Phenolic Profiling and In-Vitro Bioactivities of Corn (Zea mays L.) Tassel Extracts by Combining Enzyme-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Materials
2.3. Conventional Extraction
2.4. Experimental Design and Statistical Analysis
2.5. Enzyme-Assisted Extraction
2.6. Total Phenolic Compounds and HPLC Analysis
2.7. Antioxidant Activity
2.8. Antimicrobial Activity
2.9. Cytotoxic Activity
2.10. Statistical ANALYSIS
3. Results and Discussion
3.1. Optimization of Phenolic Extraction Conditions with the Use of Cellulase, Protease, and Their Mixture (1:1)
3.2. Identification of the Phenolic Compounds in Corn Tassel Extract Obtained by Enzyme-Assisted Method
3.3. Antioxidant Activity of Corn Tassel Extracts
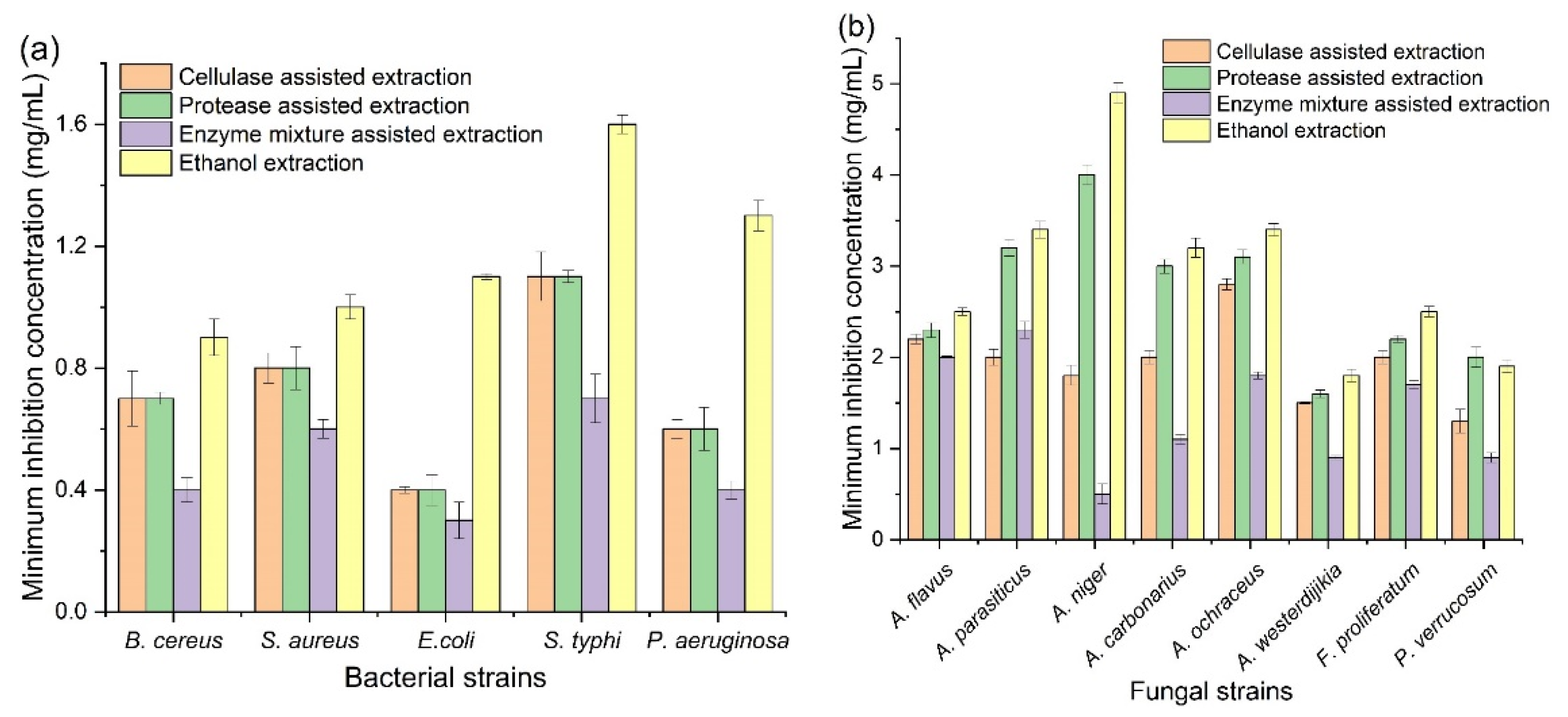
3.4. Antimicrobial Activity of Corn Tassel Extracts
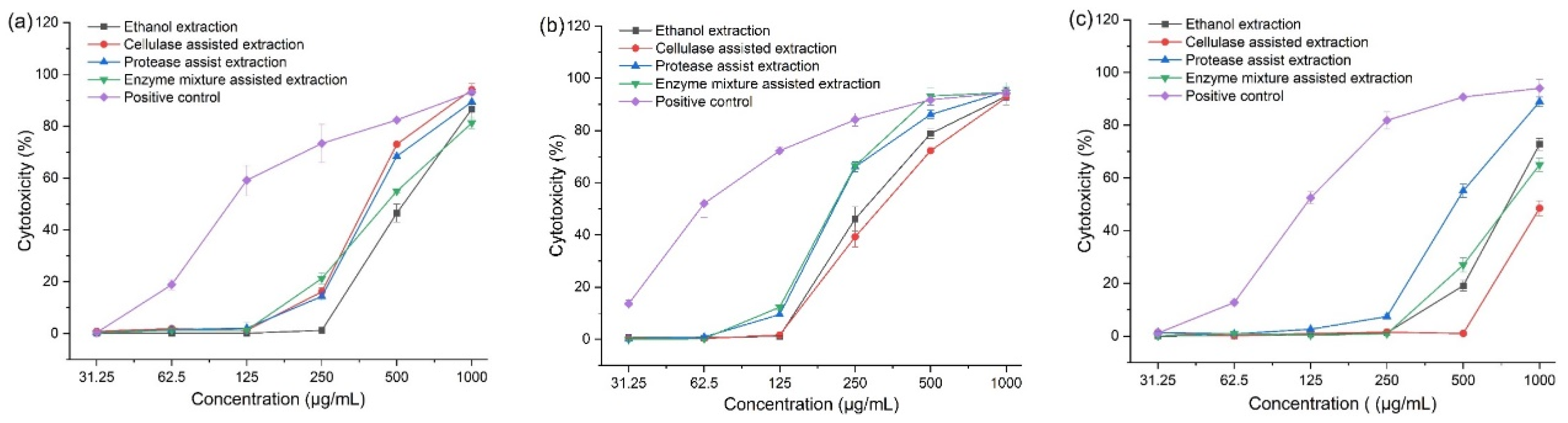
3.5. Cytotoxic Activity of Corn Tassel Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- USDA National Agricultural Statistics Service. Crop Production 2019 Summary (January 2020). Available online: https://usda.library.cornell.edu/concern/publications/k3569432s (accessed on 12 January 2022).
- Knoema. Sugarcane Area Harvested. Available online: https://knoema.com/atlas/topics/Agriculture/Crops-Production-Area-Harvested/Sugar-cane-area-harvested (accessed on 28 March 2020).
- Mohsen, S.M.; Ammar, A.S. Total Phenolic Contents and Antioxidant Activity of Corn Tassel Extracts. Food Chem. 2009, 112, 595–598. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Teranishi, R. Volatiles of Corn Tassels: Possible Corn Ear WORM Attractants. J. Agric. Food Chem. 1980, 28, 771–774. [Google Scholar] [CrossRef]
- Ceska, O.; Styles, E.D. Flavonoids from Zea mays Pollen. Phytochemistry 1984, 23, 1822–1823. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Núñez, M.J. Effect of Solvent, Temperature, and Solvent-to-Solid Ratio on the Total Phenolic Content and Antiradical Activity of Extracts from Different Components of Grape Pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Krakowska, A.; Rafińska, K.; Walczak, J.; Buszewski, B. Enzyme-assisted Optimized Supercritical Fluid Extraction to Improve Medicago Sativa Polyphenolics Isolation. Ind. Crops Prod. 2018, 124, 931–940. [Google Scholar] [CrossRef]
- Younis, M.I.; Xiaofeng, R.; Alzubaidi, A.K.; Mahmoud, K.F.; Altemimi, A.B.; Cacciola, F.; Raza, H.; Pratap-Singh, A.; Abedelmaksoud, T.G. Optimized Green Extraction of Polyphenols from Cassia javanica L. Petals for Their Application in Sunflower Oil: Anticancer and Antioxidant Properties. Molecules 2022, 27, 4329. [Google Scholar] [CrossRef]
- Fernández, K.; Vega, M.; Aspé, E. An Enzymatic Extraction of Proanthocyanidins from País Grape Seeds and Skins. Food Chem. 2015, 168, 7–13. [Google Scholar] [CrossRef]
- Abedelmaksoud, T.G.; Mohsen, S.M.; Duedahl-Olesen, L.; Elnikeety, M.M.; Feyissa, A.H. Optimization of Ohmic Heating Parameters for Polyphenoloxidase Inactivation in not-from-Concentrate Elstar Apple Juice using RSM. J. Food Sci. Technol. 2018, 55, 2420–2428. [Google Scholar] [CrossRef]
- Elsayed, N.; El-Din, H.S.; Altemimi, A.B.; Ahmed, H.Y.; Pratap-Singh, A.; Abedelmaksoud, T.G. In Vitro Antimicrobial, Antioxidant and Anticancer Activities of Egyptian Citrus Beebread. Molecules 2021, 26, 2433. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Baur, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Marrez, D.A.; Naguib, M.M.; Sultan, Y.Y.; Higazy, A.M. Antimicrobial and Anticancer Activities of Scenedesmus Obliquus metabolites. Heliyon 2019, 5, e01404. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemoth. 2001, 48, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Marrez, D.A.; Sultan, Y.Y. Antifungal Activity of the Cyanobacterium Microcystis Aeruginosa against Mycotoxigenic Fungi. J. Appl. Pharm. Sci. 2016, 11, 191–198. [Google Scholar] [CrossRef]
- Senthilraja, P.; Kathiresan, K. In Vitro Cytotoxicity MTT Assay In Vero, HepG2 and MCF-7 Cell Lines Study of Marine Yeast. J. Appl. Pharm. Sci. 2015, 5, 80–84. [Google Scholar] [CrossRef]
- Miron, T.; Herrero, M.; Ibáñez, E. Enrichment of Antioxidant Compounds from Lemon Balm (Melissa Officinalis) by Pressurized Liquid Extraction and Enzyme-Assisted Extraction. J. Chromatogr. A 2013, 1288, 1–9. [Google Scholar] [CrossRef]
- Pinelo, M.; Zornoza, B.; Meyer, A.S. Selective Release of Phenols from Apple Skin: Mass Transfer Kinetics during Solvent and enzyme-assisted extraction. Sep. Purif. Technol. 2008, 63, 620–627. [Google Scholar] [CrossRef]
- Cerda, A.; Martínez, M.E.; Soto, C.; Poirrier, P.; Perez-Correa, J.R.; Vergara-Salinas, J.R.; Zúñiga, M.E. The enhancement of Antioxidant Compounds Extracted from Thymus Vulgaris using Enzymes and the Effect of Extracting Solvent. Food Chem. 2013, 139, 138–143. [Google Scholar] [CrossRef]
- De Vries, R.P.; Visser, J. Aspergillus Enzymes involved in Degradation of Plant Cell Wall Polysaccharides. Microbiol. Mol. Biol. Rev. 2001, 65, 497–522. [Google Scholar] [CrossRef]
- Shahidi, F. Antioxidant Properties of Food Phenolics. Phenol. Food Nutraceut. 2004. Available online: https://www.researchgate.net/publication/287473903_Antioxidant_properties_of_food_phenolics (accessed on 28 March 2020).
- Kamonwannasit, S.; Nantapong, N.; Kumkrai, P.; Luecha, P.; Kupittayanant, S.; Chudapongse, N. Antibacterial Activity of Aquilaria Crassna Leaf Extract against Staphylococcus Epidermidis by Disruption of Cell Wall. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1–7. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.N.J.I.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to Different Experimental Organ Systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Deng, Z. Protective Effects of Flavonoids from Corn Silk on Oxidative Stress Induced by Exhaustive Exercise in Mice. Afr. J. Biotechnol. 2011, 10, 3163–3167. [Google Scholar]
- Shallan, M.A.; Ali, M.A.; Meshrf, W.A.; Marrez, D.A. In Vitro Antimicrobial, Antioxidant and Anticancer Activities of Globe Artichoke (Cynara Cardunculus var. scolymus L.) Bracts and Receptacles Ethanolic Extract. Biocatal. Agric. Biotechnol. 2020, 29, 101774. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, and Ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Solaiman, M.A.; Ali, M.A.; Abdel-Moein, N.M.; Mahmoud, E.A. Synthesis of Ag-NPs Developed by Green-Chemically Method and Evaluation of Antioxidant Activities and Anti-inflammatory of Synthesized Nanoparticles against LPS-Induced NO in RAW 264.7 Macrophages. Biocatal. Agric. Biotechnol. 2020, 29, 101832. [Google Scholar] [CrossRef]
- Duangpapeng, P.; Lertrat, K.; Lomthaisong, K.; Paul Scott, M.; Suriharn, B. Variability in Anthocyanins, Phenolic Compounds and Antioxidant Capacity in the Tassels of Collected Waxy Corn Germplasm. Agronomy 2019, 9, 158. [Google Scholar] [CrossRef]
- Wille, J.; Berhow, A.; Bioactives, M. Derived from Ripe Corn Tassels: A possible New Natural Skin Whitener, 4-Hydroxy-1-Oxindole-3-Acetic Acid. Curr. Bioact. Compd. 2011, 7, 126–134. [Google Scholar] [CrossRef]
- Wang, L.-C.; Yu, Y.-Q.; Fang, M.; Zhan, C.-G.; Pan, H.-Y.; Wu, Y.-N.; Gong, Z.-Y. Antioxidant and Antigenotoxic Activity of Bioactive Extracts from Corn Tassel. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2014, 34, 131–136. [Google Scholar] [CrossRef]
- Fukamachi, K.; Imada, T.; Ohshima, Y.; Xu, J.; Tsuda, H. Purple Corn Color Suppresses Ras PROTEIN Level and Inhibits 7, 12-Dimethylbenz [a] Anthracene-Induced Mammary Carcinogenesis in the Rat. Cancer Sci. 2008, 99, 1841–1846. [Google Scholar] [PubMed]
- Guo, H.; Guan, H.; Yang, W.; Liu, H.; Hou, H.; Chen, X.; Liu, Z.; Zang, C.; Liu, Y.; Liu, J. Pro-apoptotic and Anti-proliferative Effects of Corn Silk Extract on Human Colon Cancer Cell Lines. Oncol. Lett. 2017, 13, 973–978. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sánchez, J.G.; Arismendi, S.M.; Menacho, L.M.P. Characteristics and Functional Properties of Purple Corn (Zea mays L.) var. Subnigroviolaceo. Sci. Agropecu. 2014, 5, 211–217. [Google Scholar]
- Wu, T.; Guo, X.; Zhang, M.; Yang, L.; Liu, R.; Yin, J. Anthocyanins in black Rice, Soybean and Purple Corn Increase Fecal Butyric Acid and Prevent liver Inflammation in High Fat Diet-Induced Obese Mice. Food Funct. 2017, 8, 3178–3186. [Google Scholar] [CrossRef]
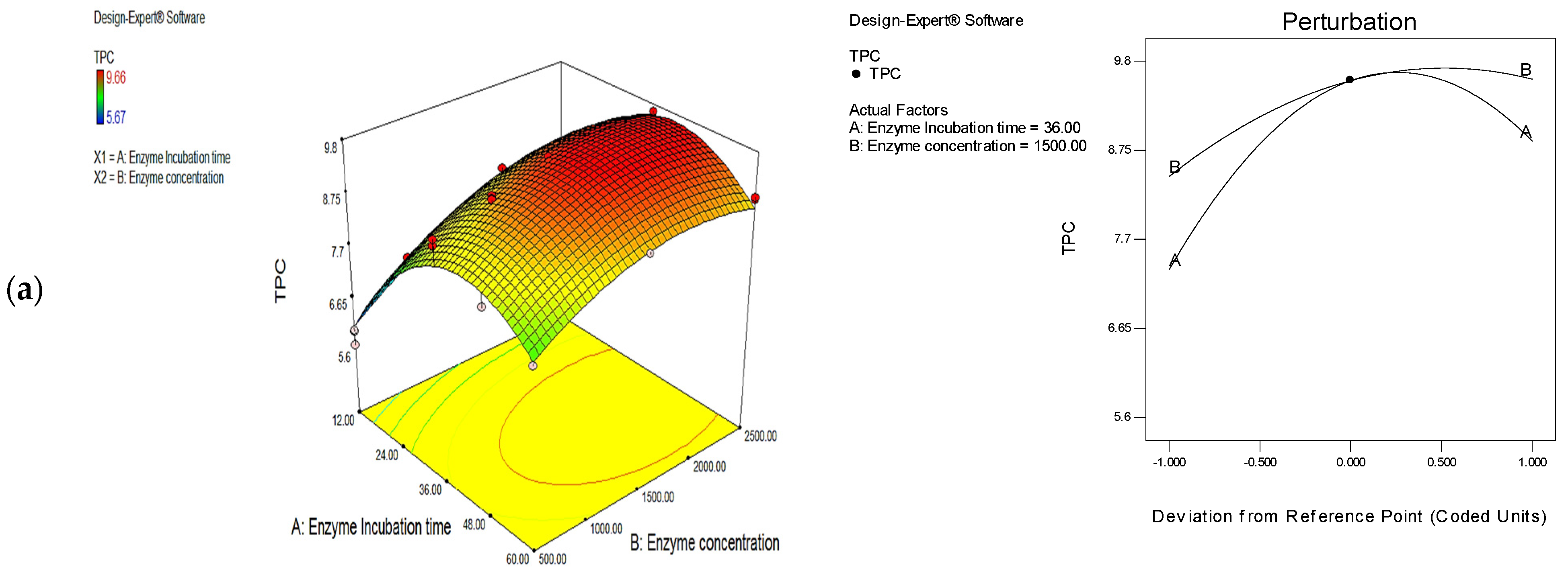
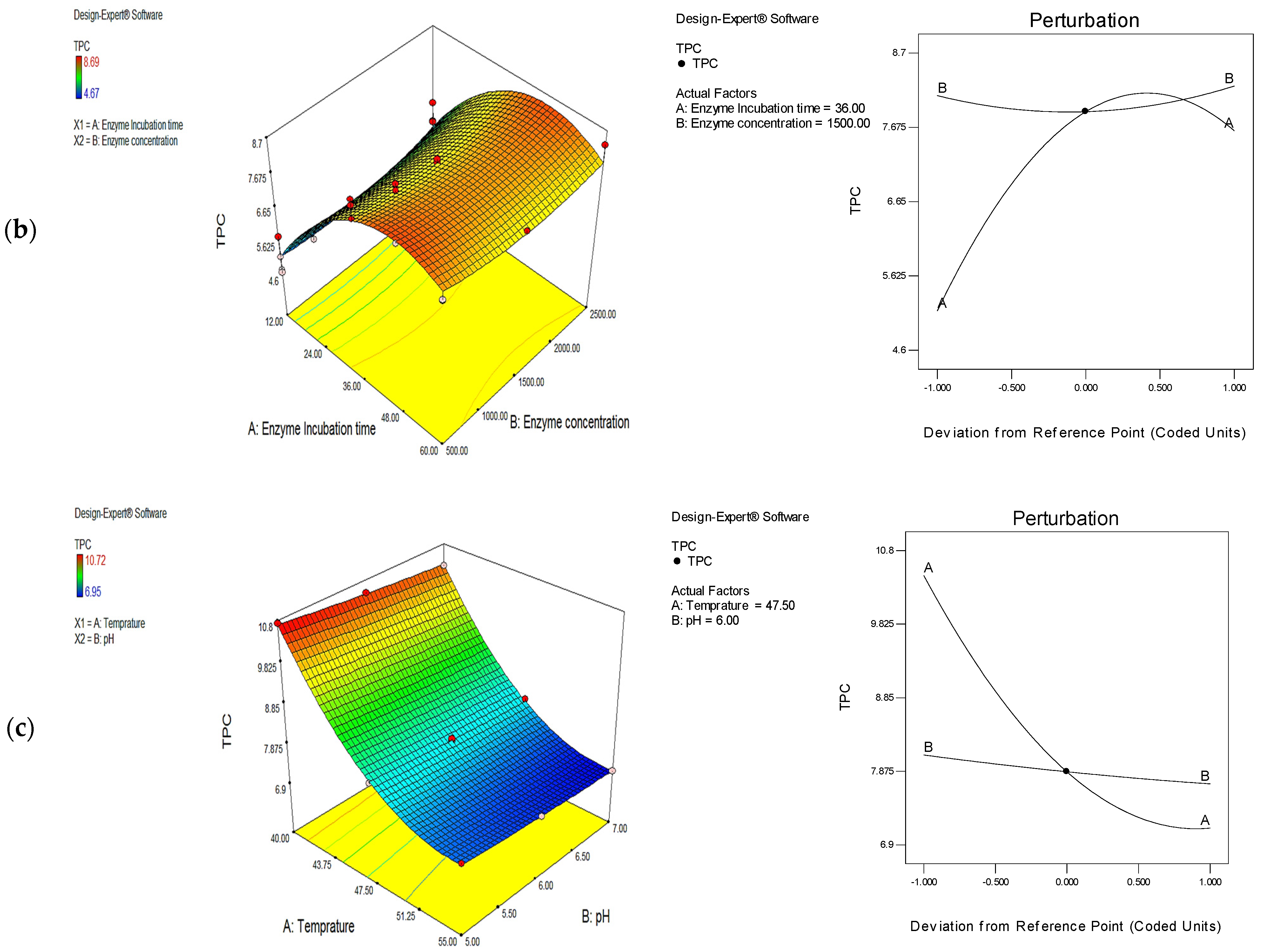
| Treatment | Enzyme Incubation Time (h) | Enzyme Concentration (U/g) | TPC (mg/g) | Desirability | |
|---|---|---|---|---|---|
| Predicted Value | Actual Value * | ||||
| Cellulase | 39.32 | 1978.60 | 9.78 | 9.56 ± 0.11 b | 1.000 |
| Protease | 47.38 | 500.00 | 8.45 | 8.48 ± 0.12 c | 0.941 |
| Enzyme mixture (40 °C, pH 5.0) | - | - | 10.70 | 10.74 ± 0.10 a | 0.995 |
| Ethanol 60% | - | - | - | 6.75 ± 0.11 d | - |
| Phenolic Compounds | Cellulase (µg/g) | Proteases (µg/g) | Cellulase–Protease Mixture (µg/g) |
|---|---|---|---|
| Gallic acid | 967.88 ± 34.00 | 314.87 ± 8.74 | 1694.36 ± 54.75 |
| Chlorogenic acid | 659.8 ± 41.56 | 97.83 ± 2.09 | 1172 ± 86.38 |
| Caffeic acid | 370.48 ± 20.46 | 9.75 ± 0.63 | 661.81 ± 32.40 |
| Naringenin | 259.05 ± 20.55 | 118.76 ± 2.24 | 270.01 ± 28.44 |
| Rutin | 181.24 ± 7.21 | 159.92 ± 1.18 | 211.24 ± 11.05 |
| Ferulic acid | 26.61 ± 2.87 | 20.39 ± 0.91 | 180.39 ± 4.93 |
| Syringic acid | 148.18 ± 6.09 | 66.08 ± 1.09 | 167.22 ± 5.13 |
| Catechin | 198.41 ± 16.96 | 114.62 ± 3.66 | 134.23 ± 4.51 |
| Ellagic acid | nd | 6.99 ± 0.29 | 74.95 ± 4.50 |
| Methyl gallate | 26.18 ± 3.87 | 8.77 ± 0.80 | 58.82 ± 5.39 |
| Kaempferol | 6.31 ± 0.47 | nd | 40.86 ± 3.55 |
| Coumaric acid | 36.91 ± 1.68 | nd | 36.83 ± 0.50 |
| Pyrocatechol | 21.30 ± 1.54 | 11.86 ± 0.82 | 18.85 ± 0.50 |
| Taxifolin | nd | 6.11 ± 0.29 | 13.80 ± 0.83 |
| Corn Tassel Extract | Antioxidant Activity (%) | |
|---|---|---|
| DPPH Assay | ABTS Assay | |
| Ethanol extract | 75.25 ± 0.10 e | 80.25 ± 0.16 e |
| Cellulose-assisted extract | 86.11 ± 0.12 c | 90.29 ± 0.14 b |
| Protease-assisted extract | 83.20 ± 0.13 d | 88.70 ± 0.18 c |
| Mixed-enzyme-assisted extract | 92.98 ± 0.09 a | 95.18 ± 0.15 a |
| BHT | 87.69 ± 0.14 b | 83.74 ± 0.17 d |
| Treatment | IC50 (µg/mL) | ||
|---|---|---|---|
| Caco2 | A549 | Wi-38 | |
| Ethanol extraction | 624.57 ± 17.66 a | 332.14 ± 11.73 a | 778.66 ± 61.38 b |
| Cellulase-assisted extraction | 410.51 ± 15.41c | 359.56 ± 18.11 a | 1073.50 ± 49.16 a |
| Protease-assisted extraction | 461.98 ± 18.87 b | 213.45 ± 13.66 b | 578.89 ± 17.77 c |
| Mixed-enzyme-assisted extract | 392.62 ± 24.3 c | 210.66 ± 8.9 b | 809.85 ± 37.25 b |
| Doxorubicin | 110.83 ± 6.82 d | 61.42 ± 5.79 c | 122.72 ± 7.8 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, N.; Marrez, D.A.; Ali, M.A.; El-Maksoud, A.A.A.; Cheng, W.; Abedelmaksoud, T.G. Phenolic Profiling and In-Vitro Bioactivities of Corn (Zea mays L.) Tassel Extracts by Combining Enzyme-Assisted Extraction. Foods 2022, 11, 2145. https://doi.org/10.3390/foods11142145
Elsayed N, Marrez DA, Ali MA, El-Maksoud AAA, Cheng W, Abedelmaksoud TG. Phenolic Profiling and In-Vitro Bioactivities of Corn (Zea mays L.) Tassel Extracts by Combining Enzyme-Assisted Extraction. Foods. 2022; 11(14):2145. https://doi.org/10.3390/foods11142145
Chicago/Turabian StyleElsayed, Nesren, Diaa A. Marrez, Mohamed A. Ali, Ahmed Ali Abd El-Maksoud, Weiwei Cheng, and Tarek Gamal Abedelmaksoud. 2022. "Phenolic Profiling and In-Vitro Bioactivities of Corn (Zea mays L.) Tassel Extracts by Combining Enzyme-Assisted Extraction" Foods 11, no. 14: 2145. https://doi.org/10.3390/foods11142145
APA StyleElsayed, N., Marrez, D. A., Ali, M. A., El-Maksoud, A. A. A., Cheng, W., & Abedelmaksoud, T. G. (2022). Phenolic Profiling and In-Vitro Bioactivities of Corn (Zea mays L.) Tassel Extracts by Combining Enzyme-Assisted Extraction. Foods, 11(14), 2145. https://doi.org/10.3390/foods11142145