and tracing of caspase-3 involved cell apoptosis. Sens. Actuators B Chem. 2022, 358, 131485. [Google Scholar] [CrossRef]
- Van Acker, T.; Buckle, T.; Van Malderen, S.J.M.; van Willigen, D.M.; van Unen, V.; van Leeuwen, F.W.B.; Vanhaecke, F. High-resolution imaging and single-cell analysis via laser ablation-inductively coupled plasma-mass spectrometry for the determination of membranous receptor expression levels in breast cancer cell lines using receptor-specific hybrid tracers. Anal. Chim. Acta 2019, 1074, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, I.; Keeney, F.; Altieri, D.C. Protocol for assessing real-time changes in mitochondrial morphology, fission and fusion events in live cells using confocal microscopy. STAR Protoc. 2021, 2, 100767. [Google Scholar] [CrossRef] [PubMed]
- Fakai, M.I.; Abd Malek, S.N.; Karsani, S.A. Induction of apoptosis by chalepin through phosphatidylserine externalisations and DNA fragmentation in breast cancer cells (MCF7). Life Sci. 2019, 220, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, S.; Schmidt, J.H.; Lavrik, I.N. Quantification of apoptosis and necroptosis at the single cell level by a combination of Imaging Flow Cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods 2015, 423, 99–103. [Google Scholar] [CrossRef]
- Yang, K.; Luo, M.; Li, H.; Abdulrehman, G.; Kang, L. Effects of jasplakinolide on cytotoxicity, cytoskeleton and apoptosis in two different colon cancer cell lines treated with m-THPC-PDT. Photodiagnosis Photodyn. Ther. 2021, 35, 102425. [Google Scholar] [CrossRef]
- Wu, W.; Löbmann, K.; Schnitzkewitz, J.; Knuhtsen, A.; Pedersen, D.S.; Rades, T.; Grohganz, H. Dipeptides as co-formers in co-amorphous systems. Eur. J. Pharm. Biopharm. 2019, 134, 68–76. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Khammuang, S.; Sarnthima, R.; Sanachai, K. Purification and identification of novel antioxidant peptides from silkworm pupae (Bombyx mori) protein hydrolysate and molecular docking study. Biocatal. Agric. Biotechnol. 2022, 42, 102367. [Google Scholar] [CrossRef]
- Xia, L.; Wu, Y.; Kang, S.; Ma, J.; Yang, J.; Zhang, F. CecropinXJ, a silkworm antimicrobial peptide, induces cytoskeleton disruption in esophageal carcinoma cells. Acta Biochim. Biophys. Sin. 2014, 46, 867–876. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, F.; Liu, Z.; Ma, J.; Yang, J. Expression and characterization of cecropinXJ, a bioactive antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in Escherichia coli. Exp. Ther. Med. 2013, 5, 1745–1751. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, H.; Admassu, H.; Sulieman, A.A.; Wei, F.A. Health benefits of bioactive peptides produced from muscle proteins: Antioxidant, anti-cancer, and anti-diabetic activities. Process Biochem. 2022, 116, 116–125. [Google Scholar] [CrossRef]
- Velliquette, R.A.; Fast, D.J.; Maly, E.R.; Alashi, A.M.; Aluko, R.E. Enzymatically derived sunflower protein hydrolysate and peptides inhibit NFκB and promote monocyte differentiation to a dendritic cell phenotype. Food Chem. 2020, 319, 126563. [Google Scholar] [CrossRef]
- Sun, C.; Tang, X.; Shao, X.; Han, D.; Zhang, H.; Shan, Y.; Gooneratne, R.; Shi, L.; Wu, X.; Hosseininezhad, M. Mulberry (Morus atropurpurea Roxb.) leaf protein hydrolysates ameliorate dextran sodium sulfate-induced colitis via integrated modulation of gut microbiota and immunity. J. Funct. Foods 2021, 84, 104575. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, X.; Luo, X.; Ding, J.; Fan, F.; Li, P.; Shen, X.; Fang, Y. Encapsulation of selenium-containing peptides in xanthan gum-lysozyme nanoparticles as a powerful gastrointestinal delivery system. Food Res. Int. 2022, 156, 111351. [Google Scholar] [CrossRef] [PubMed]
- Askari Rizvi, S.F.; Zhang, H. Emerging trends of receptor-mediated tumor targeting peptides: A review with perspective from molecular imaging modalities. Eur. J. Med. Chem. 2021, 221, 113538. [Google Scholar] [CrossRef]
- Vale, N.; Duarte, D.; Silva, S.; Correia, A.S.; Costa, B.; Gouveia, M.J.; Ferreira, A. Cell-penetrating peptides in oncologic pharmacotherapy: A review. Pharmacol. Res. 2020, 162, 105231. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Xia, L.-J.; Li, J.-Y.; Zhang, F.-C. CecropinXJ inhibits the proliferation of human gastric cancer BGC823 cells and induces cell death in vitro and in vivo. Int. J. Oncol. 2015, 46, 2181–2193. [Google Scholar] [CrossRef][Green Version]
- Ragozzino, E.; Brancaccio, M.; Di Costanzo, A.; Scalabrì, F.; Andolfi, G.; Wanderlingh, L.G.; Patriarca, E.J.; Minchiotti, G.; Altamura, S.; Summa, V.; et al. 6-Bromoindirubin-3′-oxime intercepts GSK3 signaling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice. Sci. Rep. 2019, 9, 18091. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves de Oliveira-Júnior, R.; Marcoult-Fréville, N.; Prunier, G.; Beaugeard, L.; Beserra de Alencar Filho, E.; Simões Mourão, E.D.; Michel, S.; Quintans-Júnior, L.J.; Guedes da Silva Almeida, J.R.; Grougnet, R.; et al. Polymethoxyflavones from Gardenia oudiepe (Rubiaceae) induce cytoskeleton disruption-mediated apoptosis and sensitize BRAF-mutated melanoma cells to chemotherapy. Chem. Biol. Interact. 2020, 325, 109109. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.-S.; Zhou, X.-H.; Miao, Y.-H.; Wang, P.; Zhang, Q.-Q.; Huang, Q. In situ observation of mitochondrial biogenesis as the early event of apoptosis. iScience 2021, 24, 103038. [Google Scholar] [CrossRef]
- Chapter 11—Regulated Cell Death. In Goodman’s Medical Cell Biology, 4th ed.; Goodman, S.R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 315–336. [Google Scholar]
- Wang, Q.; Xie, L.; Wang, Y.; Jin, B.; Ren, J.; Dong, Z.; Chen, G.; Liu, D. Djhsp70s, especially Djhsp70c, play a key role in planarian regeneration and tissue homeostasis by regulating cell proliferation and apoptosis. Gene 2022, 820, 146215. [Google Scholar] [CrossRef]
- Abroudi, A.; Samarasinghe, S.; Kulasiri, D. A comprehensive complex systems approach to the study and analysis of mammalian cell cycle control system in the presence of DNA damage stress. J. Theor. Biol. 2017, 429, 204–228. [Google Scholar] [CrossRef]
- Su, L.; Xu, G.; Shen, J.; Tuo, Y.; Zhang, X.; Jia, S.; Chen, Z.; Su, X. Anticancer bioactive peptide suppresses human gastric cancer growth through modulation of apoptosis and the cell cycle. Oncol. Rep. 2010, 23, 3–9. [Google Scholar] [CrossRef]
- Gupta, N.; Bhagyawant, S.S. Bioactive peptide of Cicer arietinum L. induces apoptosis in human endometrial cancer via DNA fragmentation and cell cycle arrest. 3 Biotech 2021, 11, 63. [Google Scholar] [CrossRef]
- Barras, D.; Widmann, C. Promises of apoptosis-inducing peptides in cancer therapeutics. Curr. Pharm. Biotechnol. 2011, 12, 1153–1165. [Google Scholar] [CrossRef]
| Group | Concentration (mg/mL) | Cells were Treated with SPPHs for 24 h | |||
|---|---|---|---|---|---|
| G0/G1 | S | G2/M | Apoptosis Rate | ||
| Blank | - | 34.41 ± 0.74 d | 31.51 ± 1.46 b | 33.52 ± 1.16 a | 2.95 ± 0.65 d |
| Doxorubicin | 0.001 | 38.82 ± 2.05 c | 36.79 ± 1.73 a | 24.76 ± 3.71 b | ND |
| SPPH1 | 0.31 | 41.26 ± 2.24 bc | 23.83 ± 1.54 c | 34.74 ± 3.28 a | 42.23 ± 2.61 b |
| SPPH2 | 0.31 | 40.24 ± 1.36 c | 24.41 ± 9.42 c | 33.51 ± 0.11 a | 30.07 ± 4.74 c |
| SPPH3 | 0.02 | 38.73 ± 0.72 c | 24.35 ± 1.49 c | 36.97 ± 1.92 a | 32.80 ± 2.21 c |
| SPPH3 | 0.04 | 42.25 ± 1.73 ab | 21.46 ± 4.09 c | 36.77 ± 3.10 a | 35.13 ± 5.50 c |
| SPPH3 | 0.08 | 42.54 ± 0.88 ab | 25.80 ± 3.38 c | 32.23 ± 3.36 a | 36.70 ± 7.54 bc |
| SPPH3 | 0.16 | 43.06 ± 0.80 ab | 25.55 ± 1.26 c | 32.28 ± 1.15 a | 59.20 ± 0.53 a |
| SPPH3 | 0.31 | 45.17 ± 2.20 a | 21.25 ± 4.24 c | 33.77 ± 2.44 a | 61.63 ± 0.68 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Mu, L.; Zou, Y.; Wang, W.; Zhao, H.; Wu, X.; Liao, S. Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In Vitro. Foods 2022, 11, 2367. https://doi.org/10.3390/foods11152367
Li W, Mu L, Zou Y, Wang W, Zhao H, Wu X, Liao S. Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In Vitro. Foods. 2022; 11(15):2367. https://doi.org/10.3390/foods11152367
Chicago/Turabian StyleLi, Weixin, Lixia Mu, Yuxiao Zou, Weifei Wang, Haifeng Zhao, Xuli Wu, and Sentai Liao. 2022. "Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In Vitro" Foods 11, no. 15: 2367. https://doi.org/10.3390/foods11152367
APA StyleLi, W., Mu, L., Zou, Y., Wang, W., Zhao, H., Wu, X., & Liao, S. (2022). Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In Vitro. Foods, 11(15), 2367. https://doi.org/10.3390/foods11152367







