Abstract
Current estimates place the amount of spent coffee grounds annually generated worldwide in the 6 million ton figure, with the sources of spent coffee grounds being classified as domestic (i.e., household), commercial (i.e., coffee houses, cafeterias and restaurants), and industrial (i.e., soluble and instant coffee industries). The majority of the produced spent coffee grounds are currently being inappropriately destined for landfills or to a form of energy recovery (e.g., incineration) as a refuse-derived fuel. The disposal of spent coffee in landfills allows for its anaerobic degradation with consequent generation and emission of aggressive greenhouse gases such as methane and CO2, and energy recovery processes must be considered an end-of-life stage in the lifecycle of spent coffee grounds, as a way of delaying CO2 emissions and of avoiding emissions of toxic organic volatile compounds generated during combustion of this type of waste. Aside from these environmental issues, an aspect that should be considered is the inappropriate disposal of a product (SCG) that presents unique thermo-mechanical properties and textural characteristics and that is rich in a diversity of classes of compounds, such as polysaccharides, proteins, phenolics, lipids and alkaloids, which could be recovered and used in a diversity of applications, including food-related ones. Therefore, researchers worldwide are invested in studying a variety of possible applications for spent coffee grounds and products thereof, including (but not limited to) biofuels, catalysts, cosmetics, composite materials, feed and food ingredients. Hence, the aim of this essay was to present a comprehensive review of the recent literature on the proposals for utilization of spent coffee grounds in food-related applications, with focus on chemical composition of spent coffee, recovery of bioactive compounds, use as food ingredients and as components in the manufacture of composite materials that can be used in food applications, such as packaging.
1. Introduction
Spent coffee grounds (SCG) are roasted and ground coffee beans that were depleted of some of their water-soluble compounds. They are the solid residues obtained after coffee beverage preparation and can be found in a variety of places including homes and commercial establishments that serve coffee [1]. Disposal of SCG is quite a problem from an environmental point of view. These residues are usually thrown directly in the trash, and thus mostly end up in landfill sites, being highly pollutant due to significant amounts of organic substances that demand great quantities of oxygen to decompose [1]. Aside from environmental implications, SCG presents an additional disposal problem, because they can be used for adulteration of roasted and ground coffee and are very difficult to detect [2,3]. Thus, the soluble coffee industry must be extra careful with its disposal, and most of the time this residue is simply used as a boiler fuel by the same industry. Many recent studies have focused on finding alternative uses for SCG, and some applications are compiled in Table 1 and illustrated in Figure 1.
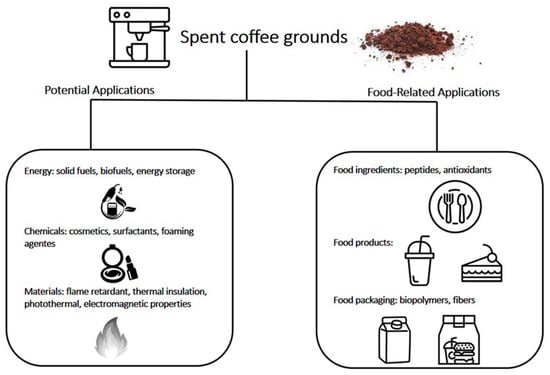
Figure 1.
Summarized illustrative overview of potential uses for spent coffee grounds.
As is quite common for agricultural wastes in general [4], the first attempts towards finding alternative uses for SCG were concentrated on applications as solid fuels, fertilizers and as supplements for animal feed [1]. Among these applications, recent studies have proposed its potential use in a sustainable biorefinery route [5]. Such an approach focuses on the integrated use of various processes such as extraction, transesterification, hydrolysis, fermentation, and pyrolysis, resulting in several fuel types, including not only solid fuel pellets, but also biogas, bio-oil, biodiesel, and bioethanol. Applications in composting and soil amendment have been proven successful [6,7,8], and SCG were shown to favor the assimilation of nutrients by plants, even though it should be used in small quantities. Although a recent study has indicated that SCG is not suitable for vermicomposting [9], another research indicated that spent coffee grounds can be viewed as a source of biochelates to obtain fortified foods [10]. The chelating treatment increased SCG Fe content 108-fold and Zn content 18,000-fold. Functionalized SCG was able to significantly increase both Fe and Zn levels in lettuces. Fe levels increased by approximately 30%, whereas Zn levels showed a more significant increase, up to 400%.
SCG has also been suggested as an alternative feed source for livestock. Earlier studies have focused on the use of SCG as a feed ingredient in ruminant rations. Early in vitro studies with livestock observed that dry matter digestibility decreased as the amount of SCG increased [11], and this observation still holds for later studies [12]. In these studies, SCG was used to replace feed ingredients that were nutritionally richer, thus reducing the energy and nutrient content of the ration. Nonetheless, a recent study evaluated the potential use of SCG at lower doses as a functional ingredient in the diet of dairy sheep [13]. Increasing doses of SCG up to 100 g/kg (feed dry matter) resulted in increases in milk yield, protein and fat content. Additionally, no differences were found in intake, apparent dry matter digestibility and feeding behavior, indicating that inclusion of SCG provided improvements in milk production and composition without impairing feeding behavior or apparent digestibility.

Table 1.
Recent proposals for alternative uses of spent coffee grounds.
Table 1.
Recent proposals for alternative uses of spent coffee grounds.
| Starting Material | Technology | Product/Application | Ref. |
|---|---|---|---|
| Energy | |||
| wood sawdust and SCG | pellet pressing | solid fuel pellets | [14] |
| SCG and larch sawdust or spruce shavings | high-pressure hydraulic briquetting press | solid fuel briquettes | [15] |
| SCG | pyrolysis | biochar/carbon cloth electrode/electricity generation and storage | [16] |
| SCG and reduced graphene oxide | vacuum-assisted impregnation | composite phase change material/solar energy storage | [17] |
| SCG | drying + oil extraction + transesterification | biodiesel | [18] |
| SCG | carbonization + CO2 activation | energy storage | [19] |
| Chemicals | |||
| SCG extract and Xanthophyllomyces dendrorhous | drying, sterilization and water extraction | astaxanthin/cosmetics, supplements and food | [20] |
| SCG extract and Millerozyma farinosa | drying, sterilization and water extraction | glutathione/medicine, food supplements and cosmetics | [21] |
| SCG | defatting + drying + hot water extraction + incubation with ammonium sulfate + centrifugation + ultra-filtration + vacuum drying | surfactants | [22] |
| SCG | solvent extraction (ultrasound, microwave or β-cyclodextrin-assisted) | phenolics | [23] |
| SCG | high-pressure temperature extraction | chlorogenic acids and caffein | [24] |
| SCG | subsequent extractions (hexane, water and ammonium sulfate) + centrifugation+ ultrafiltration + vacuum drying | foaming agent | [25] |
| Adsorbents | |||
| SCG | mixture with KOH and carbonization | biochar/ammonia removal from water | [26] |
| SCG | torrefaction | biochar/removal of diesel mixed in water | [27] |
| SCG | activation with NaOH, CaCO3 and carbonization | activated carbon/removal of methylene blue and methyl orange from water | [28] |
| SCG | no treatment | biosorbent/heavy metal (Cd) removal from aqueous solution | [29] |
| SCG | H3PO4 pyrolysis | microporous AC/removal of explosives from water | [30] |
| SCG | pyrolysis at 500 °C | biochar/removal of Norfloxacin (antibiotic) from water | [31] |
| SCG | washing with NaOH following by drying | biosorbent/recovery of dissolved metals (Fe, Al, Ca, Co, Mn, Ni, and Zn) from acid sulfate soil drainage | [32] |
| Materials | |||
| SCG and epoxy resin | mixing and curing | Flame retardant in polymer | [33] |
| SCG and Polylactide (PLA) powder | decolorization, micro- and nano-processing and extrusion | polylactic acid composite for 3D-printing | [34] |
| SCG and pectin | continuous casting | biocomposite pectin film | [35] |
| SCG and plaster | mixture with water and drying | thermal insulation material | [36] |
| SCG and hydrogels | near infra-red laser irradiation and incorporation into poly(N-isopropylacrylamide) hydrogels | photothermal materials | [37] |
| SCG | carbonization + composite mixture (cyanate ester, graphene nanoplates and epoxy resin) +hot pressing | composite with electromagnetic interference (EMI) shielding properties | [38] |
| Food products and ingredients | |||
| SCG | acid extraction followed by precipitation | bioactive peptides with antihypertensive and antioxidant potentials | [39] |
| SCG | microwave-assisted extraction of antioxidants, fermentation (Saccharomyces cerevisiae) and distillation | fermented and distilled alcoholic beverages | [40] |
| SCG | hydrolysis followed by fermentation with wine yeasts | alcoholic beverages | [41] |
| SCG | ethanolic extraction followed by addition of caramel, water, glucose syrup and vanillin | coffee-flavored liquor | [42] |
| SCG | extraction with isopropanol | antimycotic and anti-ochratoxigenic material; potential food ingredient with moderate cytotoxic and antibacterial activities | [43] |
Many applications of SCG that are of interest for the food industry are related to the fact that moderate daily consumption of coffee can be associated with positive health effects [44]. Recent studies have confirmed that both green and roasted coffee extracts present inverse correlation with several diseases such as depression, Alzheimer’s, cancer, Parkinson’s, and diabetes, among others [45,46,47,48,49,50]. Coffee also shows protective effects on various systems including nervous, skeletal, reproductive, immune, and cardiovascular [51,52,53]. These health-related benefits are due to coffee’s rich phytochemistry, which includes substances such as caffeine and other biologically active compounds predominantly belonging to the polyphenol and alkaloid classes [1,54]. These substances are present not only in coffee beans or the associated beverage, but also in the solid wastes generated during coffee preparation, e.g., spent coffee grounds. Thus, in the following section, the chemical composition of SCG will be addressed to be later associated with potential food-related applications: the recovery of bioactive substances and development of food products and polymers that can be used in food packaging.
2. Chemical Composition
A comparative overview of the chemical composition of spent coffee grounds in comparison to green and roasted coffee is summarized in Table 2. The SCG proximate composition is expected to be somewhat similar to that of roasted coffee beans because SCG represents the solid matrix that remains after water extraction of soluble roasted coffee components. Protein contents are in the range of 12 to 15 g/100 g for roasted coffees, and of 10 to 17 g for SCG [55,56], so there are no significant differences in terms of protein contents. This class of compounds remains relatively stable during roasting and is not significantly extracted during beverage preparation. Nonetheless, reported contents are based on determination of total nitrogen, being probably overestimated due to the presence of other nitrogen-containing substances (e.g., caffeine, melanoidins, Maillard reaction products). Mineral contents range from 4 to 5 g/100 g in roasted coffee and are much lower in SCG (0.1 to 1 g/100 g), due to their significant extraction during beverage preparation. Lipid contents are reported to be in the range of 15 to 20 g/100 g and of 11 to 16 g/100 g for Arabica and Robusta roasted coffees, respectively. In the case of spent coffee grounds, lipid contents are reported to be in the same range of 22 to 27 g/100 g [1]. Given that lipids are relatively preserved intact during roasting (i.e., minor losses) and are not significantly extracted during beverage preparation, the higher values observed for SCG in comparison to roasted coffees are attributed to the differences in the dry mass basis used for calculations. Results for roasted coffee are expressed on dry basis of roasted coffee, whereas SCG results are expressed on dry basis of spent coffee grounds, with the dry mass of the latter being smaller due to the loss of soluble substances during extraction of the roasted and ground beans. The fact that such an effect was not present in protein contents is attributed to the extraction of nitrogen-based substances during beverage preparation. The fatty acid composition of SCG oil is reported to be similar to that of roasted coffee, with linoleic and palmitic acids being the predominant ones, respectively, at 43–50% and 32–43% [57]. The sterol content of SCG oils was determined to be in the range of ~3.5 to 11.5 g/100 g, and both the content and composition of SCG oil varied depending on the method of extraction [57,58]. However, regardless of the extraction method, the predominant sterols were sitosterol (~5 g/100 g) and stigmasterol (~4 g/100 g) [59,60]. Considering the significant contents of sterols, Nzekoue et al. [58] deemed SCG oils to be a potential alternative source of such compounds in regard to commercially produced sterols to satisfy their increasing demand in the global market [58]. Caffeine (1,3,7-trimethyl xanthine) is probably the most frequently ingested pharmacologically active substance in the world, mainly due to the popularity of coffee and other caffeinated beverages, and it is also used as adjuvant analgesic in combination with drugs such as acetaminophen, aspirin and ibuprofen [61]. Caffeine contents range from approximately 1 to 2 mg/g in roasted coffee, but the concentration in SCG is much lower because of the extraction that occurs during beverage preparation. Caffeine levels in SCG vary considerably, ranging from 0.7 to over 40 μg/mg [62]. Such variations are not only related to species (Robusta contains twice the caffeine content of Arabica), but also to extraction conditions and the type of solvent employed. High caffeine values are usually obtained with solvents that are commonly employed for decaffeination (dichloromethane, water and CO2), thus being already established as good extractants for this compound [62,63]. Naturally, the lowest caffeine contents of SCG are observed for coffees that underwent decaffeination.

Table 2.
Comparative overview of the chemical composition of spent coffee grounds in comparison to green and roasted coffee (g/100 g dry basis).
Coffee beans are a rich source of polysaccharides, mostly cellulose, galactomannans and arabinogalactans [64]. Except for cellulose, roasting increases the polysaccharides’ solubilities because the cell wall structure loosens as the coffee beans swell and the degrees of polymerization and of branching of the hemicelluloses are significantly altered. Hence, a considerable portion of the hemicellulose polysaccharides will be removed by water extraction during coffee beverage preparation. Oligomers and monomers are rapidly converted into Maillard and pyrolysis products during roasting. Arabinoses present as side-chains in the arabinogalactan molecules are deemed more susceptible to thermal degradation than galactoses and mannoses as roasting conditions become more severe [65]. Regardless of this increase in solubility, a substantial fraction of these polysaccharides are retained as insoluble material bound to the SCG matrix [66,67]. Galactomannans were determined to comprise 50% of the polysaccharide fraction of SCGs, whereas cellulose and arabinogalactans account for the other 50% fraction in equal proportions [67,68,69]. Average monosaccharide compositions of SCG were reported in the ranges of 37 to 46% for mannose, 27 to 32% for galactose, 20 to 24% for glucose, and 7% for arabinose [56].
As previously mentioned, SCG are rich in polysaccharides, mostly cellulose and hemicellulose. The polysaccharide fraction comprises neutral detergent fibers (45.2%) and acid detergent fiber (29.8%) [70]. The total dietary fiber (TDF) content of SCG ranges from 45 to 51 g/100 g, of which 35 to 48 g/100 g are insoluble fibers and 2 to 8 g/100 g are soluble fibers [71,72]. TDF contents of SCG are in the same range of fiber-rich powders from fruit peels such as orange (~49–51 g/100 g), prickly pear (~41 g/100 g) and feijoa (~46–48 g/100 g) [73,74] but higher than some commonly used fiber sources such as rice and wheat bran (~27–45 g/100 g) [75] and some fruit peel powders including pequi (40–43 g/100 g) and mango (40–41 g/100 g) [73,76]. Spent coffee grounds have lower TDF contents in comparison to other coffee processing residues, including coffee silverskin (~62 g/100 g) [77] and coffee husks and pulp (~66 g/100 g) [78], and also buriti peels (~89 g/100 g) [79]. However, Vilela et al. [71] demonstrated that, when treated with alkaline hydrogen peroxide, total dietary fiber content of SCG increased to 70 g/100 g.
Aside from differences in technological functionalities, the insoluble (IDF) and soluble (SDF) natures of dietary fibers will also present distinct physiological activities in the human body, with IDF being associated with the ability to increase fecal bulk and decrease intestinal transit and SDF associated with increased solution viscosity and reduced glycemic response and plasma cholesterol, as well as prebiotic action [75]. IDF fibers in SCG are in the range of 80 to 90% of the total dietary fiber. IDF contents of SCG are in the same range as those of lemon (~42 g/100 g), orange (~52 g/100 g) and carrot (~34–51 g/100 g) peels and of pomegranate bagasse (~29–30 g/100 g). Insoluble dietary fiber contents of SCG are significantly higher than the contents in apple, orange, dates and tomatoes (~5–12 g/100 g), but much lower than in buriti peels (~88 g/100 g) [75,79,80]. On the other hand, SDF contents of spent coffee grounds are lower than SDF contents of agricultural residues such as carrot peel and pomegranate bagasse (~9.8–19.9 g/100 g) [75,80]. SCG dietary fibers were categorized as antioxidant dietary fibers by Murthy and Naidu [72], since they exhibited antioxidant activities similar to those of red wine products, thus, being considered a potential dietary supplement.
The antioxidant activity of food products is usually associated with the contents and types of phenolic compounds in their composition. Phenolic compounds in coffees mostly comprise chlorogenic acids (CGA), corresponding to a class of water-soluble esters in which one or two moieties of caffeic or ferulic acids are esterified to a quinic acid molecule, with the most abundant CGA being caffeoylquinic, dicaffeoylquinic, feruloylquinic, and p-coumaroylquinic acids. The antioxidant activity and nutraceutical potential of green coffees were attributed to the CGA class [81,82,83]. Although CGA contents were repeatedly demonstrated to significantly decrease during roasting [84], comparative evaluations demonstrated that aqueous solutions from green and roasted coffees presented similar antioxidant activities. A slight decrease in CGA content was observed for light roasts, followed by an increase in darker roasts, with this behavior being respectively attributed to loss of phenolic compounds during light roasting and to the successive formation of Maillard reaction or pyrolysis antioxidant products in dark roasts. Therefore, the antioxidant potential of spent coffee grounds is probably due to the combined effect of the remaining CGA as well as Maillard reaction products that were not extracted during beverage preparation.
3. Applications
Recent reports and reviews on the use of SCG and other coffee processing by-products are available [85], including applications in the fabrication of construction materials [86], cosmetics [87], recovery of bioactive substances [88], biopolymers and biocatalysts [89,90], food packaging [91], materials and energy [92], as well as multiple uses considering a biorefinery approach [5,93,94,95,96,97,98,99]. In the next sections, we will comment on the latest findings on applications of SCG that are of particular interest for the food industry.
3.1. SCG as a Source of Bioactive Compounds
As mentioned in the previous section, SCG are rich in polysaccharides, polymers that can be used as dietary fibers, and present immunostimulatory activity [62]. Therefore, a few recent studies on the extraction of polymeric sugars from SCG are available in the literature. Bhaturiwala [100] proposed a series of sequential treatments for extraction of both oligosaccharides and phenolics from SCG. The treatments consisted of roasting at 150 °C for 30 min, followed by heating with water at 90 °C and then hydrolysis of the pretreated materials (both the solid and liquid residues) with β-mannanase. The enzymatic hydrolysis was performed at 45 °C for 20 h, with the solutions later heated for 10 min at 90 °C for enzyme deactivation. Both the materials obtained from the solid and liquid residues were then lyophilized for analysis. The total amount of reducing sugars increased to 106.10 (mg glucose/g) in the combined treated materials from an initial content of 5.32 (mg glucose/g raw SCG, while the total phenolic content (estimated by Folin–Cilcateau) increased from 45.68 mg gallic acid/g in the untreated SCG up to 291.86 (mg gallic acid/g lyophilized material). FTIR analysis indicated the breakage of the β-glycosidic bonds between cellulose and hemicellulose, confirming the efficiency of the extraction procedures. Zhang et al. [101] evaluated the extraction of polyphenols and polysaccharides from SCG using water with ultrasound assistance. The authors employed central composite design to maximize the yields of both polysaccharides and polyphenols. Polyphenol and polysaccharide yields of 1.0 and 1.1% wt. were obtained under optimal conditions (1:23 solid/water ratio, 213 W ultrasound power, 30 min extraction and 68 °C extraction temperature). Wongsiridetchai and collaborators [102] characterized the mannooligosaccharides obtained from SCG and evaluated their prebiotic properties. SCGs were submitted to an alkali treatment with NaOH and then digested by mannanase from Bacillus subtilis GA2. Characterization results showed that the alkali treated SCG mainly comprised mannose, mannobiose, mannotriose, and mannopentose. The results showed that the obtained mixture of manno-oligosaccharides had prebiotic properties (promotion of lactic acid bacteria growth, tolerance under gastrointestinal conditions, and pathogen growth inhibition), confirming the potential of SCGs as a prebiotic agent.
The recovery of phenolic compounds from SCG has been extensively investigated, given the considerable attention that this class of compounds has been receiving due to their beneficial effects on human health [62]. Different extraction techniques have been employed, with the earlier studies focusing on traditional solid–liquid extraction [72,103] and lately employing other techniques such as ultrasound [104,105], subcritical water extraction [106], pressurized liquid extraction [24,107], supramolecular solvent extraction [108] and microwave-assisted extraction [109], among others, in order to improve extraction conditions.
A simple solid–liquid extraction procedure was proposed by Ramón-Gonçalves and collaborators [110] for extracting polyphenols from spent coffee grounds. Extraction conditions were optimized based on chemometric analysis, and the best conditions obtained in this study were 15 min extraction at 60 °C using a 25:75 (v/v) ethanol–water solution. Chlorogenic acid, p-coumaric acid, trans-ferulic acid, rutin, naringin and resveratrol were extracted, with variations in quantities depending on the original coffee variety. Chlorogenic and p-coumaric acids were the polyphenols found in higher amounts. Both the composition and bioactivity of ethanolic extracts obtained from spent espresso coffee grounds were investigated in a recent study [111]. SCG samples were based on pure and mixed Arabica and Robusta coffees from distinct provenances but using the same brewing parameters. The following bioactive compounds were found: α- and β-tocopherols, chlorogenic acids, 4-hydroxybenzoic acid, vanillin and tyrosol. Among the evaluated samples, the extract obtained from 100% Robusta Guatemala coffee showed the highest α- to β-tocopherol ratio of antioxidant potential, and promising anti-proliferative activity toward human lung carcinoma cells. However, the significant variability in terms of chemical composition and bioactivities found in the evaluated samples should be considered in further studies. Arauzo and collaborators [112] extracted phenolic compounds from spent coffee grounds by a hydrothermal delignification method followed by ultrasound-assisted extraction. The hydrothermal treatment was carried out with three distinct solvents: water, methanol and a water–methanol mixture. The highest extraction of total phenolic content (11.7–20.3 mg GAE/mg of dry sample) was achieved with the ultrasound-assisted extraction applied to the hydrochars using methanol as a solvent, with the mildest delignification process conditions providing the highest extraction of phenols from the hydrochars. The hydrochars were considered promising solid biofuels, with fuel ratios of 0.3 to 0.7, energy yields ranging from 83 to 95.2%, and HHV values in the range of 28.5 to 33.2 MJ/kg.
The study by Bitencourt and collaborators [113] employed supercritical extraction methods for recovery of phenolics from SCG. Extraction was performed at 333 K and 40 MPa, in one or two steps, using the following solvents: pure supercritical CO2; ethanol; and a supercritical mixture of CO2 with ethanol (90:10 w/w). The use of high pressure was important to increase the overall extraction yields. Extractions performed with ethanol as solvent presented the highest overall extraction yield and total phenolics content. Liquid–solid extraction assisted by sonication was employed by Zengin et al. [114] for obtaining SCG extracts using several solvents: H2O, MeOH, MeOH:H2O (50:50), and EtOH:H2O (70:30). The ethanolic extract was the richest in caffeine and presented the highest antioxidant activities. However, the hydroalcoholic and methanolic extracts were shown to be the most effective in terms of enzymatic inhibitory activity against acetylcholinesterase, butyrylcholinesterase, α-amylase, α-glucosidase, and tyrosinase, while the water extracts displayed lower activity. Such results confirm SCG as a potential source of bioactive metabolites with biological activity. Nonetheless, variations in solvent polarity led to differences in the biological activities of the products, confirming that extraction is a crucial step in the recovery of bioactive compounds from SCG [114].
Isopropanol extracts obtained from SCG were investigated in terms of their antimycotic and anti-ochratoxigenic potentials [43]. The substances encountered in higher amounts were caffeine and the following phenolics: (R)-(+)-rosmarinic, syringic, gallic, sinapic, salicylic, chlorogenic and caffeic acids. Apigenin-7-glucoside, naringin, epicatechin, and catechin were the predominant flavonoids. The obtained extracts showed degradation efficiency against the growth of Aspergillus strains. The SCG extracts provided detoxification in liquid media for aflatoxins (AFs) and ochratoxin A (OCA) and antifungal effect against toxigenic fungal strains, especially A. flavus and A. ochraceus. Both ethanolic and aqueous extracts were also evaluated with respect to their antibacterial, antiradical and antiproliferative potentials [115]. Although slightly higher yields were obtained with water extraction, the amount of extracted phenolics was significantly higher with ethanol. In antiproliferative assays, SCG extracts exhibited a significant decrease in viability of both lung and cervical carcinoma (C33A) cell cultures, increased apoptotic cells and promoted cell cycle arrest. The higher total phenols content and antiradical activity of SCG ethanolic extracts were thus related to their antiproliferative activity in cancer cells and their antibacterial activity against clinical isolates [115].
A recent study [116] proposed the use of a tertiary amine (N,N-dimethylcyclohexylamine, CyNMe2) to simultaneously separate SCG into three fractions: a carbohydrate fraction; a phenolic fraction; and a lipid fraction consisting mostly of palmitic and linoleic acid. Although the yields of each fraction were influenced by extraction conditions, it was observed that 24 h extractions within a temperature range of 35–25 °C led to significant yields of carbohydrates (68–83%), phenolics (479–535 mg g−1), and lipids (9.97–11.54%). This study is of particular relevance in terms of technological features, given that the employed solvent was able to produce satisfactory yields of each fraction at once.
Although most of the works on the extraction of bioactive substances from SCG have focused on the extraction of polysaccharides, polyphenols and caffeine, a few recent studies have looked at the possibility of using SCG as a source of protein and peptides [117,118]. Valdés and collaborators [117] performed a comparative evaluation of two methods for protein extraction from SCG. It was observed that a urea-based extraction buffer allowed for a more efficient extraction of proteins in comparison to the use of Tris-HCl buffers. The highest protein content was obtained from espresso SCG. Furthermore, the produced protein hydrolysates were shown to have antioxidant and ACE-inhibitory activities. The study developed by Ramirez et al. [118] focused on obtaining protein hydrolysates with potential bioactivity after fermentation of SCG using Bacillus clausii. Fermentation was able to increase the amounts of total proteins, soluble proteins, and protein hydrolysates by 2.7, 2.2, and 1.2-fold, respectively. It is worth noting that there was an increase in the amounts of seven peptides that displayed potential antioxidant capacity, angiotensin-converting enzyme activity, and dipeptidyl peptidase-IV-inhibitor activity.
3.2. SCG in Food Products
As well established in the previous sections, extracts obtained from SCG can be a rich source of antioxidants, so they could be used as alternatives to synthetic antioxidants in many food-related applications, including inhibition of lipid oxidation, and thus ethanol (with and without heating) and hot water extracts were evaluated for that purpose [119]. It was observed that both ethanol and water SCG extracts presented high antioxidant activity and DPPH radical scavenging capacity. Among the evaluated extraction methods, heated ethanol extraction (80 °C for 1 h) was the most effective for extraction of antioxidants and in preventing lipid oxidation in oil emulsion and raw meat systems. However, the method was not able to prevent oxidative changes in cooked meat packaged in oxygen permeable bags for more than 3 days. Nonetheless, the potential of SCG extracts in avoiding lipid oxidation in meat products was established, and results could probably be improved by introducing modifications in the extraction methods and/or the antioxidant delivery method.
A few studies have dealt with the use of SCG in the production of beverages. Sampaio et al. [120] employed a three-step process to produce a spirit from SCG. The steps consisted of: (i) hydrothermal treatment, (ii) fermentation of the obtained extract supplemented with sucrose to ethanol, and (iii) distillation. Coffee was the most representative aroma based on aroma analysis, and sensory evaluation results indicated that the produced beverage presented pleasant flavor, with both coffee taste and smell. A process developed by Machado [40] was based on microwave-assisted water extraction of SCG, followed by fermentation of the obtained extract with Saccharomyces cerevisiae, after being supplemented with sucrose, and then distillation of the fermented broth. Two fermented and two distilled beverages were obtained, with ethanol contents varying from approximately 10 to 40% for the fermented and distilled beverages, respectively. The substances that affected both the aroma and flavor of the produced beverages were characterized by GC-MS, with almost 60 volatile components being identified. The fermented beverages were characterized by higher alcohols, including isobutanol and isoamylic alcohol, and esters that had a positive contribution to the beverage aroma. Alcohols as major compounds and volatile acids and esters as minor compounds were most abundant in the distillate beverages, contributing to the fruity flavor and floral aroma of the drink [40]. Overall, the distillate beverages presented better sensory quality, although the authors concluded that both the fermented and distilled beverages obtained based on SCG extracts presented acceptable organoleptic qualities. A recent study evaluated the potential of producing fermented beverages from SCG extract employing non-Saccharomyces wine yeasts, namely Torulaspora delbrueckii and Pichia kluyveri [41]. An SCG hydrolysate was prepared based on a sequential acidic/enzymatic hydrolysis method, pasteurized, and later fermented. It was observed that adding the yeast extracts improved the generation of succinic and lactic acids and the production of odor-active compounds such as short-chain esters. Although the study indicated the potential of developing novel fermented SCG-based products, no sensory data were available, so at this point there is no knowledge on the organoleptic aspects of the produced beverages. Masino and collaborators [42] employed extracts prepared by mixing SCG with absolute ethanol to prepare coffee-flavored liquor mixtures with water, caramel, and glucose syrup. It was concluded that the prepared hydroalcoholic solutions could be used as a substitute for coffee extracts or flavorings in the formulation of coffee-flavored liquor.
The fact that SCG can be viewed as rich sources of fibers has prompted some studies on its use in bakery products. Martinez-Saez et al. [121] evaluated the use of SCG as a food ingredient in biscuits. The results confirmed SCG as natural sources of antioxidant insoluble fibers and essential amino acids. SCG were added to biscuit formulations together with low-calorie sweeteners and oligofructose. Results indicated that SCG could be used directly as food ingredient in biscuits (up to 4% w/w) without affecting the final nutritional or sensory quality of the product. SCG and the residue obtained after supercritical extraction of its oil content (SCGR) were used for the preparation of cookies [122]. Both SCG and SCGR were compared in terms of textural properties, bioactivity, prebiotic activity, and sensory analysis. No significant differences were observed in the probiotic viability count of both types of cookies, indicating that the polysaccharide profile did not change significantly with the oil extraction. Results from the sensory analysis indicated reasonable acceptability (score 7 out of 10) for cookies with up to 7% (w/w) SCGR or SCG in comparison to the control samples with overall acceptability of 9. Use of larger concentrations of SCGR increased the bitterness of the cookies and adversely affected both the texture and overall acceptability. Nonetheless, this study confirmed the potential of SCG as bakery ingredients. In addition, the extracted SCG oil was shown to be rich in polyphenols and presented a mild bactericidal effect, and thus could also be used as a functional ingredient in other food products.
SCG was also evaluated as a functional ingredient in sponge cakes [123]. It was observed that flour substitution with SCG (2, 4 and 6%) led to a reduction in the degree of browning, this being attributed to the lower glycemic sugar protein contents in comparison to the control sample. SCG supplementation resulted in production of sponge cakes with significantly higher volume, weight, and specific volume in comparison to the control sample. Texture profile analysis data indicated that adding SCG to the cake formulation resulted in decreases in hardness, resilience, cohesiveness, springiness, gumminess, and chewiness of the product. Addition of SCG had a deleterious effect on the organoleptic properties, although cakes prepared with 2 and 4% SCGs presented significantly higher sensory scores in comparison to cakes supplemented with 6% SCGs. The oil extracted from spent coffee grounds was used by Meerasri and Sothornvit [124] as a partial replacement for butter (10 to 30%) in cookies, with the substitution content of 20% being the limit at which cookie textural and organoleptic properties were considered acceptable by trained panelists. The partial replacement of butter by SCG oil increased both the phenolic contents and the antioxidant properties and softened the texture of the cookies.
3.3. SCG in Polymers
Polymeric materials have been extensively used as packaging materials given that they are lightweight in nature, relatively easy to produce, resistant to corrosion, and have appropriate mechanical and thermal properties, among other characteristics [125]. In the case of food products, packaging aims mainly to avoid food deterioration during several steps including production, storage, distribution, sales and even handling at home. Lately, the functions of food packaging have been extended to include increased shelf-life, waste minimization and marketing [126].
Some of the recent advances in terms of polymer-based food packaging research are associated with reducing their environmental impact by employing biodegradable polymers as alternative materials to conventional fossil fuel-based plastics. Since agricultural wastes such as SCG are a rich source of polysaccharides, a few studies have focused on the production of biopolymeric films using the polysaccharide-rich fraction of SCG as precursor material [127,128]. Galactomannans, which represent the main fraction of polysaccharides present in SCG, are high molecular weight molecules with low branching degree, comprising a backbone of (β1→4)-linked mannose residues and sporadic units of (β1→4)-linked glucose, with O-6 single (α1→6)-linked galactoses and single (α1→5)-linked arabinose residues. SCG galactomannans are insoluble in water and form highly viscous and stable aqueous solutions of ZnCl2 that have film-forming ability, thus being quite interesting raw materials for the production of edible and biodegradable films or coatings for food applications [91,127,128].
Biofilms that were predominantly composed of galactomannans were produced using SCG submitted to alkaline and enzymatic treatments [127,128]. The treatment with alkaline hydrogen peroxide was employed to remove lignin and phenolics, whereas a subsequent enzymatic treatment was used to partially remove cellulose. It was observed that interactions between the film matrix polysaccharides had a direct influence on the film properties. The authors reported that cellulose plays an important role in film formation as it influences crosslinking and, although tensile strength values were similar for films with and without enzymatic treatment, cellulose removal increased both Young’s modulus and elongation at break. Film failures during the mechanical tests were attributed to the presence of aggregates, probably of polysaccharides mediated by chlorogenic acids. Nonetheless, it was shown that the developed biofilms have extensive application potential not only in the food industry, but also in the engineering field in general.
Besides pure polymers, polymer composites have also been extensively investigated in recent years. In this context, SCG have been evaluated as fillers, aiming to improve the polymeric matrices’ mechanical, thermal, and barrier properties, and as additives that can improve specific properties such as antioxidant characteristics [125]. A compilation of recent studies that deal with the application of SCG in polymer composites is shown in Table 3. One of the major difficulties found in the addition of SCG as fillers is the poor compatibility between the filler and the polymeric matrix. Therefore, several studies employed chemical treatment of the SCG to modify the respective surface properties and improve SCG particle adhesion to distinct polymeric matrices, as shown in Table 2. Furthermore, SCG and other types of natural fibers are mainly composed of cellulose, hemicelluloses and lignin, and these polysaccharides have hydrophilic hydroxyl groups in their structures. These groups form new hydrogen bonds with water molecules present in the air, thus absorbing moisture. Pectin and waxy substances hold these water molecules, hindering free hydroxyl groups from bonding with the polar matrix. As a result, a poor interfacial bonding between the polysaccharides and matrix occurs.

Table 3.
Some recent applications of SCG as fillers in polymers.
Most of the studies dealing with SCG in polymer composites have used petroleum-based polymeric matrices. Nonetheless, to obtain a truly green composite comprising 100% renewable materials, not only the fiber but also the polymer should be bio-based [131]. With that in mind, a study by Tarazona et al. [131] demonstrated that it was possible to obtain a polymeric matrix by epoxidation of waste soybean oil and use SCG as a reinforcement to improve the composite mechanical properties. Other studies have prepared composites using pectin [35] and corn starch [135]. Although the employed polymers were of commercial grade, these can be easily recovered from food and agricultural wastes, so there is much potential in obtaining completely waste-based polymer composites using SCG and other agricultural residues.
A few studies have employed some forms of extract obtained from SCG as additives in polymer composites. In a recent study developed by da Silva et al. [138] coffee oil extracted from SCG was used as a plasticizer for development of a composite based on cellulose obtained from recycled paper coffee cups. The paper cups were milled, modified with lactic acid, and then mixed with poly(lactic acid) (PLA). The addition of coffee oil improved both the flexibility and processibility of the resulting polymer. The aqueous extract obtained from spent coffee grounds (after water extraction at 100 °C for 5 min) was employed together with citric acid as an additive in polyvinyl alcohol (PVA)/starch films [139]. The incorporation of SCG extract improved antioxidant release and antimicrobial activity, while showing a synergistic effect with citric acid on film antibacterial activity. Although there was a slight decrease in tensile strength with the addition of citric acid, the produced films were shown to be adequate for the development of new green antimicrobial materials for application in food packaging. SCG extracts were also used as additives in a poly(lactic acid) (PLA) polymer matrix using diatomaceous earth as reinforcing filler [140]. Improvements in both mechanical and oxygen barrier properties were observed for the polymers characterized by the co-presence of diatomite and SCG extract, indicating a possible synergistic effect of the two additives. Getashew et al. [141] developed packaging films using gelatin extracted from fish skin impregnated with SCG extracts obtained by subcritical water extraction. The films presented high antioxidant and antimicrobial activities against several food poisoning bacteria, including Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes and Escherichia coli. These results confirmed the potential of using SCG and other types of agricultural and food wastes in the production of active food packaging.
Spent coffee grounds were incorporated at 20% wt. into PLA by extrusion to develop a green composite [136]. Two oligomers of lactic acid, one not functionalized and the other functionalized with maleic anhydride, were mixed with SCG during an extrusion process, resulting in composites with ductility increased by approximately 280%, which was attributed to the high lipid content of SCG. Increased tensile strength was also observed, with the functionalized PLA oligomer contributing to an increased strength of about 60% due to increased chemical interactions between the biopolyester and the SCG fillers promoted by the maleic anhydride functional groups. The resultant composites were deemed promising alternatives for the production of disposable food-serving utensils and tableware. Papadaki and co-workers [142] investigated the incorporation of an antioxidant-rich extract obtained from spent coffee grounds into whey protein edible films with the aim of improving the functional properties of the films. The incorporated SCG extract promoted an increase in both the tensile strength and the elastic modulus of the whey protein films. The inclusion of SCG phenolics into the protein films improved their UV-light resistance and increased the antioxidant properties of the films. The antioxidant capacity of films remained high after a 12-month storage period at 5 °C, decreasing only 16.8% in relation to its original activity. The supplemented films were thus considered promising active packaging material for long-term storage of foods with lipid oxidative susceptibility.
Recent comprehensive reviews are available in the scientific literature on the topic of composite materials produced with spent coffee grounds and other coffee by-products that could be used for food applications [91,92,143].
4. Conclusions and Future Perspectives
Spent coffee grounds recently have been extensively researched as precursor material for a variety of value-added applications, including biofuels, catalysts, cosmetics, composites, and food ingredients. Spent coffee grounds are rich in a diversity of classes of compounds, such as polysaccharides, proteins, phenolics, lipids and alkaloids, and as such, are suitable candidates for applications in food-related areas. An extensive body of work has been recently published on food-related applications of SCG with a focus on recovery of food-grade bioactive compounds (e.g., chlorogenic acids and its derivatives, and caffeine) that can be further used in food formulations, food ingredients (e.g., fiber), and precursors for composite materials for food packaging (e.g., biopolymeric films and composite fillers). The repurposing, recycling, and upcycling of SCG, avoiding or delaying its disposal in landfills or use as refuse-derived fuel, not only minimize environmentally negative impacts in the coffee production and consumption chain but also allow for an extended service lifecycle of coffee. Increased revenues would also be another advantage of extending the coffee service lifecycle if the soluble and instant coffee industries could themselves further process SCG into value-added food-related products, employing the concepts of biorefinery and circular economy. Most of the current research has been focused on developing a single product out of SCG, and a handful of published works have presented possible scenarios for application of biorefinery concepts to coffee by-products. However, with the development and use of innovative technologies (e.g., ultrasound, microwave and others) for extraction, fractionation and purification of compounds from spent coffee grounds, a wider spectrum of applications can be envisioned for SCG-derived products. Future investigations should consider detoxification and subsequent hydrolysis of SCG compounds, thus allowing the application of a diversity of biotechnological processes for the conversion of SCG into food-grade ingredients, alleviating the need to use actual agricultural and food products for such endeavors.
Author Contributions
Conceptualization, A.S.F. and L.S.O.; investigation, A.S.F. and L.S.O.; writing—original draft preparation A.S.F. and L.S.O.; writing—review and editing A.S.F. and L.S.O.; funding acquisition, A.S.F. All authors have read and agreed to the published version of the manuscript.
Funding
A.S.F. and L.S.O. acknowledge financial support from the Brazilian National Council for Scientific and Technological Development, CNPq (Grants 310456/2021-5; 310575/2020-6). A.S.F. acknowledges financial support from Minas Gerais State Agency for Research and Development, FAPEMIG (Grant # APQ-01037-21).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Franca, A.S.; Oliveira, L.S. Coffee. In Integrated Processing Technologies for Food and Agricultural By-Products; Pan, Z., Zhang, R., Zicari, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–438. [Google Scholar] [CrossRef]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Performance of diffuse reflectance infrared Fourier transform spectroscopy and chemometrics for detection of multiple adulterants in roasted and ground coffee. LWT 2013, 53, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Reis, N.; Botelho, B.G.; Franca, A.S.; Oliveira, L.S. Simultaneous detection of multiple adulterants in ground roasted coffee by ATR-FTIR spectroscopy and data fusion. Food Anal. Methods 2017, 10, 2700–2709. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Sharma, N.; Bhatia, S.; Verma, A.; Soni, S.; Batra, N. Insights on sustainable approaches for production and applications of value added products. Chemosphere 2022, 286, 131623. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Kumar, M.D.; Preethi; Atabani, A.E.; Kumar, G. Biorefinery of spent coffee grounds waste: Viable pathway towards circular bioeconomy. Bioresour. Technol. 2020, 302, 122821. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Morais, S.; Mendes, E.; Pereira, J.A.; Baptista, P.; Casal, S. Improvement of vegetables elemental quality by espresso coffee residues. Food Chem. 2014, 148, 294–299. [Google Scholar] [CrossRef]
- Cruz, R.; Mendes, E.; Torrinha, Á.; Morais, S.; Pereira, J.A.; Baptista, P.; Casal, S. Revalorization of spent coffee residues by a direct agronomic approach. Food Res. Int. 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Cruz, R.; Gomes, T.; Ferreira, A.; Mendes, E.; Baptista, P.; Cunha, S.; Pereira, J.A.; Ramalhosa, E.; Casal, S. Antioxidant activity and bioactive compounds of lettuce improved by espresso coffee residues. Food Chem. 2014, 145, 95–101. [Google Scholar] [CrossRef]
- Hanc, A.; Hrebeckova, T.; Grasserova, A.; Cajthaml, T. Conversion of spent coffee grounds into vermicompost. Bioresour. Technol. 2021, 341, 125925. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.Á. Spent coffee grounds as a source of smart biochelates to increase Fe and Zn levels in lettuces. J. Clean. Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Sikka, S.S.; Bakshi, M.P.; Ichhponani, J.S. Evaluation in vitro of spent coffee grounds as a livestock feed. Agric. Wastes 1985, 13, 315–317. [Google Scholar] [CrossRef]
- Xu, C.C.; Cai, Y.; Zhang, J.G.; Ogawa, M. Fermentation quality and nutritive value of a total mixed ration silage containing coffee grounds at ten or twenty percent of dry matter. J. Anim. Sci. 2007, 85, 1024–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Otálora, X.D.; Ruiz, R.; Goiri, I.; Rey, J.; Atxaerandio, R.; Martin, D.S.; Orive, M.; Iñarra, B.; Zufia, J.; Urkiza, J.; et al. Valorisation of spent coffee grounds as functional feed ingredient improves productive performance of Latxa dairy ewes. Anim. Feed Sci. Technol. 2020, 264, 114461. [Google Scholar] [CrossRef]
- Nosek, R.; Tun, M.M.; Juchelkova, D. Energy utilization of spent coffee grounds in the form of pellets. Energies 2020, 13, 1235. [Google Scholar] [CrossRef] [Green Version]
- Brunerová, A.; Roubík, H.; Brožek, M.; Haryanto, A.; Hasanudin, U.; Iryani, D.; Herák, D. Valorization of bio-briquette fuel by using spent coffee ground as an external additive. Energies 2020, 13, 54. [Google Scholar] [CrossRef] [Green Version]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Hu, X.; Huang, H.; Hu, Y.; Lu, X.; Qin, Y. Novel bio-based composite phase change materials with reduced graphene oxide-functionalized spent coffee grounds for efficient solar-to-thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 219, 110790. [Google Scholar] [CrossRef]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental impacts of biodiesel production from waste spent coffee grounds and its implementation in a compression ignition engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Wang, H.-L.; Yu, G.-T.; Huang, G.-M.; Lin, J.-H. Meso-pore dominant activated carbon from spent coffee grounds for high-performance electrochemical capacitors in organic electrolyte. J. Environ. Chem. Eng. 2021, 9, 106418. [Google Scholar] [CrossRef]
- Hirono-Hara, Y.; Mizutani, Y.; Murofushi, K.; Iwahara, K.; Sakuragawa, S.; Kikukawa, H.; Hara, K.Y. Effect of spent coffee grounds extract on astaxanthin production by Xanthophyllomyces dendrorhous. Bioresour. Technol. Rep. 2022, 17, 100953. [Google Scholar] [CrossRef]
- Hirono-Hara, Y.; Mizutani, Y.; Murofushi, K.; Iwahara, K.; Sakuragawa, S.; Kikukawa, H.; Hara, K.Y. Glutathione fermentation by Millerozyma farinosa using spent coffee grounds extract and seawater. Bioresour. Technol. Rep. 2021, 15, 100777. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Insights into the composition, structure-function relationship, and molecular organization of surfactants from spent coffee grounds. Food Hydrocoll. 2022, 124, 107204. [Google Scholar] [CrossRef]
- Solomakou, N.; Loukri, A.; Tsafrakidou, P.; Michaelidou, A.-M.; Mourtzinos, I.; Goula, A.M. Recovery of phenolic compounds from spent coffee grounds through optimized extraction processes. Sustain. Chem. Pharm. 2022, 25, 100592. [Google Scholar] [CrossRef]
- da Silva, M.F.; Pettinato, M.; Casazza, A.A.; Maciel, M.I.; Perego, P. Design and evaluation of non-conventional extraction for bioactive compounds recovery from spent coffee (Coffea arabica L.) grounds. Chem. Eng. Res. Des. 2022, 177, 418–430. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Foaming agents from spent coffee grounds: A mechanistic understanding of the modes of foaming and the role of coffee oil as antifoam. Food Hydrocoll. 2021, 112, 106354. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Vo, T.-D.-H.; Tran, T.; Nguyen, T.-N.; Le, T.-N.-C.; Bui, X.-T.; Bach, L.-G. Biochar derived from the spent coffee ground for ammonium adsorption from aqueous solution. Case Stud. Chem. Environ. Eng. 2021, 4, 100141. [Google Scholar] [CrossRef]
- Lee, K.-T.; Cheng, C.-L.; Lee, D.-S.; Chen, W.-H.; Vo, D.-V.N.; Ding, L.; Lam, S.S. Spent coffee grounds biochar from torrefaction as a potential adsorbent for spilled diesel oil recovery and as an alternative fuel. Energy 2022, 239, 122467. [Google Scholar] [CrossRef]
- Block, I.; Günter, C.; Rodrigues, A.D.; Paasch, S.; Hesemann, P.; Taubert, A. Carbon adsorbents from spent coffee for removal of methylene blue and methyl orange from water. Materials 2021, 14, 3996. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, J.-G. Adsorption characteristics of spent coffee grounds as an alternative adsorbent for cadmium in solution. Environments 2020, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Charmas, B.; Zięzio, M.; Tomaszewski, W.; Kucio, K. Smart preparation of microporous carbons from spent coffee grounds. Comprehensive characterization and application in explosives removal from water samples. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128889. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Vo, T.-D.-H.; Nguyen, T.-B.; Dat, N.D.; Huu, B.T.; Nguyen, X.-C.; Tran, T.; Le, T.-N.-C.; Duong, T.-G.-H.; Bui, M.-H.; et al. Adsorption of norfloxacin from aqueous solution on biochar derived from spent coffee ground: Master variables and response surface method optimized adsorption process. Chemosphere 2022, 288, 132577. [Google Scholar] [CrossRef]
- Shahabi-Ghahfarokhi, S.; Rahmati-Abkenar, M.; Matson, J.G.; Karimi, H.; Yu, C.; Hogland, W.; Klavinš, M.; Ketzer, M. Removal and potential recovery of dissolved metals from acid sulfate soil drainage by spent coffee-grounds and dissolved organic carbon. Environ. Adv. 2022, 8, 100193. [Google Scholar] [CrossRef]
- Vahabi, H.; Jouyandeh, M.; Parpaite, T.; Saeb, M.R.; Ramakrishna, S. Coffee Wastes as Sustainable Flame Retardants for Polymer Materials. Coatings 2021, 11, 1021. [Google Scholar] [CrossRef]
- Li, S.; Shi, C.; Sun, S.; Chan, H.; Lu, H.; Nilghaz, A.; Tian, J.; Cao, R. From brown to colored: Polylactic acid composite with micro/nano-structured white spent coffee grounds for three-dimensional printing. Int. J. Biol. Macromol. 2021, 174, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Mendes, J.F.; Martins, J.T.; Manrich, A.; Neto, A.R.; Pinheiro, A.C.; Mattoso, L.H.; Martins, M.A. Development and physical-chemical properties of pectin film reinforced with spent coffee grounds by continuous casting. Carbohydr. Polym. 2019, 210, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lachheb, A.; Allouhi, A.; Marhoune, M.E.; Saadani, R.; Kousksou, T.; Jamil, A.; Rahmoune, M.; Oussouaddi, O. Thermal insulation improvement in construction materials by adding spent coffee grounds: An experimental and simulation study. J. Clean. Prod. 2019, 209, 1411–1419. [Google Scholar] [CrossRef]
- Chen, X.-E.; Mangindaan, D.; Chien, H.-W. Green sustainable photothermal materials by spent coffee grounds. J. Taiwan Inst. Chem. Eng. 2022, 346, 104259. [Google Scholar] [CrossRef]
- Guo, Z.; Ren, P.; Zhang, Z.; Dai, Z.; Lu, Z.; Jin, Y.; Ren, F. Fabrication of carbonized spent coffee grounds/graphene nanoplates/cyanate ester composites for superior and highly absorbed electromagnetic interference shielding performance. J. Mater. Sci. Technol. 2022, 102, 123–131. [Google Scholar] [CrossRef]
- Ribeiro, E.; de Souza Rocha, T.; Prudencio, S.H. Potential of green and roasted coffee beans and spent coffee grounds to provide bioactive peptides. Food Chem. 2021, 348, 129061. [Google Scholar] [CrossRef]
- Machado, E.; Mussatto, S.; Teixeira, J.; Vilanova, M.; Oliveira, J. Increasing the sustainability of the coffee agro-industry: Spent coffee grounds as a source of new beverages. Beverages 2018, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Lu, Y.; Liu, S.Q. The potential of spent coffee grounds hydrolysates fermented with Torulaspora delbrueckii and Pichia kluyveri for developing an alcoholic beverage: The yeasts growth and chemical compounds modulation by yeast extracts. Curr. Res. Food Sci. 2021, 4, 489–498. [Google Scholar] [CrossRef]
- Masino, F.; Montevecchi, G.; Calvini, R.; Foca, G.; Antonelli, A. Sensory evaluation and mixture design assessment of coffee-flavored liquor obtained from spent coffee grounds. Food Qual. Prefer. 2022, 96, 104427. [Google Scholar] [CrossRef]
- Badr, A.N.; El-Attar, M.M.; Ali, H.S.; Elkhadragy, M.F.; Yehia, H.M.; Farouk, A. Spent coffee grounds valorization as bioactive phenolic source acquired antifungal, anti-mycotoxigenic, and anti-cytotoxic activities. Toxins 2022, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.-H.; Park, J.-H.; Im, S.-S.; Song, D.-K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costabile, A.; Sarnsamak, K.; Hauge-Evans, A.C. Coffee, type 2 diabetes and pancreatic islet function—A mini-review. J. Funct. Foods 2018, 45, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, G.; Mirshekar, M.A.; Amirpour, M.; Montazerifar, F.; Miri, S.; Shourestani, S. Sub-chronic administration of brewed coffee on rat behavior and cognition and oxidative stress Alzheimer\textquotesingles disease model. Clin. Nutr. Exp. 2019, 28, 62–73. [Google Scholar] [CrossRef] [Green Version]
- AlAmri, O.D.; Albeltagy, R.S.; Akabawy, A.M.; Mahgoub, S.; Abdel-Mohsen, D.M.; Moneim, A.E.; Amin, H.K. Investigation of antioxidant and anti-inflammatory activities as well as the renal protective potential of green coffee extract in high fat-diet/streptozotocin-induced diabetes in male albino rats. J. Funct. Foods 2020, 71, 103996. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, J.; dos Santos, L.S.; de Souza, R.G.; Lima, L.G.; Mattos, D.S.; Viana, B.P.; Fonseca Bastos, A.C.S.; Muzzi, L.; Conte-Júnior, C.A.; Gimba, E.R.P.; et al. Bioactive compounds, antioxidant activity and antiproliferative effects in prostate cancer cells of green and roasted coffee extracts obtained by microwave-assisted extraction (MAE). Food Res. Int. 2021, 140, 110014. [Google Scholar] [CrossRef]
- Zhu, M.; Jatoi, A. Colorectal Cancer, Crohn-Like Lymphoid Reactions, Survival—And the Power of a Good Cup of Coffee! Mayo Clin. Proc. 2022, 97, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Açıkalın, B.; Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol. 2021, 114, 625–632. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Álvarez, R.; Muñoz-Durango, K. Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- LIczbiński, P.; Bukowska, B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Desbrow, B.; Anoopkumar-Dukie, S.; Davey, A.K.; Arora, D.; McDermott, C.; Schubert, M.M.; Perkins, A.V.; Kiefel, M.J.; Grant, G.D. A review of the bioactivity of coffee, caffeine and key coffee constituents on inflammatory responses linked to depression. Food Res. Int. 2015, 76, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Franca, A.S.; Oliveira, L.S. Chemistry of Defective Coffee Beans. In Progress in Food Chemistry; Koeffer, E.N., Ed.; Nova Publishers: New York, NY, USA, 2008; pp. 105–138. [Google Scholar]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, Functional, and Structural Properties of Spent Coffee Grounds and Coffee Silverskin. Food Bioprocess Technol. 2015, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Bijla, L.; Aissa, R.; Bouzid, H.A.; Sakar, E.H.; Ibourki, M.; Gharby, S. Spent Coffee Ground Oil as a Potential Alternative for Vegetable Oil Production: Evidence from Oil Content, Lipid Profiling, and Physicochemical Characterization. Biointerface Res. Appl. Chem. 2021, 12, 6308–6320. [Google Scholar] [CrossRef]
- Nzekoue, K.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef]
- Marx, S.; Venter, R.; Karmee, S.K.; Louw, J.; Truter, C. Biofuels from spent coffee grounds: Comparison of processing routes. Biofuels 2020, 13, 537–543. [Google Scholar] [CrossRef]
- Bijla, L.; Aissa, R.; Laknifli, A.; Bouyahya, A.; Harhar, H.; Gharby, S. Spent coffee grounds: A sustainable approach toward novel perspectives of valorization. J. Food Biochem. 2022, e14190. [Google Scholar] [CrossRef]
- Devasagayam, T.P.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Biomembr. 1996, 1282, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.S.; Franca, A.S. Potential of spent coffee grounds as sources of dietary fiber with antioxidant activity. In Food Waste: Practices, Management and Challenges; Riley, G.L., Ed.; Nova Publishers: New York, NY, USA, 2016; pp. 51–70. [Google Scholar]
- Franca, A.S. Coffee: Decaffeination. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 232–236. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Oosterveld, A.; Voragen, A.G.; Schols, H.A. Effect of roasting on the carbohydrate composition of Coffea arabica beans. Carbohydr. Polym. 2003, 54, 183–192. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Simões, J.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Extractability and structure of spent coffee ground polysaccharides by roasting pre-treatments. Carbohydr. Polym. 2013, 97, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.P.; Coimbra, M.A. Microwave superheated water extraction of polysaccharides from spent coffee grounds. Carbohydr. Polym. 2013, 94, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Nunes, F.M.; Domingues, M.R.; Coimbra, M.A. Galactomannans in Coffee. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 173–182. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Vilela, W.F.; Leão, D.P.; Franca, A.S.; Oliveira, L.S. Effect of peroxide treatment on functional and technological properties of fiber-rich powders based on spent coffee grounds. ETP Int. J. Food Eng. 2016, 2, 42–47. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2010, 5, 897–903. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; García-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. The dietary fiber profile of fruit peels and functionality modifications induced by high hydrostatic pressure treatments. Food Sci. Technol. Int. 2017, 23, 396–402. [Google Scholar] [CrossRef]
- dos Santos Opuski de Almeida, J.; Dias, C.O.; Arriola, N.D.; de Freitas, B.S.; de Francisco, A.; Petkowicz, C.L.; Araujo, L.; Guerra, M.P.; Nodari, R.O.; Amboni, R.D. Feijoa (Acca sellowiana) peel flours: A source of dietary fibers and bioactive compounds. Food Biosci. 2020, 38, 100789. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2014, 52, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Cangussu, L.B.; Melo, J.C.; Franca, A.S.; Oliveira, L.S. Chemical Characterization of Coffee Husks, a By-Product of Coffea arabica Production. Foods 2021, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) fruit by-products flours: Evaluation as source of dietary fibers and natural antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Martin-Sánchez, A.; Sánchez-Zapata, E.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Navarro, C.J.; Pérez-Álvarez, J.A. Chemical, physico-chemical and functional properties of pomegranate (Punica granatum L.) bagasses powder co-product. J. Food Eng. 2012, 110, 220–224. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Dziki, D.; Kowalska, I.; Pecio, Ł.; Durak, A.; Sęczyk, Ł. Lipoxygenase inhibitors and antioxidants from green coffee—Mechanism of action in the light of potential bioaccessibility. Food Res. Int. 2014, 61, 48–55. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; Fang, H.; Tian, L.; Liu, Y.; Niu, J. Chlorogenic acid improves health in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets: Involvement of lipid metabolism, antioxidant ability, inflammatory response, and intestinal integrity. Aquaculture 2011, 545, 737169. [Google Scholar] [CrossRef]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Macheiner, L.; Schmidt, A.; Mayer, H.K. A novel basis for monitoring the coffee roasting process: Isomerization reactions of 3-caffeoylquinic and 4-caffeoylquinic acids. LWT 2021, 152, 112343. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Saberian, M.; Li, J.; Donnoli, A.; Bonderenko, E.; Oliva, P.; Gill, B.; Lockrey, S.; Siddique, R. Recycling of spent coffee grounds in construction materials: A review. J. Clean. Prod. 2021, 289, 125837. [Google Scholar] [CrossRef]
- dos Santos, É.M.; de Macedo, L.M.; Tundisi, L.L.; Ataide, J.A.; Camargo, G.A.; Alves, R.C.; Oliveira, M.B.P.P.; Mazzola, P.G. Coffee by-products in topical formulations: A review. Trends Food Sci. Technol. 2021, 111, 280–291. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of spent coffee grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Saratale, G.D.; Bhosale, R.; Shobana, S.; Banu, J.R.; Pugazhendhi, A.; Mahmoud, E.; Bhatia, S.K.; Atabani, A.E.; Mulone, V.; Yoon, J.-J.; et al. A review on valorization of spent coffee grounds (SCG) towards biopolymers and biocatalysts production. Bioresour. Technol. 2020, 314, 123800. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A. Potential applications of by-products from the coffee industry in polymer technology–Current state and perspectives. Waste Manag. 2021, 121, 296–330. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee By-Products and Their Suitability for Developing Active Food Packaging Materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Sisti, L.; Celli, A.; Totaro, G.; Cinelli, P.; Signori, F.; Lazzeri, A.; Bikaki, M.; Corvini, P.; Ferri, M.; Tassoni, A.; et al. Monomers, materials and energy from coffee by-products: A review. Sustainability 2021, 13, 6921. [Google Scholar] [CrossRef]
- Karmee, S.K. A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag. 2018, 72, 240–254. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Bio-refinery approach for spent coffee grounds valorization. Bioresour. Technol. 2018, 247, 1077–1084. [Google Scholar] [CrossRef]
- Battista, F.; Zuliani, L.; Rizzioli, F.; Fusco, S.; Bolzonella, D. Biodiesel, biogas and fermentable sugars production from Spent coffee Grounds: A cascade biorefinery approach. Bioresour. Technol. 2021, 342, 125952. [Google Scholar] [CrossRef]
- Araujo, M.N.; dos Santos, K.C.; do Carmo Diniz, N.; de Carvalho, J.C.; Corazza, M.L. A biorefinery approach for spent coffee grounds valorization using pressurized fluid extraction to produce oil and bioproducts: A systematic review. Bioresour. Technol. Rep. 2022, 18, 101013. [Google Scholar] [CrossRef]
- Atabani, A.E.; Ali, I.; Naqvi, S.R.; Badruddin, I.A.; Aslam, M.; Mahmoud, E.; Almomani, F.; Juchelková, D.; Atelge, M.R.; Khan, T.M. A state-of-the-art review on spent coffee ground (SCG) pyrolysis for future biorefinery. Chemosphere 2022, 286, 131730. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Lee, Y.G.; Song, Y.; Nguyen, D.-T.; Bae, H.-J. An integrated process for conversion of spent coffee grounds into value-added materials. Bioresour. Technol. 2022, 346, 126618. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P. Food waste valorization advocating Circular Bioeconomy-A critical review of potentialities and perspectives of spent coffee grounds biorefinery. J. Clean. Prod. 2019, 211, 1553–1566. [Google Scholar] [CrossRef]
- Bhaturiwala Rizwan, A.; Modi Hasmukh, A. Extraction of oligosaccharides and phenolic compounds by roasting pretreatment and enzymatic hydrolysis from spent coffee ground. J. Appl. Biol. 2020, 8, 75–81. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, J.; Wang, S.; Rupasinghe, H.P.; He, Q. Experimental exploration of processes for deriving multiple products from spent coffee grounds. Food Bioprod. Process. 2021, 128, 21–29. [Google Scholar] [CrossRef]
- Wongsiridetchai, C.; Jonjaroen, V.; Sawangwan, T.; Charoenrat, T.; Chantorn, S. Evaluation of prebiotic mannooligosaccharides obtained from spent coffee grounds for nutraceutical application. LWT 2021, 148, 111717. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of Natural Antioxidants from Spent Coffee Grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef]
- Severini, C.; Derossi, A.; Fiore, A.G. Ultrasound-assisted extraction to improve the recovery of phenols and antioxidants from spent espresso coffee ground: A study by response surface methodology and desirability approach. Eur. Food Res. Technol. 2016, 243, 835–847. [Google Scholar] [CrossRef]
- Abrahão, F.R.; Rocha, L.C.; Santos, T.A.; do Carmo, E.L.; Pereira, L.A.; Borges, S.V.; Pereira, R.G.F.A.; Botrel, D.A. Microencapsulation of bioactive compounds from espresso spent coffee by spray drying. LWT 2019, 103, 116–124. [Google Scholar] [CrossRef]
- Mayanga-Torres, P.C.; Lachos-Perez, D.; Rezende, C.A.; Prado, J.M.; Ma, Z.; Tompsett, G.T.; Timko, M.T.; Forster-Carneiro, T. Valorization of coffee industry residues by subcritical water hydrolysis: Recovery of sugars and phenolic compounds. J. Supercrit. Fluids 2019, 120, 75–85. [Google Scholar] [CrossRef]
- Shang, Y.-F.; Xu, J.-L.; Lee, W.-J.; Um, B.-H. Antioxidative polyphenolics obtained from spent coffee grounds by pressurized liquid extraction. S. Afr. J. Bot. 2017, 109, 75–80. [Google Scholar] [CrossRef]
- Torres-Valenzuela, L.S.; Ballesteros-Gómez, A.; Sanin, A.; Rubio, S. Valorization of spent coffee grounds by supramolecular solvent extraction. Sep. Purif. Technol. 2019, 228, 115759. [Google Scholar] [CrossRef]
- Pettinato, M.; Casazza, A.A.; Perego, P. The role of heating step in microwave-assisted extraction of polyphenols from spent coffee grounds. Food Bioprod. Process. 2019, 114, 227–234. [Google Scholar] [CrossRef]
- Ramón-Gonçalves, M.; Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Extraction, identification and quantification of polyphenols from spent coffee grounds by chromatographic methods and chemometric analyses. Waste Manag. 2019, 96, 15–24. [Google Scholar] [CrossRef]
- Balzano, M.; Loizzo, M.R.; Tundis, R.; Lucci, P.; Nunez, O.; Fiorini, D.; Giardinieri, A.N.; Frega, G.; Pacetti, D. Spent espresso coffee grounds as a source of anti-proliferative and antioxidant compounds. Innov. Food Sci. Emerg. Technol. 2020, 59, 102254. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Lucian, M.; Du, L.; Olszewski, M.P.; Fiori, L.; Kruse, A. Improving the recovery of phenolic compounds from spent coffee grounds by using hydrothermal delignification coupled with ultrasound assisted extraction. Biomass Bioenergy 2020, 139, 105616. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Mello, F.M.; Cabral, F.A.; Meirelles, A.J. High-pressure fractionation of spent coffee grounds oil using green solvents. J. Supercrit. Fluids 2020, 157, 104689. [Google Scholar] [CrossRef]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Díaz-Hernández, G.C.; Alvarez-Fitz, P.; Maldonado-Astudillo, Y.I.; Jiménez-Hernández, J.; Parra-Rojas, I.; Flores-Alfaro, E.; Salazar, R. Ramírez, M. Antibacterial, Antiradical and Antiproliferative Potential of Green, Roasted, and Spent Coffee Extracts. Appl. Sci. 2022, 12, 1938. [Google Scholar] [CrossRef]
- Rathnakumar, K.; Osorio-Arias, J.C.; Krishnan, P.; Martínez-Monteagudo, S.I. Fractionation of spent coffee ground with tertiary amine extraction. Sep. Purif. Technol. 2021, 274, 119111. [Google Scholar] [CrossRef]
- Valdés, A.; Castro-Puyana, M.; Marina, M.L. Isolation of proteins from spent coffee grounds. Polyphenol removal and peptide identification in the protein hydrolysates by RP-HPLC-ESI-Q-TOF. Food Res. Int. 2020, 137, 109368. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, K.; Pineda-Hidalgo, K.V.; Rochín-Medina, J.J. Fermentation of spent coffee grounds by Bacillus clausii induces release of potentially bioactive peptides. LWT 2021, 138, 110685. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, D.; Eun, J.; Moon, S. Antioxidant effect of extracts from the coffee residue in raw and cooked meat. Antioxidants 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampaio, A.; Dragone, G.; Vilanova, M.; Oliveira, J.M.; Teixeira, J.A.; Mussatto, S.I. Production, chemical characterization, and sensory profile of a novel spirit elaborated from spent coffee ground. LWT 2013, 54, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Saez, N.; García, A.T.; Pérez, I.D.; Rebollo-Hernanz, M.; Mesías, M.; Morales, F.J.; Martín-Cabrejas, M.A.; del Castillo, M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017, 216, 114–122. [Google Scholar] [CrossRef]
- Sharma, A.; Ray, A.; Singhal, R.S. A biorefinery approach towards valorization of spent coffee ground: Extraction of the oil by supercritical carbon dioxide and utilizing the defatted spent in formulating functional cookies. Future Foods 2021, 4, 100090. [Google Scholar] [CrossRef]
- Hussein, A.; Ali, H.; Bareh, G.; Farouk, A. Influence of spent coffee ground as fiber source on chemical, rheological and sensory properties of sponge cake. Pak. J. Biol. Sci. 2013, 22, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Meerasri, J.; Sothornvit, R. Novel development of coffee oil extracted from spent coffee grounds as a butter substitute in bakery products. J. Food Process. Preserv. 2022, e16687. [Google Scholar] [CrossRef]
- Zhang, M.; Biesold, G.M.; Choi, W.; Yu, J.; Deng, Y.; Silvestre, C.; Lin, Z. Recent advances in polymers and polymer composites for food packaging. Mater. Today 2022, 53, 134–161. [Google Scholar] [CrossRef]
- Han, J.-W.; Ruiz-Garcia, L.; Qian, J.-P.; Yang, X.-T. Food Packaging: A Comprehensive Review and Future Trends. Compr. Rev. Food Sci. 2018, 17, 860–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, M.J.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Polysaccharide-rich fraction of spent coffee grounds as promising biomaterial for films fabrication. Carbohydr. Polym. 2020, 233, 115851. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.O.; Batista, M.J.; Ávila, A.F.; Franca, A.S.; Oliveira, L.S. Development and characterization of biopolymeric films of galactomannans recovered from spent coffee grounds. J. Food Eng. 2021, 289, 110083. [Google Scholar] [CrossRef]
- Wu, H.; Hu, W.; Zhang, Y.; Huang, L.; Zhang, J.; Tan, S.; Cai, X.; Liao, X. Effect of oil extraction on properties of spent coffee ground–plastic composites. J. Mater. Sci. 2016, 51, 10205–10214. [Google Scholar] [CrossRef]
- Moustafa, H.; Guizani, C.; Dupont, C.; Martin, V.; Jeguirim, M.; Dufresne, A. Utilization of Torrefied Coffee Grounds as Reinforcing Agent To Produce High-Quality Biodegradable PBAT Composites for Food Packaging Applications. ACS Sustain. Chem. Eng. 2017, 5, 1906–1916. [Google Scholar] [CrossRef]
- Tarazona, E.R.; Oliveira, L.S.; Rubio, J.C.; Franca, A.S. Preparation, preliminary characterization and mechanical properties of epoxy composites reinforced with spent coffee grounds. In Proceedings of the 8th International Conference on Mechanical and Aerospace Engineering (ICMAE), Prague, Czech Republic, 22–25 July 2017. [Google Scholar] [CrossRef]
- Thiagamani, S.M.; Nagarajan, R.; Jawaid, M.; Anumakonda, V.; Siengchin, S. Utilization of chemically treated municipal solid waste (spent coffee bean powder) as reinforcement in cellulose matrix for packaging applications. Waste Manag. 2017, 69, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Essabir, H.; Raji, M.; Laaziz, S.A.; Rodrique, D.; Bouhfid, R.; el kacem Qaiss, A. Thermo-mechanical performances of polypropylene biocomposites based on untreated, treated and compatibilized spent coffee grounds. Compos. B Eng. 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Senthil Muthu Kumar, T.; Yorseng, K.; Rajini, N.; Siengchin, S.; Ayrilmis, N.; Varada Rajulu, A. Mechanical and thermal properties of spent coffee bean filler/poly(3-hydroxybutyrate-co-3-hydroxyvalerate) biocomposites: Effect of recycling. Process. Saf. Environ. Prot. 2019, 124, 187–195. [Google Scholar] [CrossRef]
- Gazonato, E.C.; Maia, A.A.; da Silva Moris, V.A.; de Paiva, J.M. Thermomechanical Properties of Corn Starch Based Film Reinforced with Coffee Ground Waste as Renewable Resource. Mater. Res. 2019, 22. [Google Scholar] [CrossRef]
- Terroba-Delicado, E.; Fiori, S.; Gomez-Caturla, J.; Montanes, N.; Sanchez-Nacher, L.; Torres-Giner, S. Valorization of Liquor Waste Derived Spent Coffee Grains for the Development of Injection-Molded Polylactide Pieces of Interest as Disposable Food Packaging and Serving Materials. Foods 2022, 11, 1162. [Google Scholar] [CrossRef]
- Gupta, A.; Mohanty, A.K.; Misra, M. Biocarbon from spent coffee ground and their sustainable biocomposites with recycled water bottle and bale wrap: A new life for waste plastics and waste food residues for industrial uses. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106759. [Google Scholar] [CrossRef]
- da Silva, A.P.; de Paula Pereira, M.; Passador, F.R.; Montagna, L.S. PLA/coffee grounds composites: A study of photodegradation and biodegradation in soil. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2020; Volume 394, p. 2000091. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Kamwilaisak, K.; Saengprachatanarug, K.; Mongkolthanaruk, W.; Souvanh, M.; Pongsa, U.; Chindaprasirt, P. Polyvinyl alcohol (pva)/starch bioactive packaging film enriched with antioxidants from spent coffee ground and citric acid. J. Polym. Environ. 2018, 26, 3762–3772. [Google Scholar] [CrossRef]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getachew, A.T.; Ahmad, R.; Park, J.-S.; Chun, B.-S. Fish skin gelatin based packaging films functionalized by subcritical water extract from spent coffee ground. Food Packag. Shelf Life 2021, 29, 100735. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Lappa, I.K.; Andriotis, H.; Eriotou, E.; Mandala, I.; Kopsahelis, N. Tuning the physical and functional properties of whey protein edible films: Effect of pH and inclusion of antioxidants from spent coffee grounds. Sustain. Chem. Pharm. 2022, 27, 100700. [Google Scholar] [CrossRef]
- Bomfim, A.; Oliveira, D.; Voorwald, H.; Benini, K.; Dumont, M.-J.; Rodrigue, D. Valorization of Spent Coffee Grounds as Precursors for Biopolymers and Composite Production. Polymers 2022, 14, 437. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).