Abstract
This study evaluated the effects of three polysaccharides, xanthan gum (XG), kappa-carrageenan (CA), and guar gum (GG), on the foaming and emulsifying properties of egg white liquid (EWL) and explored the intermolecular interactions and aggregation states in the initial polysaccharide–EWL complex. The results showed that the addition of XG and GG significantly improved the foaming stability of EWL on the one hand, from 66% to 78% and 69%, respectively (p < 0.05). On the other hand, the addition of XG and GG significantly improved the foam uniformity and density, and the average foam area decreased from 0.127 to 0.052 and 0.022 mm2, respectively (p < 0.05). The addition of XG and CA significantly improved the emulsification activity index (from 13.32 to 14.58 and 14.36 m2/mg, respectively, p < 0.05) and the emulsion stability index (from 50.89 to 53.62 and 52.18 min, respectively, p < 0.05), as well as the interfacial protein adsorption at the oil–water interface; it also reduced the creaming index. However, GG negatively affected these indicators. Furthermore, the electrostatic and hydrophobic interactions among molecules in EWL due to XG and the electrostatic, hydrogen bonding, and hydrophobic interactions among molecules in EWL due to CA ultimately led to the irregular aggregation of egg white proteins. Hydrophobic interactions and disulfide bonds between molecules in EWL–containing GG formed filamentous aggregations of egg white proteins. This work reveals that molecules in the polysaccharide–egg white complexes aggregate by interaction forces, which in turn have different effects on the foaming and emulsifying properties of egg white proteins.
1. Introduction
Egg white contains 9.7–10.6% protein, including ovalbumin (54%), ovomucoid (11%), ovotransferrin (12%), ovomucin (3.5%), and lysozyme (3.4%) [1]. The foaming property of egg white depends on the surface activity and film formation of these proteins. With the increasing demand for foam food, more manufacturers use bubbles as food ingredients to meet consumer demand. The performance of egg white foam directly affects the competitiveness of egg white in the market. The better the foaming of egg white protein is, the larger the cake volume obtained by the same quality of egg white liquid in the process of preparing cake [2]. The emulsifying properties are also produced by the amphiphilic nature of egg white protein. In an oil–water mixture, the protein is aligned at the oil–water interface and forms a viscoelastic interfacial film to stabilize droplets and make the emulsion even and delicate. Hydrophobic interactions are the key force in determining the adsorption performance of proteins at the oil–water interface [3]. The hydrophobic amino acids of ovalbumin account for approximately 50% of the total amino acids, which are mostly distributed inside of the spherical protein structure, and this inherent structure limits its functional characteristics [4]. Therefore, the utilization rate of eggs can be enhanced by improving the functional properties of egg white protein, which fully reflects its economic benefits.
The complexes formed by proteins and polysaccharides not only possess nutritional and health properties but also provide improved functional characteristics compared to single macromolecules. Common polysaccharides in the food industry include ionic polysaccharides and nonionic polysaccharides. Ionic polysaccharides include XG, CA, and pectin. Nonionic polysaccharides include GG, dextran, and Arabic gum. Among these, XG, CA, and GG have been widely studied in recent years due to their beneficial effects on food characteristics [5,6,7]. The molecular interaction between polysaccharides and proteins can be used to produce foods with diverse structures, appearances, textures, and functions [8]. Studies have shown that soy protein isolate and glucan under neutral conditions could form a protein–glucan graft through the Maillard reaction (glycosylation), which significantly improved the solubility, emulsifying activity index, and emulsifying stability index of soy protein [9]. However, the Maillard reaction process is difficult to control and easily produces harmful substances. Therefore, a noncovalent composite system prepared by physical mixing could provide a solution to this problem. When polysaccharides and proteins are mixed, the conformation of the protein will be changed by electrostatic attraction and hydrogen bonding between polysaccharides and proteins. These forces contribute to the complex state of biological macromolecules and directly affect the functional characteristics of egg white protein.
The objective of the present work was to investigate the effect of three polysaccharides (XG, GG, and CA) on the functional properties of egg white fluid. We also further explored the intermolecular interactions of the initial polysaccharides–egg white complex liquid to explain the molecular mechanism of different types of polysaccharides affecting the functional characteristics of protein solution.
2. Materials and Methods
2.1. Sample Collection
Eggs were obtained from the National Center for Performance Testing of Poultry (Beijing, China). XG, CA, and GG were purchased from Beijing Tianhong Shengshi Trading Co., Ltd. (Beijing, China).
2.2. Sample Preparation
Egg white liquid was collected and homogenized under a magnetic stirrer at a speed of 1200 r/min for 30 min at 25 °C. Then, 0.2% XG, CA, and GG were added to egg white liquid and placed at 4 °C for 24 h until complete hydration. The groups were named EWL (egg white liquid), EWL + XG (adding xanthan gum to egg white liquid), EWL + CA (adding kappa–carrageenan to egg white liquid), and EWL + GG (adding guar gum to egg white liquid).
2.3. Foaming Properties
Foaming ability (FA) and foaming stability (FS) were determined according to the method of Zhao et al. [10]. Egg white liquid (25 mL) was diluted four–fold using distilled water and then stirred at 12,000 r/min for 1 min using a high–speed tissue rammer (DS–1, Shanghai Precision Scientific Instrument Co., Ltd., Shanghai, China). The volume of foam (V1), total volume (V0), and foam volume after 30 min of whipping (V2) were recorded. FA and FS were calculated according to Equations (1) and (2), respectively:
2.4. Foam Microstructure
The foam microstructure was determined according to the method of Ye et al. [11]. Egg white liquid (100 mL) was stirred at 12,000 r/min for 1 min using a high–speed tissue rammer, and the volume of the foam was immediately recorded. An appropriate amount of foam was placed on the glass slide to form a thin layer and then placed under an inverted microscope (IXplore, Olympus Corporation, Tokyo, Japan) and magnified 10 times to observe the microstructure of foams.
2.5. Emulsifying Properties
Emulsifying activity index (EAI) and emulsifying stability index (ESI) were determined using the methods described by Ercelebi and Ibanoğlu [12] and Yi et al. [13]. First, 12 mL of egg white liquid and 4 mL of soybean oil (Yihai Kerry Food Marketing Co., Ltd., Shanghai, China) were homogenized at 25000 r/min for 2 min using a homogenizer (FLUKO, Shanghai fluke Technology Development Co., Ltd., Shanghai, China) to obtain the emulsion. The emulsion (50 µL) was removed from the bottom of the container and diluted with 5 mL 0.1% sodium dodecyl sulfate solution (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The diluent was measured at 500 nm by a UV–Vis spectrophotometer (Evolution 60 s, Delta Science Co., Ltd., Palmdale, CA, USA). After the emulsion was rested for 30 min, another 50 µL was taken from the bottom of the container and diluted according to the methods outlined above. The result of the emulsion formation was defined as EAI, and the result after 30 min was defined as ESI, and EAI and ESI were calculated according to Equations (3) and (4), respectively:
where T is the turbidity, A is the absorbance at 500 nm, L is the optical path length (m), φ is the oil volume fraction of the emulsion, and C is the protein concentration before the formation of the emulsion (mg/mL). The result is expressed as the surface area (m2) of the protein per unit weight (mg) used in the emulsion.
2.6. Creaming Index
Creaming index (CI) was determined by the method of Erçelebi and Ibanoğlu [12]. The emulsion (10 mL) was transferred to the test tube and stored to evaluate the stability of the emulsions. The serum layer height was measured every 30 min, and the total time was 5 h. CI was calculated according to Equation (6):
2.7. Interfacial Protein Adsorption
Interfacial protein adsorption was determined according to the method described by Li et al. [8]. The emulsion (2 mL) was put into a tube and centrifuged at 13,300× g for 45 min (D3415R, Biotool Swiss AG, Berna, Switzerland). Then the lower clear liquid was removed with a syringe, and the volume measured with a measuring cylinder. The protein concentration of aqueous phase and of liquid egg white were determined by the glycine and bicinchoninic acid protein assay kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Interfacial protein adsorption was calculated according to Equation (7):
where the total content of protein in 2 mL egg white liquid is MI (mg), and the content of protein in the lower clear liquid of emulsion is Mf (mg).
2.8. Viscosity Determination
Viscosity determination was carried out using the method described by Chang et al. [14]. Two percent NaCl or urea (Yeasen Biotech Co., Ltd., Shanghai, China) was added to each egg white liquid group and then placed on the sensing plate (C plate) of the rheometer (ARES G2, TA Instrument, New Castle, DE, USA) after hydration for 24 h. The plate spacing was 1000 nm. The excess sample at the edge of the plate was removed with a plastic scraper. The shear rate range was 0.01–1000 s–1. The relationship between the shear rate and apparent viscosity was determined. The groups were named EWL, EWL + 2% NaCl, EWL + 2% urea, EWL + polysaccharide (XG, CA, GG), EWL + polysaccharide + 2% NaCl, and EWL + polysaccharide + 2% urea.
2.9. Surface Hydrophobicity
Surface hydrophobicity was determined using the method described by Deepika et al. [15]. Egg white liquid was diluted in 0.01 mol/L phosphate buffer (pH 7.0) with a protein concentration between 0.01 and 0.2 mg/mL. Then, 4 mL of different diluent were added to 20 μL of 0.008 mol/L 8–phenylamino–1–naphthalene sulfonic acid (Shanghai Macklin Biochemical Industry Park, Shanghai, China) solution (prepared by 0.01 mol/L pH 7.2 phosphate buffer solution). After shaking and standing for 3 min, the fluorescence intensity of the samples was measured using a fluorescence spectrophotometer (FS5, Edinburgh Instruments Ltd., Edinburgh, UK) without and with 8–phenylamino–1–naphthalene sulfonic acid and recorded as FI0 and FI1. The excitation and emission wavelengths were 395 and 475 nm, respectively, and the slit correction was 5 nm. The difference between the two wavelengths was recorded as FI and was used to prepare a protein concentration curve. The surface hydrophobicity of protein was estimated by determining the slope in the initial stage of the curve.
2.10. Sulfhydryl Group Analysis
Sulfhydryl group analysis was determined using the method of Chang et al. [14]. To determine the surface sulfhydryl group content, egg white liquid was diluted to 2 mg/mL with tris glycine buffer (0.086 mol/L Tris, 0.09 mol/L glycine, 4 mmol/L ethylene diamine tetraacetic acid (EDTA), pH 8.0, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Then, 6 mL of the protein sample was added to 0.02 mL of Ellman’s test solution (0.2 g Ellman’s Reagent (DTNB) was dissolved in 50 mL buffer). The samples were allowed to react for 15 min and then centrifuged at 4000 r/min for 10 min. The absorbance of the supernatant of the samples was measured at 412 nm (SpectraMax i3x, Meigu Molecular Instrument Co., Ltd., Shanghai, China). To determine the free sulfhydryl group content, egg white liquid was prepared with tris glycine buffer (0.086 mol/L Tris, 0.09 mol/L glycine, 4 mmol/L EDTA, 8 mol/L urea, pH 8.0) with a mass concentration of 2 mg/mL. The subsequent steps were the same as those for the determination of the surface sulfhydryl group content. The sulfhydryl content was calculated according to Equation (8):
where 73.53 is obtained from 106/13,600, 13,600 is the molar extinction coefficient of Ellman’s reagent (L/(mol·cm)), A412 is the absorbance value of the sample when DTNB is added, D is the dilution factor, and C is the mass concentration of the sample (mg/mL).
2.11. Confocal Microscopy
Confocal microscopy was carried out using the method described by Kazumasa et al. [16]. The lyophilized egg white powder was dissolved in PBS buffer (Shanghai Baili Biotechnology Co., Ltd., Shanghai, China) to obtain an egg white solution with a protein concentration of 2%. The 2% fluorescein isothiocyanate (Sigma-Aldrich Co., Ltd., Shanghai, China) solution was prepared with dimethyl sulfoxide solution (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Then, 25 μL fluorescein isothiocyanate was added to 100 mL protein solution and stirred in a magnetic stirrer for 1.5 h. Then, 0.2% polysaccharide was added to the solution, and a small amount of sample was dropped onto the slide. After covering with the coverslips, they were observed with a super−resolution laser scanning confocal microscope at an emission wavelength of 485 nm (A1HD25, Nikon, Tokyo, Japan).
2.12. Statistical Analysis
Experiments of each group were conducted in triplicate. All statistical analyses were performed using SPSS 22.0 (Chicago, IL, USA). Results were considered statistically significant at p < 0.05 (Duncan’s one−way analysis), and GraphPad Prism 8.0 (GraphPad Software, SanDiego, CA, USA) was used for graphing. The number and average area of bubbles were calculated by ImageJ (National Institutes of Health, Bethesda, MD, USA).
3. Results and Discussion
3.1. Effects of Polysaccharides on the Foaming Properties of Egg White Liquid
3.1.1. Effect of Polysaccharides on FA and FS of Egg White Liquid
FA reflects the ability to add air to the protein solution, and FS represents foam volume and bubble size stability over time [10]. As shown in Figure 1, compared with the control group (EWL), FA and FS of the EWL + XG group were significantly increased (121% vs. 106%, 78% vs. 66%, p < 0.05), whereas the FA and FS of the EWL + CA group increased with no significant difference (108% vs. 106%, 67% vs. 66%). Compared with the EWL group, the FA of the EWL + GG group was significantly decreased (92% vs. 106%, p < 0.05), whereas the FS was significantly increased (69% vs. 66%, p < 0.05). The results indicate that polysaccharides significantly influence the foaming properties of EWLs.
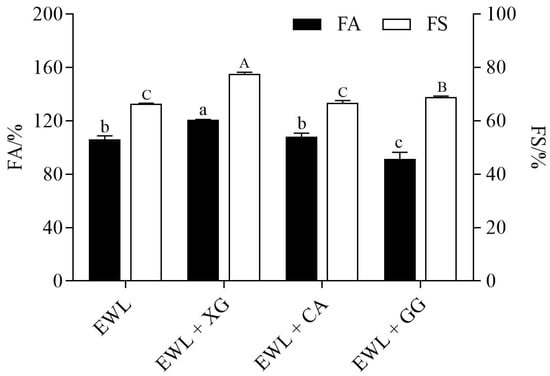
Figure 1.
Effects of polysaccharide added to egg white liquid on FA and FS. Different lowercase letters indicated significant differences (p < 0.05) between FA groups, and different uppercase letters indicated significant differences (p < 0.05) between FS groups.
While stirring EWL, egg white proteins could diffuse from the water phase and adsorb on the air–water interface because of the compatibility between their hydrophobic and gas hydrophobic regions, resulting in rapid conformational changes and rearrangements. Subsequently, a viscoelastic film was formed via intermolecular interactions [10,17]. Adding XG denatured proteins and decreased the structural order of egg white proteins because of the change in the EWL microenvironment. This is more conducive to enhancing the flexibility of diffusion and distribution of proteins at the gas–water interface and facilitates the formation of an adsorption layer, which promotes capturing and formation of bubbles [18]. Therefore, the FA of EWL + XG group was improved. However, the FA of the EWL + GG group was lower than that of the EWL group, possibly due to the reduction in the flexibility of protein molecules at the air–water interface owing to the structure of the guar polymer chain, which is described in a study of intermolecular interactions [19]. Andréa et al. [5] also found that GG negatively affects the FA of proteins.
As time passes, drainage, coalescence, and disproportionation caused the thinning of the interfacial film, and the foams commenced breakage and collapse. Drainage caused a drier foam when the liquid between bubbles seeped out under gravity, leading to liquid film rupturing between adjacent bubbles. Coalescence refers to the merging of the adjacent bubbles. Disproportionation refers to the transfer of gas from smaller to larger bubbles, resulting in the disappearance of smaller bubbles [10]. The polysaccharides were not inclined to be adsorbed to the air–water interface because of their high hydrophilicity; however, they were combined with egg white protein to cause the hydrophilic region of the protein to further protrude into the aqueous phase, which produced strong steric hindrance protection and inhibited disproportionation and coalescence of bubbles, thereby strengthening FS [17]. More importantly, XG and GG could promote intermolecular interactions to enhance the liquid viscosity, slowing the foam film rupture caused by drainage, which also contributed to FS [20]. Therefore, the FS of EWL + XG and EWL + GG groups was improved. Xie et al. [21] and Mott et al. [22] also reported that the XG solution alone did not exhibit any FA. After compounding with soybean and whey protein isolates, FA and FS of protein were improved, and the essential molecular properties of FA and FS were attributed to the polysaccharide–protein complexes.
3.1.2. Effect of Polysaccharides on Foam Structure of Egg White Liquid
The foam volume after full foaming of the EWL with polysaccharides is shown in Figure 2a. Compared to the control (EWL) group, the foam volume of the EWL + XG and EWL + GG decreased significantly (p < 0.05), whereas that of the EWL + CA group was not significantly different. The average area and number of foams are shown in Figure 2b and Table 1. The average foam area of EWL + XG and EWL + GG was significantly smaller than that of EWL and EWL + CA groups. The results indicate that the addition of the two polysaccharides (XG and GG) made the foam area smaller and denser and improved the foam structure of the EWL. In addition, the EWL + GG group had the smallest foam area, while the EWL + XG group had better foam area uniformity.
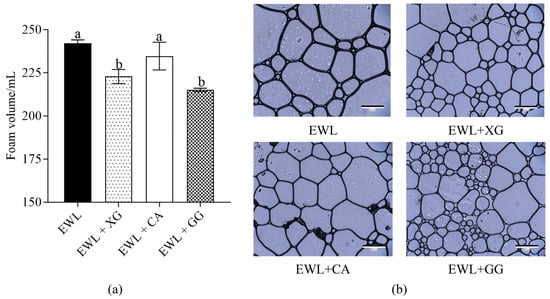
Figure 2.
Effects of polysaccharide added to egg white liquid on foam structure. (a) The foam volume after full foaming in the presence of polysaccharides of egg white liquid. Different letters indicate a significant difference (p < 0.05) between groups. (b) The microscopic images of the foam in the presence of polysaccharides of egg white liquid (10×).

Table 1.
Effects of polysaccharide added to egg white liquid on average area and quantity of foam. Different letters in the same row indicate a significant difference (p < 0.05) between groups.
FS is strongly related to foam microstructure area and distribution [23]. Ye et al. [11] and Zhao et al. [10] found that larger foams were more fragile and could easily and rapidly collapse. Previous studies have also shown that small bubbles can resist combination, expansion, or contraction [24]. In our results, the foam area of the EWL + XG and EWL + GG groups were smaller than those of the EWL + CA and EWL groups. Therefore, the EWL + XG and EWL + GG groups could enhance the stability of the bubbles to resist merging, shrinking, or expanding. Moreover, the foam of the EWL + XG group was more compact and uniform than that of the other groups and could withstand lower capillary pressure, consistent with previous studies [25]; consequently, the FA and FS of the EWL + XG group were the highest.
3.2. Effect of Polysaccharides on the Emulsifying Property of Egg White Liquid
3.2.1. Effect of Polysaccharides on Emulsifying Activity and Stability Indexes of Egg White Liquid
EAI indicates the ability of a protein to adsorb at the oil–water interface, and ESI reflects the ability of the protein to resist the aggregation and coalescence of emulsion [26]. Compared with the control group (Figure 3), the EAI (from 13.32 to 14.58 and 14.36 m2/mg, respectively) and ESI (from 50.89 to 53.62 and 52.18 min, respectively) of the EWL + XG and EWL + CA groups were significantly increased (p < 0.05), whereas the EAI (from 13.32 to 12.17 m2/mg) and ESI (from 50.89 to 39.47 min) of the EWL + GG group were significantly decreased (p < 0.05).
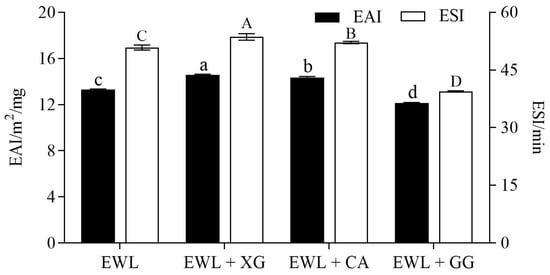
Figure 3.
Effects of polysaccharide added to egg white liquid on EAI and ESI. Different lowercase letters indicate a significant difference (p < 0.05) between EAI groups, and different uppercase letters indicate a significant difference (p < 0.05) between ESI groups.
It is necessary to use a large amount of mechanical energy provided by the homogenization process to increase the interface area and to disperse oil droplets in oil–water systems [12]. Egg white proteins can be quickly adsorbed and directionally arranged at the oil–water interface to form a facial mask [13]. XG and CA anionic groups formed more electrostatic connections with egg white proteins, which interfered with the interactions between hydrophilic residues. This would affect the tertiary structure of the protein and adjust the amphiphilic balance, which could improve the flexibility of protein molecule adsorption and arrangement at the oil–water interface, and ultimately improve the EAI of the EWL + XG and EWL + CA groups [27].
Dispersive droplets are thermodynamically unstable and influenced by coalescence, flocculation, and creaming [28]. Coalescence is the fusion of oil droplets caused by the rupture of the interface film of close oil droplets. Droplets stabilized by protein–polysaccharide complexes in the EWL + XG and EWL + CA groups tend to have greater kinetic stability by preventing coalescence owing to the high intensity of the electrostatic repulsion force [28]. Therefore, the ESI of these groups was improved.
In addition, the degree of substitution of the pyruvate group in XG (30–40%) and the sulfate group in CA (25–30%) indicated that the electrostatic effect between XG and egg white protein was stronger, and there were many binding sites, which could form more polysaccharide–protein complexes and ultimately facilitate egg white protein adsorption on the interface and exert its emulsifying properties [29]. However, the formation of polysaccharide–protein complexes may not be conducive to the surface activity of egg white proteins at the oil–water interface because of the structure of the guar polymer chain [5], leading to a reduction in the emulsifying property.
3.2.2. Effect of Polysaccharides on Adsorption of Interfacial Proteins in Emulsion
The effects of different polysaccharides on the adsorption capacity of the interfacial proteins in the emulsions are shown in Figure 4. Significant differences were observed between the experimental and control groups. Compared with the control group, the protein adsorption capacity at the oil–water interface in the EWL + XG and EWL + CA groups was significantly increased (p < 0.05), whereas the protein adsorption capacity in the EWL + GG group was significantly decreased (p < 0.05).
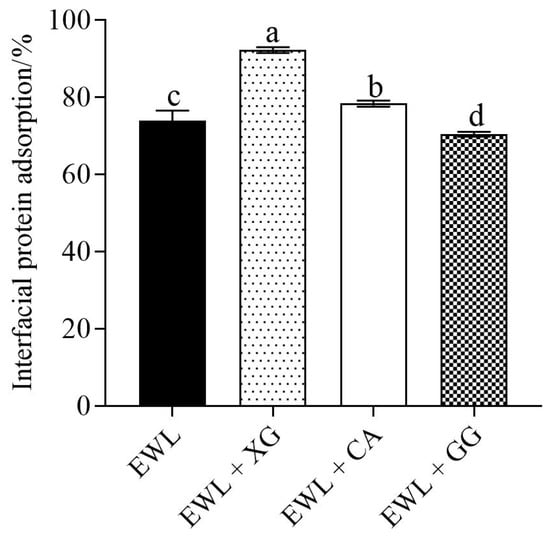
Figure 4.
Effects of polysaccharide added to egg white liquid on the interfacial protein adsorption at oil–water. Different letters indicate a significant difference (p < 0.05) between groups.
It was confirmed that XG and CA facilitated the adsorption of proteins at the oil–water interface [8]. Therefore, the EAI and ESI of the EWL + XG and EWL + CA groups improved. Flocculation of emulsions is caused by proteins unable to completely cover the droplet surface or the low interfacial film thickness, causing proteins to be adsorbed simultaneously at the surface of multiple droplets. To prevent oil droplet flocculation, the interfacial protein adsorption layer should reach a certain thickness [30]. The anionic polysaccharides, XG and CA, formed more cohesive complexes owing to the electrostatic attraction between them and the positively charged region of the protein, thereby increasing the boundary film thickness. The interfacial protein adsorption in the EWL + XG group had a higher value, with an optimal emulsifying effect. Chang et al. [31] found that the protein–pectin (negative) multilayer assembly aggregates improved emulsion stability by increasing the interface film thickness. The addition of GG leads to a decrease in interfacial protein adsorption at the oil–water interface, which directly leads to the inability of the protein to cover the droplets produced during homogenization, resulting in decreased EAI and ESI [32]. Studies have shown that the increased molecular flexibility of glycosylated proteins can promote the adsorption, swelling, and redistribution of proteins at the oil–water interface and ultimately improve the emulsification properties of proteins [33]. GG and egg white protein complexes form complexes through disulfide bonds and hydrophobic interactions, etc., which may make the flexibility of egg white protein poor and not conducive to its adsorption at the interface [34].
3.2.3. Effect of Polysaccharides on the Creaming Index of Emulsions
As shown in Figure 5, the CI of the experimental and control groups gradually increased with time. From 0 to 5 h, the CI of the EWL, EWL + GG, EWL + CA, and EWL + XG groups increased from 0% to 2.60%, 2.64%, 2.30%, and 0.84%, respectively, indicating that the EWL + XG group had the best storage stability.
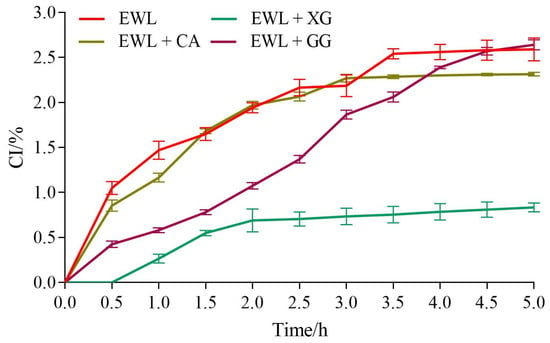
Figure 5.
The effects of polysaccharide added to egg white liquid on CI.
Among emulsion instability mechanisms, density–driven creaming is the most common phenomenon in emulsion storage and is inversely proportional to the viscosity of the liquid dispersed [31]. XG and CA improved the viscosity of the liquid to slow the continuous precipitation of the water phase in the emulsion with the thickening effect; therefore, the CI of the EWL + XG and EWL + CA groups decreased. Although GG also improved the viscosity of the liquid, its effect on the egg white protein structure limited the adsorption of proteins at the oil–water interface. Furthermore, unlike XG and CA, there was no charge repulsion effect on the surface of the droplets; thus, simply increasing the viscosity of the dispersion had no beneficial effect on CI.
3.3. Intermolecular Interaction of the Polysaccharide–Egg White Protein Complexes
3.3.1. Hydrogen Bonds and Electrostatic Interactions between Polysaccharides and Egg White Protein
Figure 6 shows the variation trend of apparent viscosity with the shear rate of EWL or the polysaccharide–EWL composite, with and without NaCl or urea. The apparent viscosity of each sample decreased with increasing shear rate, indicating the behavior of shear–thinning pseudoplastic non–Newtonian fluids. This is mainly because entangled proteins and polysaccharides are oriented along the flow direction, and these molecules are straightened, oriented, and unwrapped, resulting in shear–thinning [35].
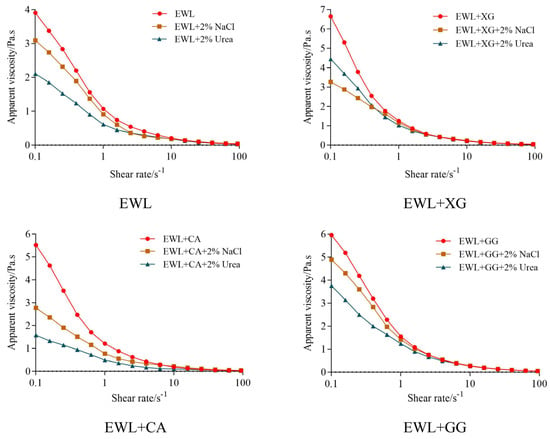
Figure 6.
Electrostatic interaction and hydrogen bonding between polysaccharides and egg white protein.
Urea and NaCl were selected as hydrogen bond–breaking and electrostatic–interfering agents to explore the effect of hydrogen bonding and electrostatic interactions on the apparent viscosity of the liquid, which could characterize the intramolecular or intermolecular interactions between polysaccharides and proteins [36,37]. With the addition of NaCl to the mixture, Cl– competed with the negative polysaccharides for the binding sites of positively charged proteins, and Na+ competed with the positively charged proteins for the binding sites of the negative polysaccharides, which shielded the electrostatic attraction between the negative polysaccharides and proteins [20]. Because of their ability to form strong hydrogen bonds, urea functional groups preferentially adsorb onto protein hydrophilic residues through hydrogen bonds, reducing the intramolecular and intermolecular interactions of proteins and polysaccharides, which leads to decreased solution viscosity [38,39]. After shielding the electrostatic and hydrogen–bond interactions, the initial apparent viscosity decreased to varying degrees because the addition of NaCl or urea blocked the intramolecular and intermolecular interactions of polysaccharides and proteins, and the phenomenon of molecular entanglement was weakened, resulting in decreased apparent viscosity [40]. After shielding the specific interaction force, the decrease in apparent viscosity indicated that compared with the EWL group, the electrostatic interaction was stronger in the EWL + XG group (3.6 Pa·s vs. 0.8 Pa·s), whereas the hydrogen bond and electrostatic interaction were stronger in the EWL + CA group (3.0 Pa·s vs. 1.8 Pa·s, 1.7 Pa·s vs. 0.8 Pa·s). The change in apparent viscosity after the addition of GG was similar to that of the EWL group.
XG and CA are polyanions in aqueous solutions because their side chains contain acidic groups, which attract egg white proteins with positive electric regions and hinder the fluidity of the protein chains [40]. CA has eight hydrogen bond donors and 25 hydrogen–bond receptors [6]. Therefore, the electrostatic interaction between XG and egg white protein was stronger, and the hydrogen bonds and electrostatic interactions between CA and egg white protein were stronger. Compared with individual proteins, amphiphilic macromolecules formed by electrostatic recombination between oppositely charged proteins and polysaccharides may change the conformation of proteins, making them more favorably adsorbed or expanded [41] and better anchored on the oil–water interface [9]. Thus, the functional properties of the EWL + XG and EWL + CA groups were enhanced.
3.3.2. Effects of Polysaccharides on the Hydrophobic Interactions between Egg White Proteins
Changes in surface hydrophobicity strongly affected the functional properties of proteins (such as solubility, interfacial characteristics, and emulsifying activity). A greater number of balanced hydrophobic groups distributed with the hydrophilic amino acids was exposed in proteins, which could improve the functional properties of the proteins [42]. As shown in Figure 7, compared with the control group, all three polysaccharides significantly reduced the surface hydrophobicity of egg white protein (p < 0.05). The reduction rates in the EWL + XG, EWL + CA and EWL + GG groups were 30.69%, 13.13%, and 16.34%, respectively.
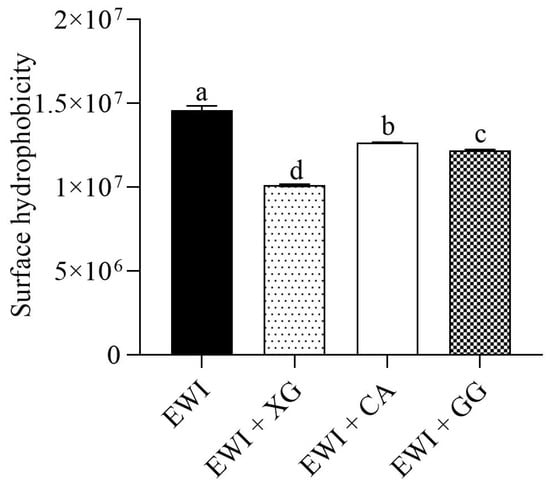
Figure 7.
Effects of polysaccharide added to egg white liquid on surface hydrophobicity. Different letters indicate significant differences (p < 0.05) between groups.
Proteins are polymorphic polymers with high structural variability [27]. Duan et al. [43] showed that the interfacial spreading ability of proteins depended on the conformation, with a certain degree of denaturation conducive to exerting the functional characteristics of the protein. The three polysaccharides changed the egg white protein environment and induced it to expose more hydrophobic regions after denaturation, which resulted in insufficient Coulomb repulsion between molecules to offset long–distance hydrophobic attraction [44] and ultimately forced hydrophobic groups to aggregate under hydrophobic interactions. Therefore, the surface hydrophobicity was reduced [45]. Khan et al. [46] and Li et al. [24] also found that the interaction between proteins and polysaccharides caused changes in protein secondary structure and aggregation, and that hydrophobic amino acids were less exposed to the protein surface in the presence of polysaccharides. However, the mechanical force in the process of stirring and homogenization destroys and disperses the aggregated and denatured proteins, causing the hydrophobic groups to be fully exposed, allowing proteins to quickly undergo the stages of diffusion, adsorption, structural rearrangement, crosslinking, and solidification to form an interfacial film of bubbles or emulsion droplets [47]. In our results, the surface hydrophobicity of the EWL + XG group significantly decreased; therefore, the hydrophobic aggregation was stronger, and the mechanical dispersion effect was easier. Consequently, the functional characteristics of EWL were significantly improved.
3.3.3. Effects of Polysaccharides on the Sulfhydryl Content of Egg White Protein
The surface sulfhydryl group is an important active group that reflects the structural changes in proteins and is closely related to their functional characteristics. XG significantly increased the surface sulfhydryl content of egg white protein by 35% (Figure 8) (p < 0.05), possibly because XG denatures more egg white protein and exposes more sulfhydryl groups. Wang et al. [48] found that the surface sulfhydryl content of rice bran protein hydrolysate increased with elevated ferulic acid and that its EAI and ESI were also improved.
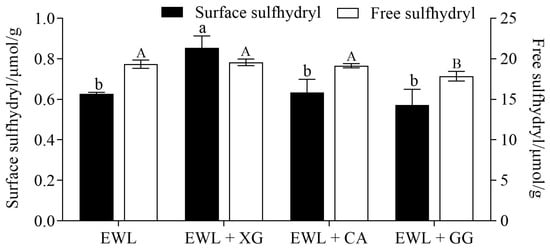
Figure 8.
Effects of polysaccharide added to egg white liquid on sulfhydryl content. Different lowercase letters show significant differences (p < 0.05) in surface sulfhydryl of egg white protein between groups, and different uppercase letters show significant differences (p < 0.05) in the free sulfhydryl of egg white protein between groups.
The change in the free sulfhydryl content reflects the breaking and formation of protein intermolecular disulfide bonds, which are related to protein denaturation and aggregation [14]. The only protein containing a free sulfhydryl group in egg whites is ovalbumin; lysozyme and ovotransferrin contain 4 and 15 disulfide bonds, respectively [49]. The addition of GG significantly reduced the free sulfhydryl group content of egg white protein from 19.34 to 17.86 μmol/g. This finding could be attributed to the fact that GG addition induces aggregation of denatured egg white protein molecules into filamentous proteins, which is related to disulfide bonding [50]. The increase in disulfide bonds led to a decrease in free sulfhydryl groups. Moreover, the molecular flexibility of the newly formed aggregates was poor, which is not conducive to forming an interfacial film [10]; thus, the FA, ESI, and EAI of the EWL + GG group were all reduced.
3.4. Effect of Polysaccharides on the Microstructure of Egg White Protein
The aggregation of protein–polysaccharide complexes significantly affects the functional characteristics of the protein so that it can be an effective emulsifier or foaming agent [51]. In Figure 9a, the green fluorescent area indicates egg white protein, and the black area indicates polysaccharides or water. In the absence of polysaccharides, egg white protein showed slight aggregation, possibly because of the electrostatic interaction between negatively charged ovalbumin and positively charged lysozyme under a pH of 8.2 [52]. With the addition of the three polysaccharides, protein aggregation increased, and the states of irregular and filamentous aggregates were observed. The EWL + CA and EWL + XG groups showed irregular aggregates, whereas the EWL + GG group showed filamentous aggregates.
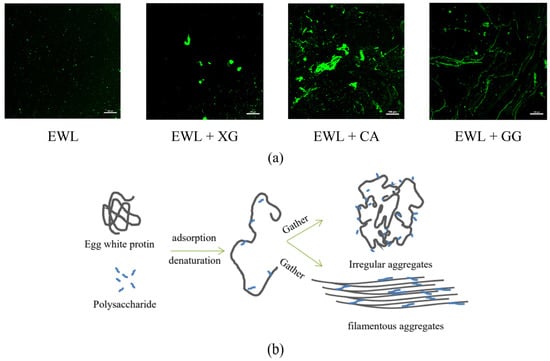
Figure 9.
(a) Effects of polysaccharide added to egg white liquid on microscopic images. (b) Electrostatic interaction and hydrogen bonding between polysaccharides and egg white protein.
Polysaccharides can be adsorbed to proteins through noncovalent interactions and cause conformational changes or unfolding of egg white protein, leading to the exposure of sequences with an aggregation tendency in the protein to bridge several protein molecules, further aggregating into larger water–soluble protein–polysaccharide complexes (Figure 9b). XG and CA assemble molecules, leading to complex aggregation; the aggregation state of the EWL + XG group is more conducive to the functional characteristics of the EWL. Mohsen and Samira [7] also found that increased spherical agglomerates enhanced protein adsorption at the interface, thus improving the emulsifying and foaming abilities. Filamentous aggregates are small oligomers or long fibrous structures of several to 100 nm formed between the side chains of denatured proteins or polypeptide chains through complex mechanisms [53]. Moderately aggregated proteins with long fibrous structures are stacked to form fibrin and filamentous aggregates [53]. This aggregation state is very rigid and not conducive to adsorption, expansion, and rearrangement at the interface [10]. Therefore, this limits the exertion of the functional characteristics of egg white proteins in the EWL + GG group.
4. Conclusions
The association between molecular interactions, aggregation states, and functional properties of polysaccharide–protein complexes was systematically investigated for the first time. Compared with adding GG and CA, adding XG has a better effect on improving the foaming and emulsifying properties of egg white liquid. The polysaccharide–egg white complex is aggregated through intermolecular interaction, which has different effects on the foaming and emulsifying properties of egg white protein. This study provides a theoretical basis and technical support for developing and utilizing special EWLs to meet the market demands of baking and other industries with strong functionality. In the future, we can develop a highly functional egg white liquid by adding polysaccharides, which can save the use cost of egg white liquid and increase the characteristics of the product.
Author Contributions
S.G. was responsible for manuscript methodology, investigation, software, data curation, writing—original draft, writing—review and editing; J.Z., J.L., and R.D. were responsible for writing—review and editing; X.S. was responsible for software and investigation; X.L. was responsible for supervision, writing—review and editing; G.X. was responsible for writing—review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the China Agriculture Research Systems (CARS-40).
Institutional Review Board Statement
The hens housing conditions were approved and conducted in accordance with the Animal Ethics Committee guidelines of China Agricultural University and national institutes of health guide for the care and use of laboratory animals. The experimental protocols and animal care in this study were approved (Approval date: 31 August 2021) by the guidelines of the Animal Care and Use Committee of China Agricultural University (AW52501202-1-1). The experiments were conducted according to the regulations and guidelines established by this committee.
Informed Consent Statement
Our research does not involve humans.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We gratefully acknowledge our colleagues at the College of Animal Science and Technology of China Agricultural University for their assistance with sample preparation.
Conflicts of Interest
We would like to declare that the work described was original research that has not been published previously and was not under consideration for publication elsewhere in whole or in part. All the authors listed have approved the manuscript that is enclosed. No conflicts of interest exist in the submission of this manuscript.
References
- Grant, M.C.; Estelle, M. Creation and characterisation of aerated food products. Trends Food Sci. Technol. 1999, 10, 283–296. [Google Scholar] [CrossRef]
- Tang, T.; Du, H.; Tang, S.; Jiang, Y.; Tu, Y.; Hu, M.; Xu, M. Effects of incorporating different kinds of peptides on the foaming properties of egg white powder. Innov. Food Sci. Emerg. Technol. 2021, 72, 102742. [Google Scholar] [CrossRef]
- Zhai, J.L.; Day, L.; Aguilar, M.I.; Wooster, T.J. Protein folding at emulsion oil/water interfaces. Curr. Opin. Colloid 2013, 18, 257–271. [Google Scholar] [CrossRef]
- Jalili–Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Andréa, A.S.; Márcia, C.T.R.V.; Valéria, P.R.M.; Luis, A.M. Ovalbumin and guar gum foam and its surface properties as influenced by sucrose and sorbitol. Int. J. Biol. Macromol. 2019, 135, 226–232. [Google Scholar] [CrossRef]
- Langendorff, V.; Cuvelier, G.; Michon, C.; Launay, B.; Parker, A.; De–Kruif, C.G. Effects of carrageenan type on the behaviour of carrageenan/milk mixtures. Food Hydrocoll. 2000, 4, 273–280. [Google Scholar] [CrossRef]
- Mohsen, D.; Samira, Y. Effect of Persian gum and Xanthan gum on foaming properties and stability of pasteurized fresh egg white foam. Food Hydrocoll. 2019, 87, 550–560. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; McClements, D.J.; Shi, M.; Shang, Q.; Liu, X.; Liu, F. Physicochemical and functional properties of lactoferrin–hyaluronic acid complexes: Effect of non–covalent and covalent interactions. LWT 2021, 151, 112121. [Google Scholar] [CrossRef]
- Axel, B.; Abraham, A.; Rachel, L.; Nissim, G. Formation and characterization of amphiphilic conjugates of whey protein isolate (WPI)/xanthan to improve surface activity. Food Hydrocoll. 2006, 21, 379–391. [Google Scholar] [CrossRef]
- Zhao, Q.; Ding, L.; Xia, M.; Huang, X.; Isobe, K.; Handa, A.; Cai, Z. Role of lysozyme on liquid egg white foaming properties: Interface behavior, physicochemical characteristics and protein structure. Food Hydrocoll. 2021, 120, 106876. [Google Scholar] [CrossRef]
- Ye, J.; Deng, L.; Wang, Y.; McClements, D.J.; Luo, S.; Liu, C. Impact of rutin on the foaming properties of soybean protein: Formation and characterization of flavonoid—protein complexes. Food Chem. 2021, 362, 130238. [Google Scholar] [CrossRef] [PubMed]
- Ercelebi, E.A.; Ibanoglu, E. Characterization of phase separation behavior, emulsion stability, rheology, and microstructure of egg white–polysaccharide mixtures. J. Food Sci. 2009, 74, C506–C512. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zheng, Q.; Pan, M.; Chiou, Y.; Li, Z.; Li, L.; Chen, Y.; Hu, J.; Duan, S.; Wei, S.; et al. Liposomal vesicles-protein interaction: Influences of iron liposomes on emulsifying properties of whey protein. Food Hydrocoll. 2019, 89, 602–612. [Google Scholar] [CrossRef]
- Chang, C.; Gao, Y.; Su, Y.; Gu, L.; Li, J.; Yang, Y. Influence of chitosan on the emulsifying properties of egg yolk hydrolysates: Study on creaming, thermal and oxidative stability. J. Sci. Food Agric. 2021, 101, 4691–4698. [Google Scholar] [CrossRef] [PubMed]
- Deepika, K.; Jiyoung, J.; Samatha, H.; Louise, W. Sugar beet pectin fractionated using isopropanol differs in galacturonic acid, protein, ferulic acid and surface hydrophobicity. Food Hydrocoll. 2016, 60, 179–185. [Google Scholar] [CrossRef]
- Kazumasa, T.; Takayuki, T.; Keiichi, N.; Kazuhiko, T. Capillary electrophoresis with a chemiluminescence detector using the two reactions of luminol and peroxyoxalate. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 1091–1101. [Google Scholar] [CrossRef]
- Salahi, M.R.; Mohebbi, M. Development of soy milk in the form of wet foam in the presences of whey protein concentrate and polysaccharides at different whipping temperatures: Study of physical, rheological and microstructural properties. LWT 2020, 137, 110444. [Google Scholar] [CrossRef]
- Joice, N.M.; Suzana, C.; Da, S.L. Egg albumin and guar gum influence on foam thixotropy. J. Texture Stud. 2009, 40, 623–636. [Google Scholar] [CrossRef]
- Miquelim, J.N.; Lannes, S.C.S.; Mezzenga, R. pH Influence on the stability of foams with protein–polysaccharide complexes at their interfaces. Food Hydrocoll. 2010, 24, 398–405. [Google Scholar] [CrossRef]
- He, W.; Xiao, N.; Zhao, Y.; Yao, Y.; Xu, M.; Du, H.; Wu, N.; Tu, Y. Effect of polysaccharides on the functional properties of egg white protein: A review. J. Food Sci. 2021, 86, 656–666. [Google Scholar] [CrossRef]
- Xie, Y.R.; Ettiarachchy, N.S.H. Effect of xanthan gum on enhancing the foaming properties of soy protein isolate. J. Am. Oil Chem. Soc. 1998, 75, 729–732. [Google Scholar] [CrossRef]
- Mott, C.L.; Hettiarachchy, N.S.; Qi, M. Effect of xanthan gum on enhancing the foaming properties of whey protein isolate. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 1383–1386. [Google Scholar] [CrossRef]
- Müller, F.N.; Windhab, E.J. Influence of process parameters on microstructure of food foam whipped in a rotor–stator device within a wide static pressure range. Colloids Surf. A 2005, 263, 353–362. [Google Scholar] [CrossRef]
- Li, C.H.; Dai, T.T.; Chen, J.; Li, X.; Li, T.; Liu, C.M.; Mcclements, D.J. Protein-polyphenol functional ingredients: The foaming properties of lactoferrin are enhanced by forming complexes with procyanidin. Food Chem. 2021, 339, 128145. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.F.; Wei, P.; Sun, L.; Jin, F.Y.; Wang, S. Experimental investigation of viscoelastic polymers for stabilizing foam. J. Ind. Eng. Chem. 2016, 47, 360–367. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Liu, Q.; Xia, X.; Chen, H. Improvement of the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process Biochem. 2016, 53, 116–124. [Google Scholar] [CrossRef]
- Varvara, M.; Annekathrin, M.; Peter, F. Mechanical properties of protein adsorption layers at the air/water and oil/water interface: A comparison in light of the thermodynamical stability of proteins. Adv. Colloid Interface Sci. 2014, 206, 195–206. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Zhao, G. Fabricating a Pickering Stabilizer from Okara Dietary Fibre Particulates by Conjugating with Soy Protein Isolate via Maillard Reaction. Foods 2020, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Casas, J.A.; Mohedano, A.F.; García, O.F. Viscosity of guar gum and xanthan/guar gum mixture solutions. J. Sci. Food Agr. 2000, 80, 1722–1727. [Google Scholar] [CrossRef]
- Dagorn, S.C.; Gueguen, J.; Lefebvre, J. Emulsifying Properties of Pea Globulins as Related to Their Adsorption Behaviors. J. Food Sci. 1987, 52, 335–341. [Google Scholar] [CrossRef]
- Chang, C.H.; Li, X.; Li, J.H.; Niu, F.; Zhang, M.Q.; Zhou, B.; Yang, Y.J. Effect of enzymatic hydrolysis on characteristics and synergistic efficiency of pectin on emulsifying properties of egg white protein. Food Hydrocoll. 2017, 65, 87–95. [Google Scholar] [CrossRef]
- Aziz, A.; Khan, N.M.; Ali, F.; Khan, Z.U.; Ahmad, S.; Jan, A.K.; Rehman, N.; Muhammad, N. Effect of protein and oil volume concentrations on emulsifying properties of acorn protein isolate. Food Chem. 2020, 324, 126894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, D.; Luo, W.; Lin, D.; Yan, J.; Liu, S.; Qin, W. Molecular structure and functional properties of glycinin conjugated to kappa-carrageenan and guar gum: A comparative study. Food Chem. 2022, 386, 132810. [Google Scholar] [CrossRef] [PubMed]
- Uruakpa, F.; Arntfield, S. Surface hydrophobicity of commercial canola proteins mixed with κ-carrageenan or guar gum. Food Chem. 2005, 95, 255–263. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, D.M.; Glazer, J. Rheological properties of gelatinized chestnut starch dispersions: Effect of concentration and temperature. J. Food Eng. 2012, 112, 94–99. [Google Scholar] [CrossRef]
- Martinez, K.D.; Baeza, R.I.; Millan, F.; Pilosof, A. Effect of limited hydrolysis of sunflower protein on the interactions with polysaccharides in foams. Food Hydrocoll. 2004, 19, 361–369. [Google Scholar] [CrossRef]
- Xie, J.; Ren, Y.; Xiao, Y.; Luo, Y.; Shen, M. Interactions between tapioca starch and Mesona chinensis polysaccharide: Effects of urea and NaCl. Food Hydrocoll. 2021, 111, 106268. [Google Scholar] [CrossRef]
- Lu, M.; Daniel, R. A Novel Carboxylesterase Derived from a Compost Metagenome Exhibiting High Stability and Activity towards High Salinity. Genes 2021, 12, 122. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Zhang, K.; Fang, Y.; Nishinari, K.; Phillips, G.O. Hydrogen bonding enhances the electrostatic complex coacervation between κ–carrageenan and gelatin. Colloids Surf. A 2015, 482, 604–610. [Google Scholar] [CrossRef]
- Yuan, C.; Zou, Y.; Cui, B.; Fang, Y.; Lu, L.; Xu, D. Influence of cyclodextrins on the gelation behavior of κ-carrageenan/konjac glucomannan composite gel. Food Hydrocoll. 2021, 120, 106927. [Google Scholar] [CrossRef]
- Baldursdottir, S.G.; Fullerton, M.S.; Nielsen, S.H.; Jorgensen, L. Adsorption of proteins at the oil/water interface--observation of protein adsorption by interfacial shear stress measurements. Colloids Surf. B 2010, 79, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Jiang, S.; Chen, Q.; Liu, Q.; Kong, B.H. Antioxidant activities and emulsifying properties of porcine plasma protein hydrolysates modified by oxidized tannic acid and oxidized chlorogenic acid. Process Biochem. 2019, 79, 101–113. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Zhang, Q.; Zhao, T.; Li, M.; Xu, X.; Liu, X. Effect of a multiple freeze-thaw process on structural and foaming properties of individual egg white proteins. Food Chem. 2017, 228, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Du, Z.; Liang, H.; Yang, Y.; Li, Q.; Wan, Z.; Yang, X. Adsorption and foaming properties of edible egg yolk peptide nanoparticles: Effect of particle aggregation. Curr. Res. Food Sci. 2021, 4, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Tang, W.J.; Xu, Z.; Wen, L.; Chen, M.L. Structure and functional properties of rice protein-dextran conjugates prepared by the Maillard reaction. Int. J. Food Sci. Technol. 2018, 53, 372–380. [Google Scholar] [CrossRef]
- Khan, N.M.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Effects of high hydrostatic pressure on secondary structure and emulsifying behavior of sweet potato protein. High Press. Res. 2015, 35, 189–202. [Google Scholar] [CrossRef]
- Mozafarpour, R.; Koocheki, A.; Milani, E.; Varidi, M. Extruded soy protein as a novel emulsifier: Structure, interfacial activity and emulsifying property. Food Hydrocoll. 2019, 93, 361–373. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhu, J.; Liu, H.; Liu, T.; Yu, G.; Shao, M. Covalent Interaction between High Hydrostatic Pressure-Pretreated Rice Bran Protein Hydrolysates and Ferulic Acid: Focus on Antioxidant Activities and Emulsifying Properties. J. Agric. Food Chem. 2021, 69, 7777–7785. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Xue, S.; Xu, X. Effects of ultrasound frequency mode on myofibrillar protein structure and emulsifying properties. Int. J. Biol. Macromol. 2020, 163, 1768–1779. [Google Scholar] [CrossRef]
- Koen, J.A.J.; Kristof, B.; Jan, A.D.; Martin, G.S. Amyloid-like aggregation of ovalbumin: Effect of disulfide reduction and other egg white proteins. Food Hydrocoll. 2016, 61, 895–902. [Google Scholar] [CrossRef]
- Zhao, M.; Hu, J.; Zhang, H.; Nishinari, K.; Fang, Y. Electrostatic complexation of beta–lactoglobulin aggregates with kappa–carrageenan and the resulting emulsifying and foaming properties. J. Dairy Sci. 2020, 103, 8709–8720. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Effects of pH, temperature and pulsed electric fields on the turbidity and protein aggregation of ovomucin-depleted egg white. Food Res. Int. 2017, 91, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, K.; Yagi, H.; Naiki, H.; Goto, Y. Heat-induced Conversion of β2-Microglobulin and Hen Egg-white Lysozyme into Amyloid Fibrils. J. Mol. Biol. 2007, 372, 981–991. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).