Evaluation of the Brewing Characteristics, Digestion Profiles, and Neuroprotective Effects of Two Typical Se-Enriched Green Teas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterizations
2.2. Preparation of Tea Infusions
2.3. Determination of the Content of Tea Polyphenols, Caffeine, Free Amino Acids, Soluble Sugar, and Water Extracts
2.4. Determination of Total Se
2.5. Dynamic In Vitro Digestion
2.5.1. Preparation of Simulated Digestion Solutions
2.5.2. In Vitro Digestion Using the DHSI-IV Model
2.6. Determination of Free Radical Scavenging Activity
2.7. Se-GTE Preparation
2.8. Cell Assay
2.8.1. Preparation of Aβ1–42 Oligomer
2.8.2. Cell Culture and Treatment
2.8.3. Cell Viability via CCK-8 Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Properties of Se-GT
3.2. Effect of Brewing Conditions on the Phytochemical Compositions of Se-GT
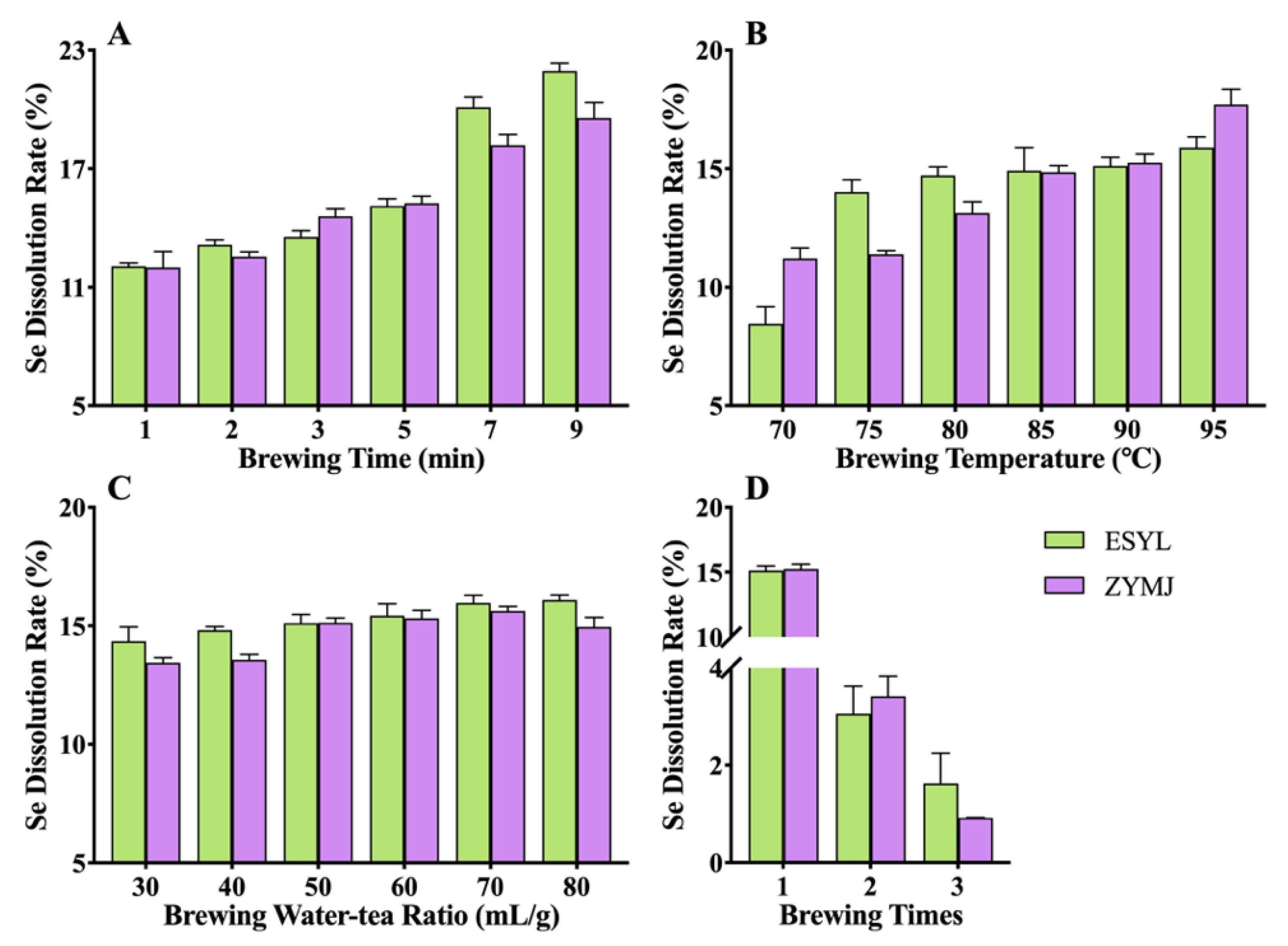
3.3. Effect of Brewing Conditions on the Se Leaching of Tea Infusions
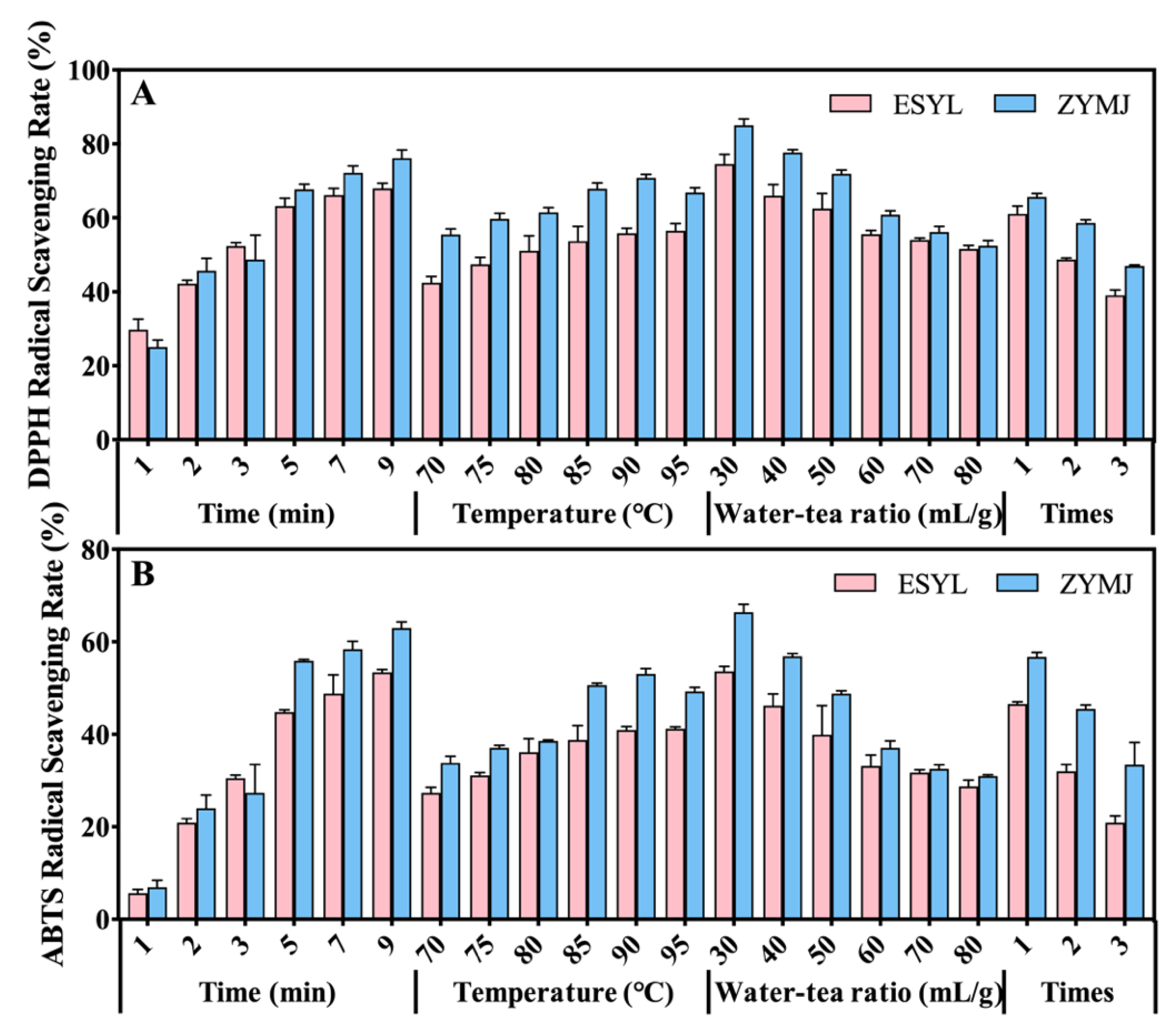
3.4. Effect of Brewing Conditions on the Antioxidant Activity of Tea Infusions
3.5. Correlation Analysis
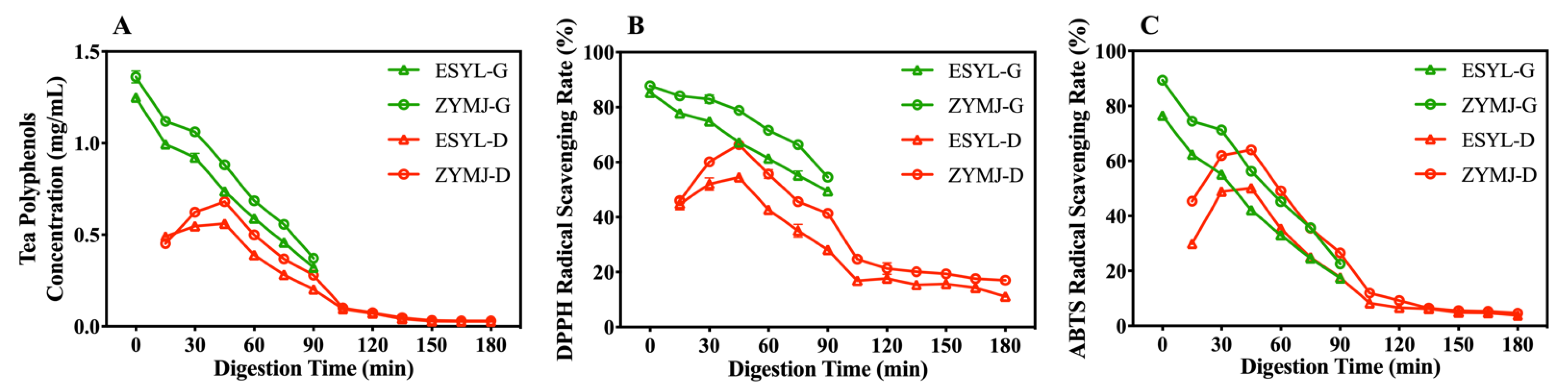
3.6. Polyphenol Release and Antioxidant Activity during In Vitro Gastroduodenal Digestion
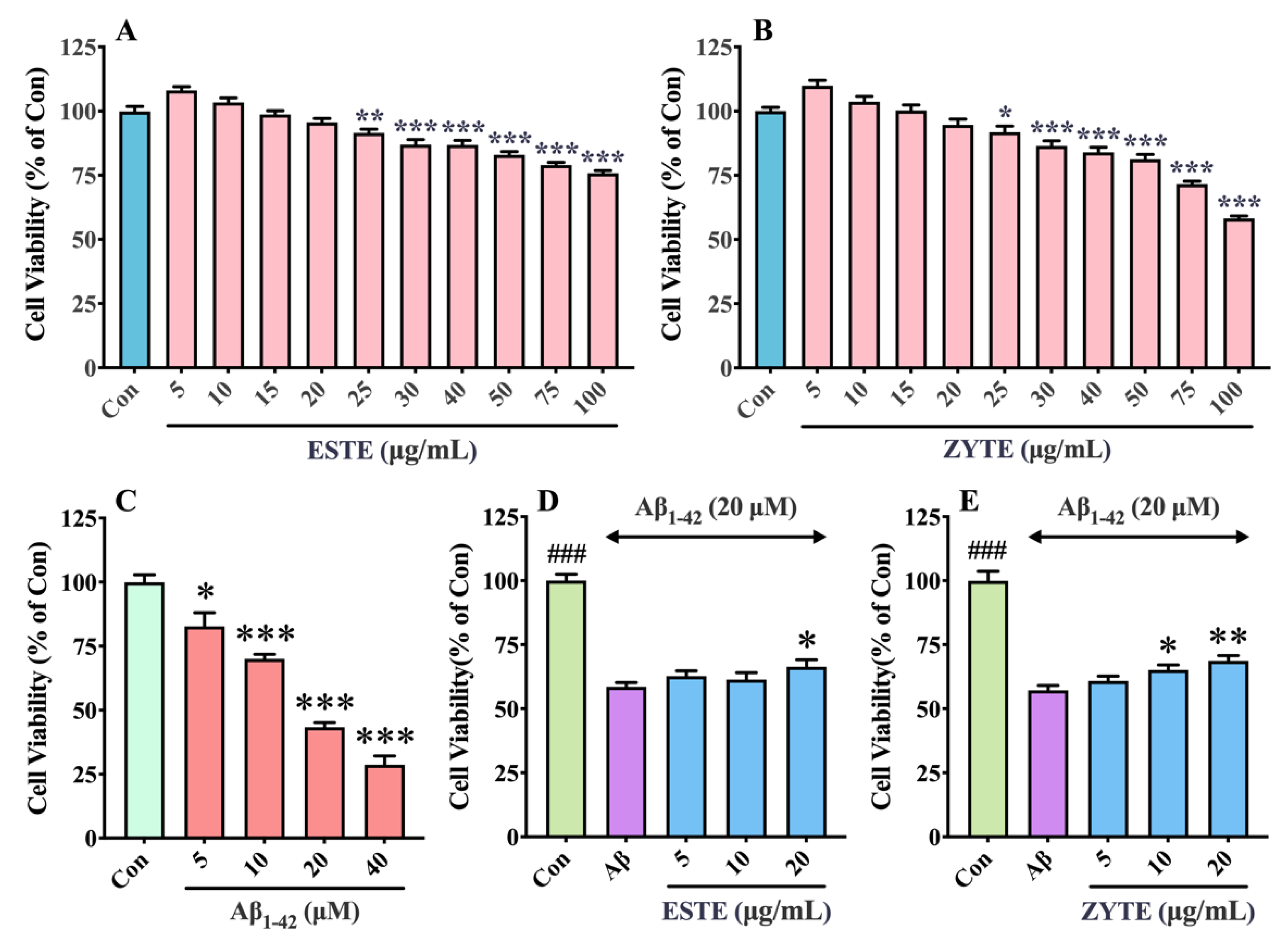
3.7. Effect of Se-GTEs on Viability of HT22 Cells against Aβ1–42 Induced Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zou, C.; Gao, Y.; Chen, J.X.; Wang, F.; Chen, G.S.; Yin, J.F. Effect of the type of brewing water on the chemical composition, sensory quality and antioxidant capacity of Chinese teas. Food Chem. 2017, 236, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health functions and related molecular mechanisms of tea components: An update review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory effects of green tea polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols in promotion of human health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 39, 112–120. [Google Scholar] [CrossRef]
- Monsen, E.R. Dietary reference intakes for the antioxidant nutrients: Vitamin C, vitamin E, selenium, and carotenoids. J. Am. Diet. Assoc. 2000, 100, 637–640. [Google Scholar] [CrossRef]
- Du, M.; Zhao, L.; Li, C.; Zhao, G.; Hu, X. Purification and characterization of a novel fungi Se-containing protein from Se-enriched Ganoderma Lucidum mushroom and its Se-dependent radical scavenging activity. Eur. Food Res. Technol. 2006, 224, 659–665. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.B.; Zhu, J.M.; Liang, L.; Wang, M.S.; Su, H. The bioavailability of selenium and risk assessment for human selenium poisoning in high-Se areas, China. Environ. Int. 2013, 52, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Ma, L.; Jin, X.; Guo, G.; Tan, R.; Liu, Z.; Zheng, L.; Ye, F.; Liu, W. Transcriptome analysis of differentially expressed genes involved in selenium accumulation in tea plant (Camellia sinensis). PLoS ONE 2018, 13, e0197506. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, X.; Lai, C.; Gao, H.; Zheng, Y.; Huang, J.; Lin, B. Selenium enrichment improves anti-proliferative effect of oolong tea extract on human hepatoma HuH-7 cells. Food Chem. Toxicol. 2021, 147, 111873. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Xu, J.; Yang, F.; An, X.; Hu, Q. Anticarcinogenic activity of selenium-enriched green tea extracts in vivo. J. Agric. Food Chem. 2007, 55, 5349–5353. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.; Zhang, S.; Luo, L.; Zeng, L. Effect of brewing conditions on phytochemicals and sensory profiles of black tea infusions: A primary study on the effects of geraniol and β-ionone on taste perception of black tea infusions. Food Chem. 2021, 354, 129504. [Google Scholar] [CrossRef]
- Safdar, N.; Sarfaraz, A.; Kazmi, Z.; Yasmin, A. Ten different brewing methods of green tea: Comparative antioxidant study. J. Appl. Biol. Biotechnol. 2016, 4, 33–40. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Cheng, X.; Thangaraj, K.; Arkorful, E.; Chen, X.; Li, X. Prediction of suitable brewing cuppages of Dahongpao tea based on chemical composition, liquor colour and sensory quality in different brewing. Sci. Rep. 2020, 10, 945. [Google Scholar] [CrossRef]
- Castiglioni, S.; Damiani, E.; Astolfi, P.; Carloni, P. Influence of steeping conditions (time, temperature, and particle size) on antioxidant properties and sensory attributes of some white and green teas. Int. J. Food Sci. Nutr. 2015, 66, 491–497. [Google Scholar] [CrossRef]
- Lin, S.-D.; Yang, J.-H.; Hsieh, Y.-J.; Liu, E.-H.; Mau, J.-L. Effect of different brewing methods on quality of green tea. J. Food Process. Preserv. 2014, 38, 1234–1243. [Google Scholar] [CrossRef]
- Lantano, C.; Rinaldi, M.; Cavazza, A.; Barbanti, D.; Corradini, C. Effects of alternative steeping methods on composition, antioxidant property and colour of green, black and oolong tea infusions. J. Food Sci. Technol. 2015, 52, 8276–8283. [Google Scholar] [CrossRef] [PubMed]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Donlao, N.; Ogawa, Y. Impacts of processing conditions on digestive recovery of polyphenolic compounds and stability of the antioxidant activity of green tea infusion during in vitro gastrointestinal digestion. LWT-Food Sci. Technol. 2018, 89, 648–656. [Google Scholar] [CrossRef]
- Li, L.; Xu, H.; Zhou, J.; Yu, J.; Copeland, L.; Wang, S. Mechanisms underlying the effect of tea extracts on in vitro digestion of wheat starch. J. Agric. Food Chem. 2021, 69, 8227–8235. [Google Scholar] [CrossRef]
- Li, Z.-T.; Zhu, L.; Zhang, W.-L.; Zhan, X.-B.; Gao, M.-J. New dynamic digestion model reactor that mimics gastrointestinal function. Biochem. Eng. J. 2020, 154, 107431. [Google Scholar] [CrossRef]
- Wu, P.; Bhattarai, R.R.; Dhital, S.; Deng, R.; Chen, X.D.; Gidley, M.J. In vitro digestion of pectin- and mango-enriched diets using a dynamic rat stomach-duodenum model. J. Food Eng. 2017, 202, 65–78. [Google Scholar] [CrossRef]
- Okello, E.J.; McDougall, G.J.; Kumar, S.; Seal, C.J. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ(1-42)) induced cytotoxicity in differentiated PC12 cells. Phytomedicine 2011, 18, 691–696. [Google Scholar] [CrossRef]
- He, Z.; Wang, M.; Zhao, Q.; Li, X.; Liu, P.; Ren, B.; Wu, C.; Du, X.; Li, N.; Liu, Q. Bis(ethylmaltolato)oxidovanadium (IV) mitigates neuronal apoptosis resulted from amyloid-beta induced endoplasmic reticulum stress through activating peroxisome proliferator-activated receptor γ. J. Inorg. Biochem. 2020, 208, 111073. [Google Scholar] [CrossRef]
- Chen, N.; Wang, J.; He, Y.; Xu, Y.; Zhang, Y.; Gong, Q.; Yu, C.; Gao, J. Trilobatin protects against Aβ25–35-induced hippocampal HT22 cells apoptosis through mediating ROS/p38/Caspase 3-dependent pathway. Front. Pharmacol. 2020, 11, 584. [Google Scholar] [CrossRef]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Bradshaw, P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J. Alzheimer’s Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Liu, M.; Zhong, X.; Yao, W.; Xiao, Q.; Wen, Q.; Yang, B.; Wei, M. Epigallocatechin gallate reduces amyloid β-induced neurotoxicity via inhibiting endoplasmic reticulum stress-mediated apoptosis. Mol. Nutr. Food Res. 2018, 62, 1700890. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Lee, Y.K.; Park, S.G.; Choi, I.S.; Ban, J.O.; Park, H.K.; Nam, S.-Y.; Yun, Y.W.; Han, S.B.; Oh, K.W.; et al. L-Theanine, an amino acid in green tea, attenuates β-amyloid-induced cognitive dysfunction and neurotoxicity: Reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-κB pathways. Free Radic. Biol. Med. 2009, 47, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, L. The neuroprotective effects of moderate and regular caffeine consumption in Alzheimer’s disease. Oxidative Med. Cell. Longev. 2021, 2021, 5568011. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Extraction Kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Fernando Soysa Nutr. J. 2015, 14, 74. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, A.; Song, Q.; Chen, H.Y.; Harmon, F.G.; Chen, Z.J. Temporal Regulation of the Metabolome and Proteome in Photosynthetic and Photorespiratory Pathways Contributes to Maize Heterosis. Plant Cell 2020, 32, 3706–3722. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Chen, G.S.; Wang, Q.S.; Yuan, H.B.; Feng, C.H.; Yin, J.F. Irreversible sediment formation in green tea infusions. J. Food Sci. 2012, 77, C298–C302. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Ji, W.B.; Yu, P.; Chen, J.X.; Wang, F.; Yin, J.F. Effect of extraction methods on the chemical components and taste quality of green tea extract. Food Chem. 2017, 248, 146–154. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, Y.N.; Chen, J.X.; Wang, F.; Du, Q.Z.; Yin, J.F. Quantitative analyses of the bitterness and astringency of catechins from green tea. Food Chem. 2018, 258, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chambers, D.; Chambers, E. Sensory and instrumental flavor changes in green tea brewed multiple times. Foods 2013, 2, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.E.; Maxwell, S.R.J.; Thorpe, G.H.G. An investigation of the antioxidant activity of black tea using enhanced chemiluminescence. Free Radic. Res. 1996, 26, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Hu, G.; Liu, K.; Yu, R.; Lu, Q.; Zhang, Y. Potential exposure to metals and health risks of metal intake from Tieguanyin tea production in Anxi, China. Environ. Geochem. Health 2019, 41, 1291–1302. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, L.; Liao, C.; Chen, L.; Wang, J.; Zeng, L. Effects of brewing conditions on the phytochemical composition, sensory qualities and antioxidant activity of green tea infusion: A study using response surface methodology. Food Chem. 2018, 269, 24–34. [Google Scholar] [CrossRef]
- Kowalska, J.; Marzec, A.; Domian, E.; Galus, S.; Ciurzynska, A.; Brzezinska, R.; Kowalska, H. Influence of tea brewing parameters on the antioxidant potential of infusions and extracts depending on the degree of processing of the leaves of Camellia sinensis. Molecules 2021, 26, 4773. [Google Scholar] [CrossRef]
- Gan, P.T.; Ting, A.S.Y. Our tea-drinking habits: Effects of brewing cycles and infusion time on total phenol content and antioxidants of common teas. J. Culin. Sci. Technol. 2017, 17, 170–183. [Google Scholar] [CrossRef]
- Komes, D.; Horžić, D.; Belščak, A.; Ganić, K.K.; Vulić, I. Green tea preparation and its influence on the content of bioactive compounds. Food Res. Int. 2010, 43, 167–176. [Google Scholar] [CrossRef]
- Sharpe, E.; Hua, F.; Schuckers, S.; Andreescu, S.; Bradley, R. Effects of brewing conditions on the antioxidant capacity of twenty-four commercial green tea varieties. Food Chem. 2016, 192, 380–387. [Google Scholar] [CrossRef]
- Perez-Burillo, S.; Gimenez, R.; Rufian-Henares, J.A.; Pastoriza, S. Effect of brewing time and temperature on antioxidant capacity and phenols of white tea: Relationship with sensory properties. Food Chem. 2018, 248, 111–118. [Google Scholar] [CrossRef]
- Zou, C.; Li, R.Y.; Chen, J.X.; Wang, F.; Gao, Y.; Fu, Y.Q.; Xu, Y.Q.; Yin, J.F. Zijuan tea- based kombucha: Physicochemical, sensorial, and antioxidant profile. Food Chem. 2021, 363, 130322. [Google Scholar] [CrossRef] [PubMed]
- Joo, C.G.; Lee, K.H.; Park, C.; Joo, I.W.; Choe, T.B.; Lee, B.C. Correlation of increased antioxidation with the phenolic compound and amino acids contents of Camellia sinensis leaf extracts following ultra high pressure extraction. J. Ind. Eng. Chem. 2012, 18, 623–628. [Google Scholar] [CrossRef]
- Wu, P.; Deng, R.; Wu, X.; Wang, Y.; Dong, Z.; Dhital, S.; Chen, X.D. In vitro gastric digestion of cooked white and brown rice using a dynamic rat stomach model. Food Chem. 2017, 237, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Bermudezsoto, M.; Tomasbarberan, F.; Garciaconesa, M. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Song, B.J.; Manganais, C.; Ferruzzi, M.G. Thermal degradation of green tea flavan-3-ols and formation of hetero- and homocatechin dimers in model dairy beverages. Food Chem. 2015, 173, 305–312. [Google Scholar] [CrossRef]
- Liu, X.Q.; Deng, Y.X.; Dai, Z.; Hu, T.; Cai, W.W.; Liu, H.F.; Li, H.; Zhu, W.L.; Li, B.Y.; Wang, Q.; et al. Sodium tanshinone IIA sulfonate protects against Aβ1-42-induced cellular toxicity by modulating Aβ-degrading enzymes in HT22 cells. Int. J. Biol. Macromol. 2020, 151, 47–55. [Google Scholar] [CrossRef]

| Chemical Constituents | ESYL (μg/g) | ZYMJ (μg/g) |
|---|---|---|
| Se | 0.58 ± 0.01 | 4.33 ± 0.18 |
| Tea polyphenols | 16.74 ± 0.69 | 21.50 ± 0.47 |
| Caffeine | 4.43 ± 0.09 | 5.41 ± 0.31 |
| Free amino acids | 7.02 ± 0.03 | 6.76 ± 0.06 |
| Soluble sugar | 19.18 ± 1.07 | 11.65 ± 1.15 |
| Water extracts | 42.59 ± 0.16 | 46.23 ± 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; He, J.; He, Z.; Zhang, N.; Liu, X.; Zhou, J.; Cheng, S.; Cai, J. Evaluation of the Brewing Characteristics, Digestion Profiles, and Neuroprotective Effects of Two Typical Se-Enriched Green Teas. Foods 2022, 11, 2159. https://doi.org/10.3390/foods11142159
Ye Y, He J, He Z, Zhang N, Liu X, Zhou J, Cheng S, Cai J. Evaluation of the Brewing Characteristics, Digestion Profiles, and Neuroprotective Effects of Two Typical Se-Enriched Green Teas. Foods. 2022; 11(14):2159. https://doi.org/10.3390/foods11142159
Chicago/Turabian StyleYe, Yuanyuan, Jiangling He, Zhijun He, Na Zhang, Xiaoqing Liu, Jiaojiao Zhou, Shuiyuan Cheng, and Jie Cai. 2022. "Evaluation of the Brewing Characteristics, Digestion Profiles, and Neuroprotective Effects of Two Typical Se-Enriched Green Teas" Foods 11, no. 14: 2159. https://doi.org/10.3390/foods11142159
APA StyleYe, Y., He, J., He, Z., Zhang, N., Liu, X., Zhou, J., Cheng, S., & Cai, J. (2022). Evaluation of the Brewing Characteristics, Digestion Profiles, and Neuroprotective Effects of Two Typical Se-Enriched Green Teas. Foods, 11(14), 2159. https://doi.org/10.3390/foods11142159








