The Effects of Different Natural Plant Extracts on the Formation of Polycyclic Aromatic Hydrocarbons (PAHs) in Roast Duck
Abstract
:1. Introduction
2. Material and Method
2.1. Material
2.2. Design of the Experiment
2.3. Roast Duck Processing and Curing Process
2.4. Determination of Total Phenolic Content and Antioxidant Capacity of Natural Extracts
2.4.1. Total Phenol Content in Natural Extracts
2.4.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.5. The Determination of PAHs
2.5.1. Determination of the PAH Content in Roast Duck
2.5.2. The Validation of the PAH Content Determination
2.6. Data Processing
3. Results and Discussion
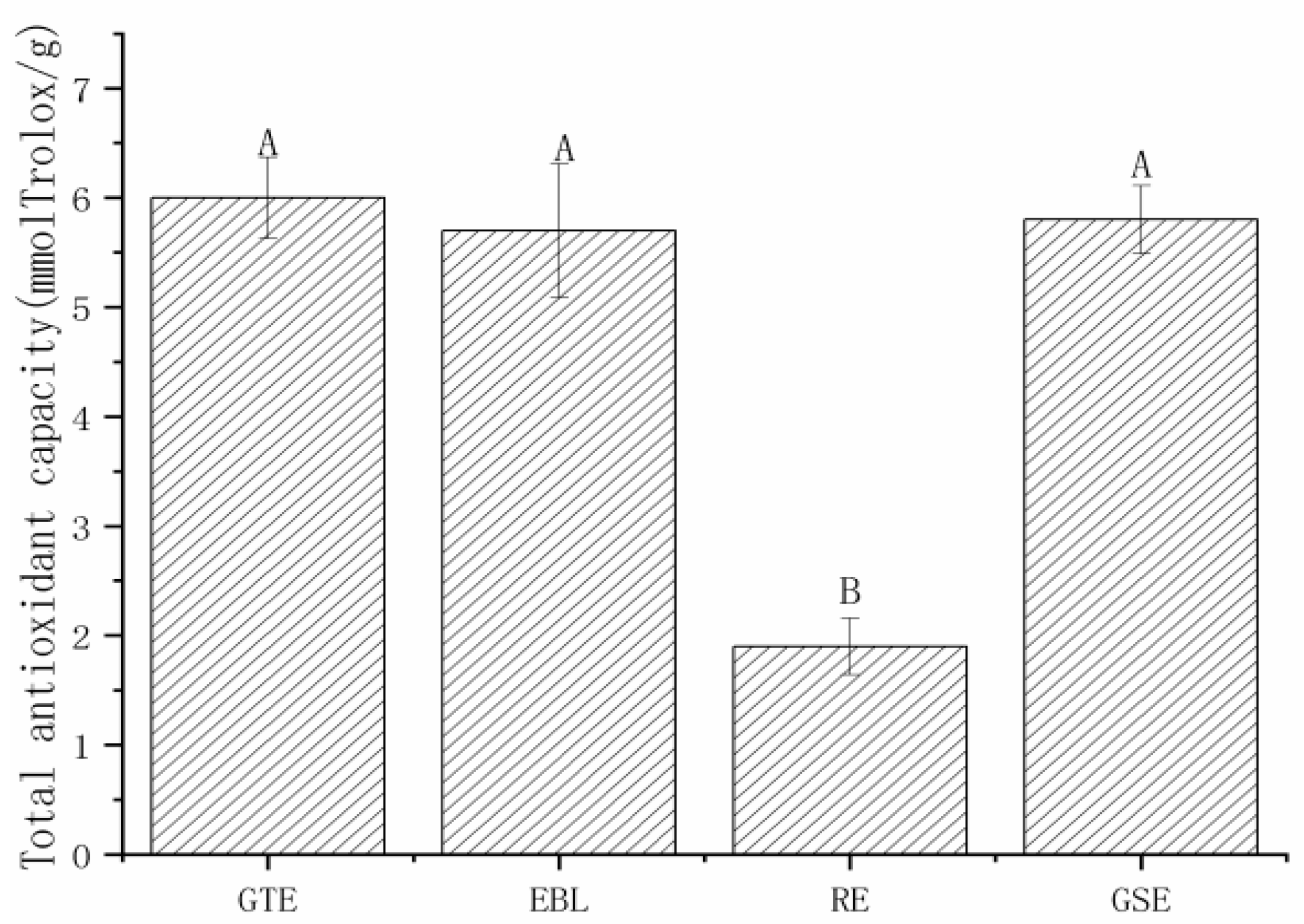
3.1. The Total Phenolic Contents and Antioxidant Capacities of the Four Natural Extracts
3.1.1. The Total Phenolic Contents of the Four Natural Extracts
3.1.2. The Antioxidant Capacities of Four Natural Extracts
3.2. The Effects of Natural Extracts on the Formation of PAHs in Roast Duck
3.2.1. Validating the Method of PAH Determination
3.2.2. The Effects of Natural Extracts on ∑16 PAH Levels
3.2.3. The Effects of Natural Extracts on the PAH4 Level
3.2.4. Effects of Natural Extracts on BaP
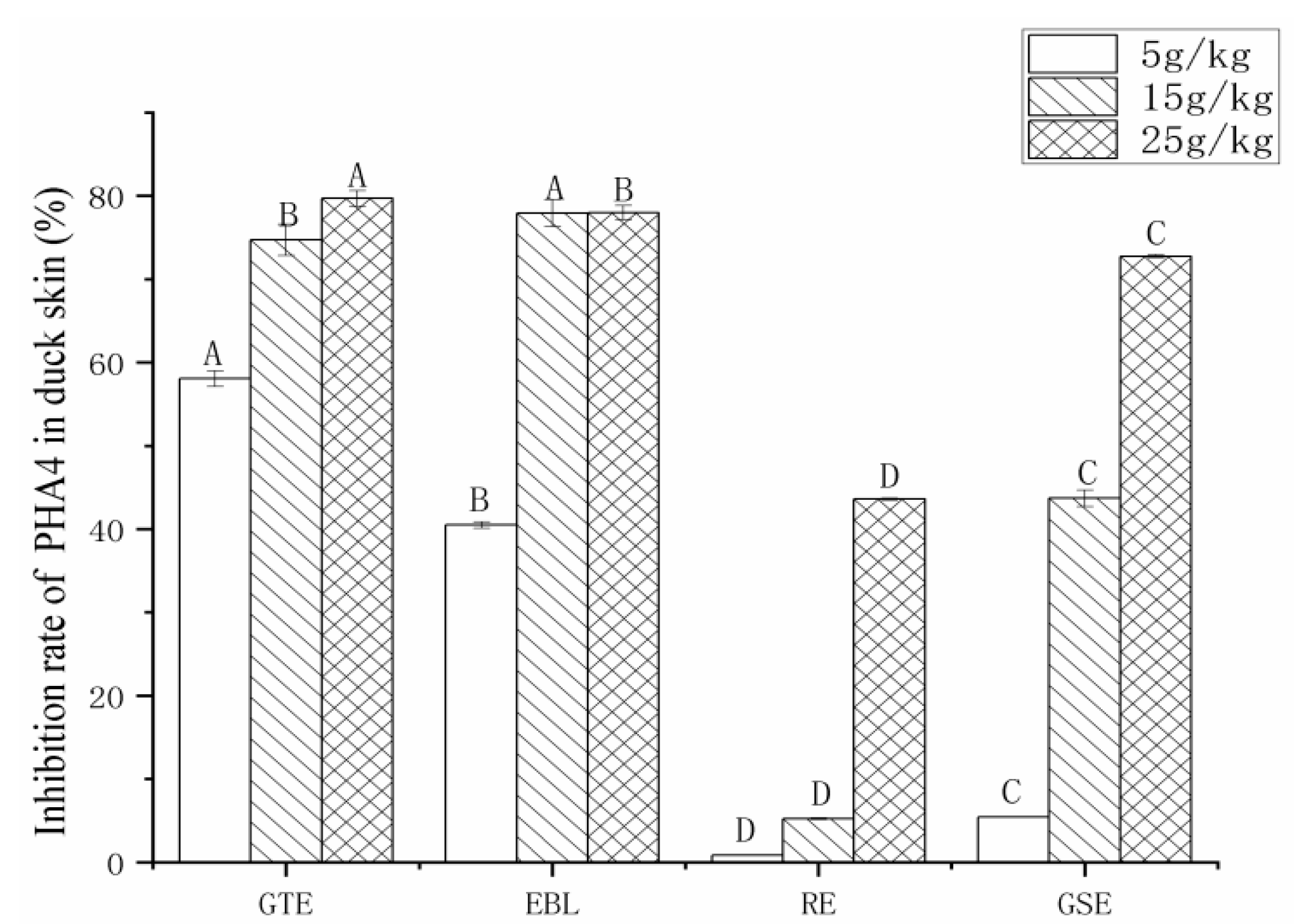
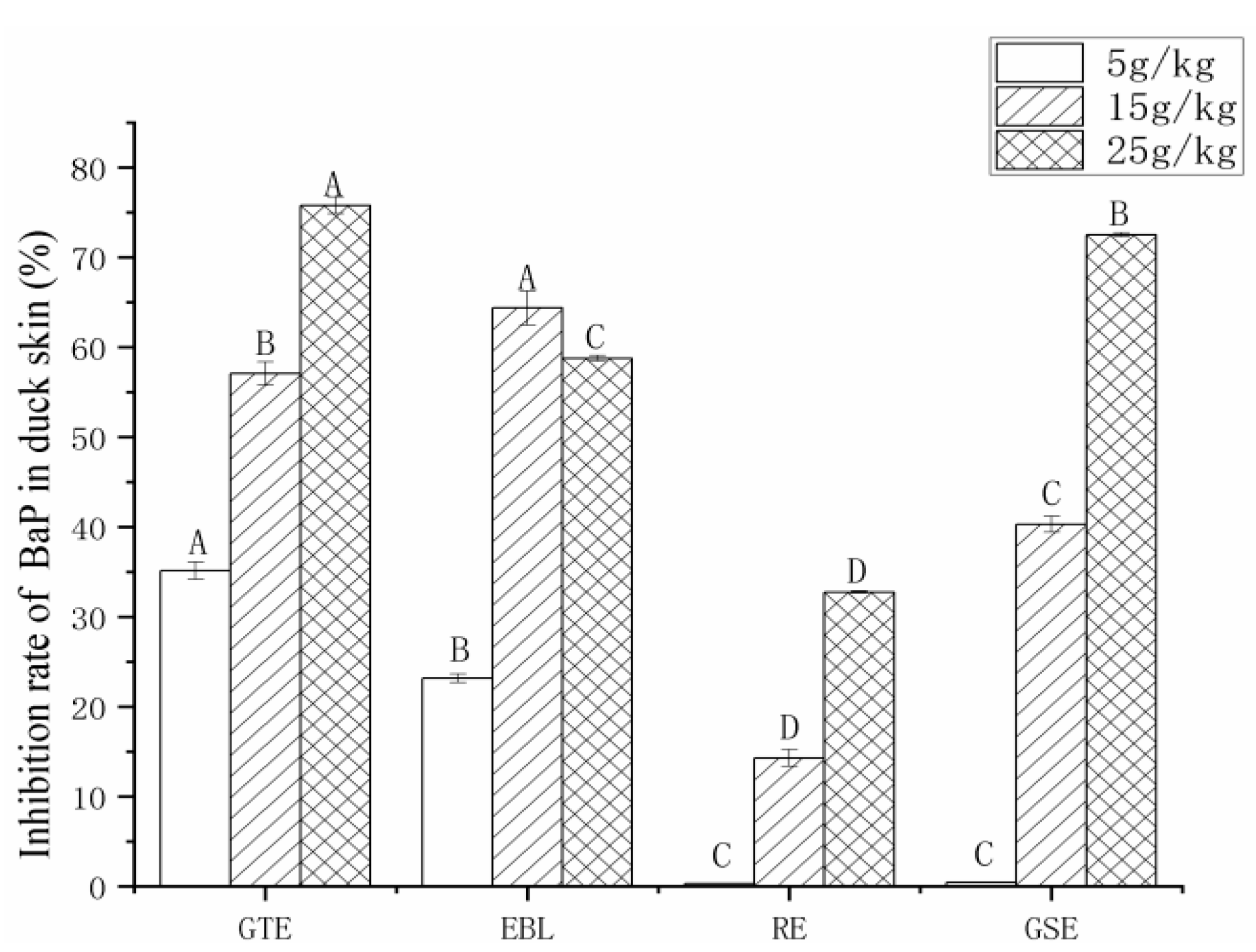
3.3. The Analysis of the PAH Inhibition Rate by Four Natural Extracts
3.3.1. The Analysis of the Inhibition Rate of the 16 PAHs
3.3.2. The Analysis of the Inhibition Rate of PAH4
3.3.3. The Analysis of the Inhibition Rate of BaP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alomirah, H.; Al-Zenki, S.; Al-Hooti, S.; Zaghloul, S.; Sawaya, W.; Ahmed, N.; Kannan, K. Concentrations and dietary exposure to polycyclic aromatic hydrocarbons (PAHs) from grilled and smoked foods. Food Control 2011, 22, 2028–2035. [Google Scholar] [CrossRef]
- Kim, K.H.; Jahan, S.A.; Kabir, E.; Brown, R.J.C. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ. Int. 2013, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Stallings-Smith, S.; Mease, A.; Johnson, T.M.; Arikawa, A.Y. Exploring the association between polycyclic aromatic hydrocarbons and diabetes among adults in the United States. Environ. Res. 2018, 166, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Hou, J.; Zhou, Y.; Hu, C.; Sun, H.; Chen, W.; Yuan, J. Dose-response relationships between polycyclic aromatic hydrocarbons exposure and platelet indices. Environ. Pollut. 2019, 245, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Afé, O.; Douny, C.; Kpoclou, Y.E.; Igout, A.; Scippo, M.L. Insight about methods used for polycyclic aromatic hydrocarbons reduction in smoked or grilled fishery and meat products for future re-engineering: A systematic review. Food Chem. Toxicol. 2020, 141, 111372. [Google Scholar] [CrossRef]
- Rozentale, I.; Zacs, D.; Bartkiene, E.; Bartkevics, V. Polycyclic aromatic hydrocarbons in traditionally smoked meat products from the Baltic states. Food Addit. Contam. Part B 2018, 11, 138–145. [Google Scholar] [CrossRef]
- Phillips, D.H. Polycyclic Aromatic Hydrocarbons in the Diet. Mutat. Res. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Kamankesh, M.; Mohammadi, A.; Hosseini, H.; Tehrani, Z.M. Rapid determination of polycyclic aromatic hydrocarbons in grilled meat using microwave-assisted extraction and dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry. Meat Sci. 2015, 103, 61–67. [Google Scholar] [CrossRef]
- Bertinetti, I.A.; Ferreira, C.D.; Fernandes Monks, J.L.; Sanches-Filho, P.J.; Elias, M.C. Accumulation of polycyclic aromatic hydrocarbons (PAHs) in rice subjected to drying with different fuels plus temperature, industrial processes and cooking. J. Food Compos. Anal. 2018, 66, 109–115. [Google Scholar] [CrossRef]
- Essumang, D.K.; Dodoo, D.K.; Adjei, J.K. Effect of smoke generation sources and smoke curing duration on the levels of polycyclic aromatic hydrocarbon (PAH) in different suites of fish. Food Chem. Toxicol. 2013, 58, 86–94. [Google Scholar] [CrossRef]
- Jahurul, M.; Jinap, S.; Zaidul, I.; Sahena, F.; Farhadian, A.; Hajeb, P. Determination of fluoranthene, benzo[b]fluoranthene and benzo[a]pyrene in meat and fish products and their intake by Malaysian. Food Biosci. 2013, 1, 73–80. [Google Scholar] [CrossRef]
- Abbas, I.; Badran, G.; Verdin, A.; Ledoux, F.; Garon, G. Polycyclic aromatic hydrocarbon derivatives in airborne particulate matter: Sources, analysis and toxicity. Environ. Chem. Lett. 2018, 16, 439–475. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, Y.N.; Zhang, J.Q.; Song, K.S.; Mu, G.Y.; Sun, C.Y.; Ju, H.Y.; Ji, M.C. The relationship of chromophoric dissolved organic matter parallel factor analysis fluorescence and polycyclic aromatic hydrocarbons in natural surface waters. Environ. Sci. Pollut. Res. 2018, 25, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Vitaglione, P.; Fogliano, V. Use of antioxidants to minimize the human health risk associated to mutagenic/carcinogenic heterocyclic amines in food. J. Chromatogr. B 2004, 802, 189–199. [Google Scholar] [CrossRef]
- Janoszka, B. HPLC-fluorescence analysis of polycyclic aromatic hydrocarbons (PAHs) in pork meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2011, 126, 1344–1353. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef]
- Gong, G.; Xue, Z.; Wu, S. Effect of natural antioxidants on inhibition of parent and oxygenated polycyclic aromatic hydrocarbons in Chinese fried bread youtiao. Food Control 2017, 87, 117–125. [Google Scholar] [CrossRef]
- Haskaraca, G.; Demirok, E.; Kolsarici, N.; Oez, F.; Oezsarac, N. Effect of green tea extract and microwave pre-cooking on the formation of heterocyclic aromatic amines in fried chicken meat products. Food Res. Int. 2014, 63, 373–381. [Google Scholar] [CrossRef]
- Viegas, O.; Yebra-Pimentel, I.; Martínez-Carballo, E.; Simal-Gandara, J.; Ferreira, I.M.P.L.V.O. Effect of beer marinades on formation of polycyclic aromatic hydrocarbons in charcoal-grilled pork. J. Agric. Food Chem. 2014, 62, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Badry, N. Effect of household cooking methods and some food additives on polycyclic aromatic hydrocarbons (PAHs) formation in chicken meat. World Appl. Sci. J. 2010, 9, 963–974. [Google Scholar]
- Jasim, K.N.; Shkhaier, S.L. Determination of benzo (a) pyrene in Iraqi Chicken, doner kebab and fish samples cooked with charcoal or gas fire. J. Fac. Med. Baghdad 2016, 58, 187–191. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, S.Y.; Moon, J.S.; Kim, S.H.; Kang, D.H.; Yoon, H.J. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Kuhnle, G.K.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Park, K.C.; Pyo, H.S.; Kim, W.S.; Yoon, K.S. Effects of cooking methods and tea marinades on the formation of benzo[a]pyrene in grilled pork belly (Samgyeopsal). Meat Sci. 2017, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, Y.; Qi, J.; Yu, Y.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Effect of Tea Marinades on the formation of polycyclic aromatic hydrocarbons in charcoal-grilled chicken wings. Food Control 2018, 93, 325–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, Z.; Shao, Z.; Yu, C.; Wang, S. Effects of Antioxidants of Bamboo Leaves and Flavonoids on 2-Amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) Formation in Chemical Model Systems. J. Agric. Food Chem. 2014, 62, 4798–4802. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, T.; Zhang, Y. Reduction of Acrylamide and Its Kinetics by Addition of Antioxidant of Bamboo Leaves (AOB) and Extract of Green Tea (EGT) in Asparagine–Glucose Microwave Heating System. J. Food Sci. 2008, 73, C60–C66. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Wu, X.; Zhang, X.; Zhang, Y. Addition of antioxidant from bamboo leaves as an effective way to reduce the formation of acrylamide in fried chicken wings. Food Addit. Contam. 2007, 24, 242–251. [Google Scholar] [CrossRef]
- Chedea, V.S.; Braicu, C.; Socaciu, C. Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem. 2010, 121, 132–139. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, H.; Ho, C.T. Effects of rosemary extracts and major constituents on lipid oxidation and soybean lipoxygenase activity. J. Am. Oil Chem. Soc. 1992, 69, 999–1002. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U. Heterocyclic Amines: 2. Inhibitory Effects of Natural Extracts on the Formation of Polar and Nonpolar Heterocyclic Amines in Cooked Beef. J. Food Sci. 2010, 70, C263–C268. [Google Scholar] [CrossRef]
- Puangsombat, K.; Smith, J.S. Inhibition of Heterocyclic Amine Formation in Beef Patties by Ethanolic Extracts of Rosemary. J. Food Sci. 2010, 75, T40–T47. [Google Scholar] [CrossRef]
- Larsson, B.K. Formation of polycyclic aromatic hydrocarbons during the smoking and grilling of food. Prog. Clin. Biol. Res. 1986, 206, 169–180. [Google Scholar]
- Chen, B.H.; Lin, Y.S. Formation of Polycyclic Aromatic Hydrocarbons during Processing of Duck Meat. J. Agric. Food Chem. 1997, 45, 1394–1403. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of Selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Re, R. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Surma, M.; Sadowska-Rociek, A.; Cielik, E. The application of d-SPE in the QuEChERS method for the determination of PAHs in food of animal origin with GC–MS detection. Eur. Food Res. Technol. 2014, 238, 1029–1036. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Chang, Y.L. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Lin, G.F.; Weigel, S.; Tang, B.; Schulz, C.; Shen, J.H. The occurrence of polycyclic aromatic hydrocarbons in Peking duck: Relevance to food safety assessment. Food Chem. 2011, 129, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moret, S.; Purcaro, G.; Conte, L.S. Polycyclic aromatic hydrocarbons (PAHs) levels in propolis and propolis-based dietary supplements from the Italian market. Food Chem. 2010, 122, 333–338. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, S.; Gong, G.; Li, G.; Zhuang, L. TBHQ and peanut skin inhibit accumulation of PAHs and oxygenated PAHs in peanuts during frying. Food Control 2017, 75, 99–107. [Google Scholar] [CrossRef]
- Union, E. Commission Regulation No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 4–8. Available online: https://www.researchgate.net/publication/284756404_Commission_Regulation_EU_No_8352011_of_19_August_2011_amending_Regulation_EC_No_18812006_as_regards_maximum_levels_for_polycyclic_aromatic_hydrocarbons_in_foodstuffs (accessed on 3 May 2022).
- Meurillon, M.; Engel, E. Mitigation strategies to reduce the impact of heterocyclic aromatic amines in proteinaceous foods. Trends Food Sci. Technol. 2016, 50, 70–84. [Google Scholar] [CrossRef]


| Compounds | Control Group | PHAs (μg/kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTE 5 g/kg | GTE 15 g/kg | GTE 25 g/kg | EBL 5 g/kg | EBL 15 g/kg | EBL 25 g/kg | RE 5 g/kg | RE 15 g/kg | RE 25 g/kg | GSE 5 g/kg | GSE 15 g/kg | GSE 25 g/kg | ||
| Nap | 24.78 ± 0.86 cd | 25.34 ± 2.61 c | 25.79 ± 2.11 bc | 21.84 ± 2.52 d | 24.69 ± 0.37 cd | 22.23 ± 0.90 d | 16.10 ± 1.63 e | 31.00 ± 1.08 a | 13.53 ± 2.61 e | 9.54 ± 0.70 f | 14.59 ± 0.54 e | 3.33 ± 1.06 g | 1.26 ± 0.47 g |
| Anl | 14.93 ± 0.17 b | nd | nd | nd | 17.83 ± 0.93 a | 8.82 ± 1.87 c | 5.20 ± 0.11 de | 5.74 ± 0.66 d | 5.70 ± 0.81 d | 1.95 ± 0.58 f | 4.91 ± 0.12 de | 4.31 ± 0.35 e | nd |
| Ane | 3.84 ± 0.43 a | 0.75 ± 0.06 ef | 0.60 ± 0.09 fg | 0.36 ± 0.07 gh | 2.38 ± 0.14 b | 1.69 ± 0.17 c | 1.62 ± 0.56 c | 1.08 ± 0.15 de | 1.45 ± 0.20 cd | nd | 1.41 ± 0.12 cd | 1.62 ± 0.05 c | nd |
| Flu | 22.64 ± 1.23 a | 10.37 ± 0.67 c | 5.87 ± 0.73 e | 10.09 ± 0.38 c | 11.91 ± 0.66 b | 8.19 ± 0.75 d | 8.84 ± 0.65 d | 4.02 ± 0.06 f | 3.84 ± 0.34 fg | 1.10 ± 0.36 i | 5.44 ± 0.54 e | 5.28 ± 0.72 e | 2.81 ± 0.24 gh |
| Phen | 91.92 ± 2.43 a | 58.01 ± 1.72 b | 57.06 ± 5.22 bc | 45.05 ± 3.61 d | 53.16 ± 1.39 c | 33.05 ± 3.45 e | 33.47 ± 3.50 e | 29.37 ± 0.64 ef | 26.38 ± 1.90 f | 14.80 ± 0.81 g | 31.75 ± 1.10 e | 29.57 ± 1.35 ef | 15.67 ± 0.96 g |
| Ant | 16.27 ± 0.72 a | 9.75 ± 0.57 c | 9.71 ± 0.58 c | 7.13 ± 0.32 de | 10.84 ± 0.52 b | 6.31 ± 0.63 ef | 6.23 ± 1.01 ef | 8.05 ± 0.07 d | 6.03 ± 0.57 fg | 3.05 ± 0.45 h | 6.81 ± 0.87 ef | 6.72 ± 0.29 ef | 5.20 ± 0.36 g |
| Flt | 31.10 ± 2.41 a | 20.73 ± 0.94 c | 20.69 ± 0.86 c | 11.62 ± 2.28 e | 27.88 ± 2.02 b | 10.62 ± 2.71 e | 6.71 ± 1.35 f | 25.54 ± 0.60 b | 19.54 ± 2.70 c | 15.18 ± 0.47 d | 18.64 ± 0.49 c | 18.67 ± 0.80 c | 11.79 ± 0.30 e |
| Pyr | 28.52 ± 1.78 a | 13.99 ± 1.80 e | 13.40 ± 1.59 e | 3.29 ± 0.65 g | 25.71 ± 0.78 b | 7.17 ± 1.02 f | 7.24 ± 0.15 f | 25.15 ± 0.43 bc | 23.29 ± 2.40 c | 17.76 ± 0.49 d | 16.56 ± 0.56 d | 16.34 ± 1.28 d | 8.74 ± 0.44 f |
| B[a]A | 9.45 ± 1.24 a | 6.13 ± 0.21 bc | 4.06 ± 0.17 de | 2.29 ± 0.50 f | 7.26 ± 0.76 b | 3.37 ± 0.93 ef | 6.77 ± 1.88 b | 9.41 ± 0.45 a | 9.39 ± 1.31 a | 5.24 ± 0.46 cd | 9.19 ± 0.28 a | 6.32 ± 0.65 bc | 3.02 ± 0.35 ef |
| Chry | 18.77 ± 0.98 a | 9.16 ± 0.67 e | 3.81 ± 0.30 g | 4.12 ± 0.42 fg | 10.56 ± 0.43 d | 2.81 ± 0.26 h | nd | 18.51 ± 0.59 a | 17.86 ± 0.32 b | 9.73 ± 0.37 e | 16.73 ± 0.25 c | 9.15 ± 0.30 e | 4.75 ± 0.37 f |
| B[b]F | 8.97 ± 0.23 a | 2.17 ± 0.24 d | 2.04 ± 0.36 d | 2.24 ± 0.43 d | 4.17 ± 0.35 c | 0.52 ± 0.34 e | nd | 8.90 ± 0.13 a | 8.67 ± 0.55 a | 5.19 ± 0.23 b | 8.88 ± 0.14 a | 5.23 ± 0.30 b | 2.34 ± 0.42 d |
| B[k]F | 9.82 ± 0.96 a | 3.35 ± 0.47 d | 2.84 ± 0.62 d | 2.76 ± 0.41 d | 4.64 ± 0.41 c | 1.86 ± 0.36 e | 1.10 ± 0.10 e | 9.98 ± 0.03 a | 7.29 ± 0.61 b | 4.82 ± 0.30 c | 7.01 ± 0.19 b | 7.34 ± 0.80 b | 4.57 ± 0.48 c |
| B[a]P | 7.42 ± 0.67 a | 1.25 ± 0.45 de | 1.37 ± 0.45 de | 0.42 ± 0.27 ef | 4.57 ± 0.47 b | 3.15 ± 0.85 c | 3.06 ± 0.25 c | 7.40 ± 0.93 a | 6.36 ± 0.71 a | 4.99 ± 0.67 b | 7.39 ± 0.52 a | 4.43 ± 0.74 b | 2.04 ± 0.42 d |
| InP | nd | nd | nd | nd | nd | nd | nd | 5.00 ± 0.02 a | 4.20 ± 0.15 c | 1.47 ± 0.28 d | 4.70 ± 0.17 b | 4.72 ± 0.34 b | nd |
| B[g,h,i]P | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| D[ah]A | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| 16 PAHs | 288.43 ± 1.82 a | 160.99 ± 3.34 c | 147.24 ± 6.70 d | 111.20 ± 3.42 f | 205.59 ± 0.98 b | 109.79 ± 5.64 f | 96.33 ± 1.70 g | 189.15 ± 0.38 b | 153.53 ± 3.87 c | 94.84 ± 1.01 g | 154.01 ± 3.73 c | 123.03 ± 5.09 e | 62.18 ± 0.95 h |
| PAH4 | 44.61 ± 1.94 a | 18.71 ± 1.29 d | 11.28 ± 0.95 ef | 9.06 ± 1.01 g | 26.55 ± 1.45 c | 9.85 ± 1.01 fg | 9.83 ± 1.69 fg | 44.22 ± 0.75 a | 42.28 ± 1.37 b | 25.16 ± 0.36 c | 42.19 ± 0.74 b | 25.12 ± 0.86 c | 12.16 ± 0.58 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Huang, X.; Tang, X.; Zhan, J.; Liu, S. The Effects of Different Natural Plant Extracts on the Formation of Polycyclic Aromatic Hydrocarbons (PAHs) in Roast Duck. Foods 2022, 11, 2104. https://doi.org/10.3390/foods11142104
Shen X, Huang X, Tang X, Zhan J, Liu S. The Effects of Different Natural Plant Extracts on the Formation of Polycyclic Aromatic Hydrocarbons (PAHs) in Roast Duck. Foods. 2022; 11(14):2104. https://doi.org/10.3390/foods11142104
Chicago/Turabian StyleShen, Xixi, Xinyuan Huang, Xiaoyan Tang, Junliang Zhan, and Suke Liu. 2022. "The Effects of Different Natural Plant Extracts on the Formation of Polycyclic Aromatic Hydrocarbons (PAHs) in Roast Duck" Foods 11, no. 14: 2104. https://doi.org/10.3390/foods11142104
APA StyleShen, X., Huang, X., Tang, X., Zhan, J., & Liu, S. (2022). The Effects of Different Natural Plant Extracts on the Formation of Polycyclic Aromatic Hydrocarbons (PAHs) in Roast Duck. Foods, 11(14), 2104. https://doi.org/10.3390/foods11142104





