Oilseed Extracts from Local Markets as Promising Coagulant Agents for Milk from Various Mammalian Species
Abstract
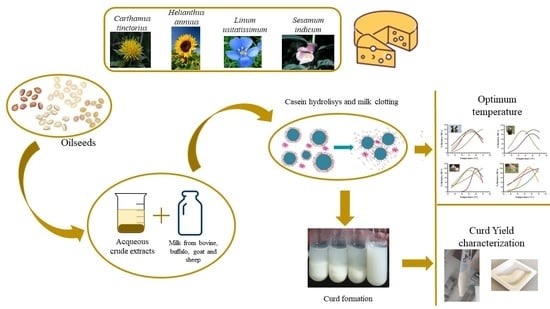
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Seed Materials and Reagents
2.1.2. Milk Samples
2.2. Experimental Procedures
2.2.1. Preparation of Aqueous Oilseed Extracts
2.2.2. Protein Concentration Determination
2.2.3. Caseinolytic Activity
2.2.4. Milk-Clotting Activity
2.2.5. Curd Yield
2.3. Data Analysis
3. Results and Discussion
3.1. Protein Content in Aqueous Oilseed Extracts
3.2. Caseinolytic Activity (CA) and Milk-Clotting Activity (MCA)
3.3. Milk-Clotting Activity (MCA) and Curd Yield in Milk of Different Animal Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camin, F.; Bontempo, L.; Ziller, L.; Franceschi, P.; Molteni, A.; Corbella, R.; Verga, I. Assessing the authenticity ofanimal rennet using δ 15 N analysis of chymosin. Food Chem. 2019, 293, 545–549. [Google Scholar] [CrossRef]
- De Koning, P.J. Coagulating enzymes in cheese making. Dairy Ind. Int. 1978, 43, 12–46. [Google Scholar]
- Ahmad, S.M.; Ahmad, M.S.; Amir Paray, M. Plant proteases as milk-clotting enzymes in cheesemaking: A review. Dairy Sci. Technol. 2013, 94, 5–16. [Google Scholar] [CrossRef]
- Liburdi, K.; Emiliani Spinelli, S.; Benucci, I.; Lombardelli, C.; Esti, M. A preliminary study of continuous milk coagulation using Cynara cardunculus flower extract and calf rennet immobilized on magnetic particles. Food Chem. 2018, 239, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Guan, R.; Jia, G.; Ma, Y.; Zhang, Y. Advances in research on calf rennet substitutes and their effects on cheese quality. Food Res. Int. 2021, 149, 110704. [Google Scholar] [CrossRef]
- Nicosia, F.D.; Puglisi, I.; Pino, A.; Caggia, C.; Lucia Randazzo, C. Plant Milk-Clotting Enzymes for Cheesemaking. Foods 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Characterization of “Lettucine”, a Serine-like Protease from Lactuca sativa Leaves, as a Novel Enzyme for Milk Clotting. J. Agric. Food Chem. 2002, 50, 2439–2443. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Perea-Gutiérrez, T.C.; Lugo-Sánchez, M.E.; Ramirez-Suarez, J.C.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Cordoba, B. Comparison of the milk-clotting properties of three plant extracts. Food Chem. 2013, 141, 1902–1907. [Google Scholar] [CrossRef]
- González-Velázquez, D.A.; Mazorra-Manzano, M.A.; Martínez-Porchas, M.; Huerta-Ocampo, J.A.; Vallejo-Córdoba, B.; Mora-Cortes, W.G.; Moreno-Hernández, J.M.; Ramírez-Suarez, J.C. Exploring the milk-clotting and proteolytic activities in different tissues of Vallesia glabra: A new source of plant proteolytic enzymes. Appl. Biochem. Biotechnol. 2021, 193, 389–404. [Google Scholar] [CrossRef]
- Hashim, M.M.; Dong, M.; Iqbal, M.F.; Li, W.; Chen, X. Ginger protease used as coagulant enhances the proteolysis and sensory quality of Peshawari cheese compared to calf rennet. Dairy Sci. Technol. 2011, 91, 431–440. [Google Scholar] [CrossRef]
- Egito, A.S.; Girardet, J.M.; Laguna, L.E.; Poirson, C.; Mollé, D.; Miclo, L.; Humbert, G.; Gaillard, J.L. Milk-clotting activity of enzyme extracts from sunflower and albizia seeds and specific hydrolysis of bovine κ-casein. Int. Dairy J. 2007, 17, 816–825. [Google Scholar] [CrossRef]
- Nasr, A.I.A.M.; Mohamed Ahmed, I.A.; Hamid, O.I.A. Characterization of partially purified milk-clotting enzyme from sunflower (Helianthus annuus) seeds. Food Sci. Nutr. 2016, 4, 733–741. [Google Scholar] [CrossRef] [Green Version]
- Roseiro, L.B.; Barbosa, M.; Ames, J.M.; Wilbey, R.A. Cheesemaking with vegetable coagulants-the use of Cynara L. for the production of ovine milk cheeses. Int. J. Dairy Technol. 2003, 56, 76–85. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Zhang, C.; Kong, X.; Hua, Y. Sesame water-soluble proteins fraction contains endopeptidases and exopeptidases with high activity: A natural source for plant proteases. Food Chem. 2021, 353, 129519. [Google Scholar] [CrossRef]
- Nandish, S.K.M.; Kengaiah, J.; Ramachandraiah, C.; Shivaiah, A.; Santhosh, S.M.; Sannaningaiah, D. Flaxseed Cysteine Protease Exhibits Strong Anticoagulant, Antiplatelet, and Clot-Dissolving Properties. Biochemistry-Moscow 2020, 85, 1113–1126. [Google Scholar] [CrossRef]
- Tan-Wilson, A.L.; Wilson, K.A. Mobilization of seed protein reserves. Physiol. Plant. 2012, 145, 140–153. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Anusha, R.; Singh, M.K.; Bindhu, O.S. Characterization of potential milk coagulants from Calotropis gigantea plant parts and their hydrolytic pattern of bovine casein. Eur. Food Res. Technol. 2014, 238, 997–1006. [Google Scholar] [CrossRef]
- Pazzola, M.; Stocco, G.; Dettori, M.L.; Bittante, G.; Vacca, G.M. Effect of goat milk composition on cheesemaking traits and daily cheese production. J. Dairy Sci. 2019, 102, 3947–3955. [Google Scholar] [CrossRef]
- Johnson, B.T. DSTAT: Software for the Meta-Analytic Review of Research Literatures; Lawrence Erlbaum: Mahwah, NJ, USA, 1989. [Google Scholar]
- Staswick, P.E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell 1990, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Sharma, A. Solid state fermentation of non-edible oil seed cakes for production of proteases and cellulases and degradation of anti-nutritional factors. J. Food Biotechnol. Res. 2018, 1, 3–8. [Google Scholar]
- Müntz, K. Proteases and proteolytic cleavage of storage proteins in developing and germinating dicotyledonous seeds. J. Exp. Bot. 1996, 47, 605–622. [Google Scholar] [CrossRef] [Green Version]
- Hemalatha, K.P.J.; Prasad, D.S. Changes in the metabolism of protein during germination of sesame (Sesamum indicum L.) seeds. Plant Foods Hum. Nutr. 2003, 58, 1–10. [Google Scholar] [CrossRef]
- Esteves, C.L.C.; Lucey, J.A.; Pires, E.M.V. Rheological properties of milk gels made with coagulants of plant origin and chymosin. Int. Dairy J. 2002, 12, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Jollès, P.; Alais, C.; Jollès, J. Étude de la Caséine κ de vache caractérisation de la liaison sensible à l’action de la présure. Biochim. Biophys. Acta 1963, 69, 511–517. [Google Scholar] [CrossRef]
- Aworh, O.C.; Muller, H.G. Cheese-making properties of vegetable rennet from sodom apple (Calotropis procera). Food Chem. 1987, 26, 71–79. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Carreira, R.L.; Silva, M.R.; Corgosinho, F.C.; Monteiro, M.R.P.; Morais, H.A. Effect of pH and temperature on the activity of enzymatic extracts from pineapple peel. Food Bioprocess Technol. 2012, 5, 1824–1831. [Google Scholar] [CrossRef]
- Liburdi, K.; Boselli, C.; Giangolini, G.; Amatiste, S.; Esti, M. An evaluation of the clotting properties of three plant rennets in the milks of different animal species. Foods 2019, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional Value and Technological Suitability of Milk from Various Animal Species Used for Dairy Production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Boyazoglu, J.; Morand-Fehr, P. Mediterranean dairy sheep and goat products and their quality: A critical review. Small Rumin. Res. 2001, 40, 1–11. [Google Scholar] [CrossRef]
- De Marchi, M.; Fagan, C.C.; O’Donnell, C.P.; Cecchinato, A.; Dal Zotto, R.; Cassandro, M.; Penasa, M.; Bittante, G. Prediction of coagulation properties, titratable acidity, and pH of bovine milk using mid-infrared spectroscopy. J. Dairy Sci. 2009, 92, 423–432. [Google Scholar] [CrossRef]
- O’Callaghan, D.J.; Mulholland, E.P.; Duffy, A.P.; O’donnell, C.P.; Payne, F.A. Evaluation of hot wire and optical sensors for on-line monitoring of curd firmness during milk coagulation. Ir. J. Agric. Food Res. 2001, 40, 227–238. [Google Scholar]
- Park, H.; Yamanaka, N.; Mikkonen, A.; Kusakabe, I.; Kobayashi, H. Purification and Characterization of Aspartic Proteinase from Sunflower Seeds. Biosci. Biotechnol. Biochem. 2000, 64, 931–939. [Google Scholar] [CrossRef]
- Walde, P.; Luisi, P.L.; Palmieri, S. Proteolytic activity in sunflower seeds (Helianthus annuus L.). J. Agric. Food Chem. 1984, 32, 322–329. [Google Scholar] [CrossRef]
- Fagan, C.C.; Castillo, M.; Payne, F.A.; O’Donnell, C.P.; O’Callaghan, D.J. Effect of cutting time, temperature, and calcium on curd moisture, whey fat losses, and curd yield by response surface methodology. J. Dairy Sci. 2007, 90, 4499–4512. [Google Scholar] [CrossRef]
- Everard, C.D.; O’Callaghan, D.J.; Mateo, M.J.; Castillo, M.; Payne, F.A.; O’Donnell, C.P. Effects of milk composition, stir-out time, and pressing duration on curd moisture and yield. J. Dairy Sci. 2011, 94, 2673–2679. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Chen, C.M.; Jaeggi, J.J. Effect of rennet coagulation time on composition, yield, and quality of reduced-fat Cheddar cheese. J. Dairy Sci. 2001, 84, 1027–1033. [Google Scholar] [CrossRef]
- Lucey, J.A.; Teo, C.T.E.T.; Munro, P.A.; Singh, H. Rheological properties at small (dynamic) and large (yield) deformations of acid gels made from heated milk. J. Dairy Res. 1997, 64, 591–600. [Google Scholar] [CrossRef]
- Riddell-Lawrence, S.; Hicks, C.L. Effect of curd firmness on stirred curd cheese yield. J. Dairy Sci. 1989, 72, 313–321. [Google Scholar] [CrossRef]
- Hussain, I.; Yan, J.; Grandison, A.S.; Bell, A.E. Effects of gelation temperature on Mozzarella-type curd made from buffalo and cows’ milk: 2. Curd yield, overall quality and casein fractions. Food Chem. 2012, 135, 1404–1410. [Google Scholar] [CrossRef]
- Stocco, G.; Pazzola, M.; Dettori, M.L.; Paschino, P.; Bittante, G.; Vacca, G.M. Effect of composition on coagulation, curd firming, and syneresis of goat milk. J. Dairy Sci. 2018, 101, 9693–9702. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Fundamentals of Cheese Science; Springer: Heidelberg, Germany, 2017. [Google Scholar]


| Enzyme Source | Extract Ratio (g/mL) | Acronymous | Protein Content (mgBSA/grDW) |
|---|---|---|---|
| Linen (Lin) | 0.1 | Lin0.1 | 6.79 (±0.30) |
| 0.2 | Lin0.2 | 4.34 (±0.27) | |
| 0.3 | Lin0.3 | 2.9 (±0.19) | |
| 0.5 | Lin0.5 | 2.25 (±0.1) | |
| Safflower (Saf) | 0.1 | Saf0.1 | 2.75 (±0.64) |
| 0.2 | Saf0.2 | 2.22 (±0.08) | |
| 0.3 | Saf0.3 | 2.55 (±0.310) | |
| 0.5 | Saf0.5 | 2.60 (±0.19) | |
| Sesame (Ses) | 0.1 | Ses0.1 | 9.10 (±0.28) |
| 0.2 | Ses0.2 | 6.48 (±0.02) | |
| 0.3 | Ses0.3 | 3.39 (±0.20) | |
| 0.5 | Ses0.5 | 3.01 (±0.19) | |
| Sunflower (Sun) | 0.1 | Sun0.1 | 15.54 (±0.14) |
| 0.2 | Sun0.2 | 16.25 (±0.70) | |
| 0.3 | Sun0.3 | 10.44 (±0.12) | |
| 0.5 | Sun0.5 | 7.08 (±0.01) |
| Enzyme Extracts | Temperature (°C) | ||||
|---|---|---|---|---|---|
| 25° | 37° | 50° | 65° | 80° | |
| Lin0.1 | n.d. | n.d. | 2.26 f (±0.01) | n.d. | n.d. |
| Lin0.2 | n.d. | 2.3 (±0.03) | 11.78 e (±0.35) | 5.2 (±0.03) | n.d. |
| Lin0.3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Lin0.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Saf0.1 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Saf0.2 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Saf0.3 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Saf0.5 | n.d. | 3.16 (±0.2) | 13.07 (±0.13) | 4.89 (±0.02) | 4.10 (±0.03) |
| Ses0.1 | n.d. | n.d. | 2.93 h (±0.02) | 4.64 k (±0.02) | n.d. |
| Ses0.2 | n.d. | n.d. | 4.71 f (±0.04) | 7.47 j (±0.52) | 3.49 x (±0.01) |
| Ses0.3 | n.d. | n.d. | 5.03 e (±0.07) | 3.12 l (±0.03) | 2.25 y (±0.03) |
| Ses0.5 | n.d. | 2.1 (±0.03) | 4.18 g (±0.03) | 7.56 j (±0.22) | 3.82 w (±0.06) |
| Sun0.1 | n.d. | n.d. | 0.31 g (±0.02) | 0.91 l (±0.008) | n.d. |
| Sun0.2 | n.d. | n.d. | 0.40 f (±0.08) | 4.28 k (±0.09) | n.d. |
| Sun0.3 | n.d. | 2.92 (±0.02) | 7.34 e (±0.10) | 6.42 j (±0.10) | 4.57 w (±0.04) |
| Sun0.5 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Density (mg/mL) | pH | Water (%) | Total Acidity (°SH) | Lactic Acid (%) | Ash (%) | Total Protein (%) | Caseins (%) | Fatty Matter (%) | Lactose (%) | Dry Matter (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bovine | 1.028 | 6.71 | 87.3 (±0.4) | 8.2 (±0.40) | 0.18 (±0.01) | 0.60 (±0.03) | 3.71 (±0.31) | 2.89 (±0.24) | 4.1 (±0.19) | 4.62 (±0.08) | 13.53 (±0.1) |
| Buffalo | 1.031 | 6.71 | 82.2 (±0.3) | 9.4 (±0.30) | 0.2 (±0.01) | 0.88 (±0.02) | 4.15 (±0.27) | 3.71 (±0.21) | 7.5 (±0.52) | 4.74 (±0.2) | 18.21 (±0.1) |
| Goat | 1.028 | 6.65 | 87.2 (±0.4) | 8.3 (±0.20) | 0.18 (±0.01) | 0.65 (±0.06) | 3.74 (±0.16) | 2.74 (0.12) | 4.0 (±0.23) | 4.51 (±0.1) | 12.36 (±0.2) |
| Sheep | 1.037 | 6.34 | 81.4 (±0.5) | 11.3 (±0.10) | 0.26 (±0.01) | 0.82 (±0.05) | 6.90 (±0.48) | 5.59 (±0.38) | 6.0 (±0.34) | 4.45 (±0.1) | 19.27 (±0.31) |
| Lin0.2 | Saf0.5 | Ses0.5 | Sun0.3 | CR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYF | CYS | CYW | CYF | CYS | CYW | CYF | CYS | CYW | CYF | CYS | CYW | CYF | CYS | CYW | ||
| Milk | Temperature (°C) | % | ||||||||||||||
| Bovine | 25 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 89 (±4.37) | 22 (±0.97) | 67 (±3.80) |
| 37 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 66 (±2.75) | 13 (±2.18) | 53 (±2.78) | |
| 50 | 42.1 ef (±3.20) | 7.4 f (±0.90) | 34.7 E (±2.50) | NA | NA | NA | 47.8 e (±2.75) | 7.9 ef (±0.51) | 36.5 E (±2.91) | 38.2 fg (±3.51) | 8.1 ef (±0.54) | 16.9 G (±1.73) | 36 g (±2.67) | 9 c (±1.01) | 27 F (±1.66) | |
| 65 | NA | NA | NA | NA | NA | NA | 35.8 (±2.46) | 7.4 (±1.54) | 28.4 (±2.92) | NA | NA | NA | NA | NA | NA | |
| 80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Buffalo | 25 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 76.1 (±4.46) | 16.4 (±1.23) | 55.6 (±4.23) |
| 37 | NA | NA | NA | 65.8 b (±4.82) | 13.6 b (±0.10) | 53.0 AB (±2.05) | 65.6 b (±4.85) | 12.9 b (±0.55) | 53.6 AB (±6.17) | 62.4 b (±2.14) | 13.0 b (±0.35) | 49.5 B (±1.85) | 73.9 a (±4.23) | 16.1 a (±1.75) | 57.7 A (±3.18) | |
| 50 | NA | NA | NA | 64.9 ef (±5.47) | 13.4 f (±0.10) | 51.6 F (±1.7) | 69.5 ef (±2.89) | 13.9 f (±0.39) | 55.5 EF (±0.72) | 65.2 f (±3.03) | 13.8 f (±0.29) | 51.4 F (±2.21) | 75.6 e (±5.01) | 16.5 e (±1.26) | 59.1 E (±4.35) | |
| 65 | NA | NA | NA | NA | NA | NA | NA | NA | NA | 44.4 j (±4.47) | 8.0 j (±0.77) | 36.9 J (±1.98) | 19.1 k (±0.25) | 4.5 k (±0.17) | 14.6 K (±5.49) | |
| 80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Goat | 25 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 70.8 a (±2.75) | 12.6 a (±0.51) | 58.2 A (±4.35) |
| 37 | 40.1 b (±2.13) | 7.5 a (±0.84) | 29.1 A (±2.52) | 39.7 a b (±0.66) | 7.5 a (±1.15) | 29.8 A (±0.78) | 44.5 a b (±0.78) | 7.8 a (±1.10) | 33.4 A (±2.75) | 45.1 a b (±2.43) | 7.9 a (±1.19) | 34.1 A (±1.71) | 53.9 a (±2.51) | 9.7 a (±0.70) | 44.2 A (±4.01) | |
| 50 | 22.5 f (±1.24) | 6.0 e (±0.15) | 16.6 EF (±1.38) | 23.8 f (±1.85) | 6.4 e (±0.62) | 17.3 EF (±0.85) | 24.4 f (±1.15) | 6.4 e (±0.65) | 18.0 EF (±1.16) | 30.2 e (±2.75) | 7.1 e (±1.34) | 21.2 E (±2.80) | 28.5 e (±0.24) | 7.4 e (±0.65) | 21.1 EF (±2.75) | |
| 65 | NA | NA | NA | NA | NA | NA | 28.6 j (±1.15) | 6.7 j (±0.83) | 21.9 J (±2.61) | NA | NA | NA | 20.2 k (±0.85) | 6.3 j (±1.12) | 13.9 K (±2.42) | |
| 80 | NA | NA | NA | NA | NA | NA | 21.1 (±1.96) | 4.3 (±0.52) | 17.7 (±1.78) | NA | NA | NA | NA | NA | NA | |
| Sheep | 25 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 84.4 (±5.23) | 23.1 (±1.70) | 60.9 (±2.45) |
| 37 | NA | NA | NA | 75.5 a (±6.02) | 13.9 b (±1.56) | 60.3 AB (±5.79) | 70.7 a (±5.23) | 12.9 b (±1.34) | 53.6 B (± 2.42) | 80.7 a (±7.62) | 15.1 b (±1.24) | 65.6 A (±5.29) | 77.3 a (±2.14) | 17.5 a (±0.32) | 59.7 AB (±2.09) | |
| 50 | NA | NA | NA | 36.0 g (±2.11) | 9.7 g (±1.02) | 26.0 H (±1.95) | 60.7 f (±3.24) | 13.9 f (±0.71) | 46.8 F (±1.24) | 57.2 f (±3.25) | 14.2 f (±1.24) | 43.0 G (±1.44) | 72.6 e (±2.54) | 15.8 e (±0.21) | 56.8 E (±1.23) | |
| 65 | NA | NA | NA | NA | NA | NA | 40.2 (±2.64) | 10.7 (±1.38) | 29.5 (±2.54) | NA | NA | NA | NA | NA | NA | |
| 80 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liburdi, K.; Cucci, S.; Esti, M. Oilseed Extracts from Local Markets as Promising Coagulant Agents for Milk from Various Mammalian Species. Foods 2022, 11, 2137. https://doi.org/10.3390/foods11142137
Liburdi K, Cucci S, Esti M. Oilseed Extracts from Local Markets as Promising Coagulant Agents for Milk from Various Mammalian Species. Foods. 2022; 11(14):2137. https://doi.org/10.3390/foods11142137
Chicago/Turabian StyleLiburdi, Katia, Sofia Cucci, and Marco Esti. 2022. "Oilseed Extracts from Local Markets as Promising Coagulant Agents for Milk from Various Mammalian Species" Foods 11, no. 14: 2137. https://doi.org/10.3390/foods11142137
APA StyleLiburdi, K., Cucci, S., & Esti, M. (2022). Oilseed Extracts from Local Markets as Promising Coagulant Agents for Milk from Various Mammalian Species. Foods, 11(14), 2137. https://doi.org/10.3390/foods11142137