Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil and Oleoresin Extraction
2.2. Treatment Description and Hamburger Preparation
2.3. Physicochemical Analyses
2.4. Lipid Oxidation
2.5. Microbiological Analyses
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Analyses
3.2. Colour
3.3. Lipid Oxidation
3.4. Antimicrobial Analyses
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, E.V.; Holt, K.G.; Mahon, B.E.; Ayers, T.; Norton, D.; Gould, L.H. Ground Beef Consumption Patterns in the United States, FoodNet, 2006 through 2007. J. Food Prot. 2012, 75, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Nsoesie, E.O.; Kluberg, S.A.; Brownstein, J.S. Online Reports of Foodborne Illness Capture Foods Implicated in Official Foodborne Outbreak Reports. Prev. Med. 2014, 67, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of Foodborne Illnesses, Hospitalizations, and Deaths to Food Commodities by Using Outbreak Data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Saux, N.L.; Spika, J.S.; Friesen, B.; Johnson, I.; Melnychuck, D.; Anderson, C.; Dion, R.; Rahman, M.; Tostowaryk, W. Ground Beef Consumption in Noncommercial Settings Is a Risk Factor for Sporadic Escherichia Coli O157:H7 Infection in Canada. J. Infect. Dis. 1993, 167, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Sánchez, J.; Correa, M.; Castañeda-Sandoval, L.M. Bacillus Cereus Un Patógeno Importante En El Control Microbiológico de Los Alimentos. Rev. Fac. Nac. Salud Pública 2016, 34, 229–242. [Google Scholar] [CrossRef]
- Shahidi, F. Nutraceuticals and Functional Foods: Whole versus Processed Foods. Trends Food Sci. Technol. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Bender, B.D.; Bárcenas, M.E. El Ajo y Sus Aplicaciones En La Conservación de Alimentos. Temas Sel. Ing. Aliment. 2013, 1, 25–36. [Google Scholar]
- Jayasena, D.D.; Jo, C. Essential Oils as Potential Antimicrobial Agents in Meat and Meat Products: A Review. Trends Food Sci. Technol. 2013, 34, 96–108. [Google Scholar] [CrossRef]
- Rodríguez Saucedo, E.N. Uso de Agentes Antimicrobianos Naturales En La Conservación de Frutas y Hortalizas|Rodríguez Sauceda|Ra Ximhai. Ra Ximhai 2011, 7, 153–170. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils-A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Sendra, E. Essential Oils in Foods: From Ancient Times to the 21st Century. Foods 2016, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Jo, C. Potential application of Essential Oils as Natural Antioxidants in Meat and Meat Products: A Review. Food Rev. Int. 2014, 30, 71–90. [Google Scholar] [CrossRef]
- Djenane, D.; Sánchez-Escalante, A.; Beltrán, J.A.; Roncalés, P. Ability of a -Tocopherol, Taurine and Rosemary, in Combination With vitamin C, to Increase the Oxidative Stability of Beef Steaks Packaged in Modified Atmosphere. Food Chem. 2002, 76, 407–415. [Google Scholar] [CrossRef]
- Kaur, R.; Gupta, T.B.; Bronlund, J.; Kaur, L. The Potential of Rosemary as a Functional Ingredient for Meat Products-A Review. Food Rev. Int. 2021, 16, 1–21. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis. L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus Officinalis L. Essential Oil Combined with Different Packaging Conditions to Extend the Shelf Life of Refrigerated Beef Meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef]
- Bouloumpasi, E.; Hatzikamari, M.; Lazaridou, A.; Chatzopoulou, P.; Biliaderis, C.G.; Irakli, M. Antibacterial and Antioxidant Properties of Oregano and Rosemary Essential Oil Distillation By-Products. Biol. Life Sci. Forum 2021, 6, 47. [Google Scholar] [CrossRef]
- Leuschner, R.G.K.; Lelsch, V. Antimicrobial Effects of Garlic, Clove and Red Hot Chilli on Listeria Monocytogenes in Broth Model Systems and Soft Cheese. Int. J. Food Sci. Nutr. 2003, 54, 127–133. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Michalczyk, M.; Macura, R.; Banaś, J.; Tesarowicz, I.; Maciejaszek, I. Effect of Adding Oregano Essential Oil, Garlic and Tomato Preparations Separately and in Combination on the Stability of Vacuum-Packed Minced Pork During Storage. Ann. Anim. Sci. 2015, 15, 221–235. [Google Scholar] [CrossRef]
- Ibrahim-Hemmat, I.; el Sabagh-Rasha, A.; El-Roos-Nahla, A.; el Fattah-Hend, A. Antimicrobial Effect of Some Essential Oils on Staphyloccosus Aureus in Minced Meat. Benha Vet. Med. J. 2016, 30, 183–191. [Google Scholar] [CrossRef]
- López, L.; El Ajo, M.T. Propiedades Farmacológicas e Indicaciones Terapéuticas. Offarm Farm. Soc. 2007, 26, 78–81. [Google Scholar]
- Amany, M.M.B.; Shaker, M.A.; Abeer, A.K. Antioxidant Activities of Date Pits in a Model Meat System. Int. Food Res. 2010, 19, 223–227. [Google Scholar]
- Berke, T.G.; Shieh, S.C. Capsicum Cultivars. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2012; Volume 1, pp. 116–130. [Google Scholar] [CrossRef]
- Ravindran, P.N.; Kallupurackal, J.A. Black Pepper. In Handbook of Herbs and Spice, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Sawston, UK, 2012; Volume 1, pp. 86–115. [Google Scholar] [CrossRef]
- Suderman, D.R. Effective Use of Flavorings and Seasonings. In Batters and Breadings in Food Processing, 2nd ed.; Kulp, K., Loewe, R., Lorenz, K., Gelroth, J., Eds.; AACC International Press: Washington, DC, USA, 2011; pp. 73–90. [Google Scholar] [CrossRef]
- FDA. 21CFR182.20 Title 21. Food and Drugs Chapter I. Food and Drug Administration Department of Health and Human Services Subchapter B. Food for Human Consumption; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2022.
- Meckelmann, S.W.; Riegel, D.W.; van Zonneveld, M.; Ríos, L.; Peña, K.; Mueller-Seitz, E.; Petz, M. Capsaicinoids, Flavonoids, Tocopherols, Antioxidant Capacity and Color Attributes in 23 Native Peruvian Chili Peppers (Capsicum spp.) Grown in Three Different Locations. Eur. Food Res. Technol. 2014, 240, 273–283. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Jiménez-Fernández, M.; Azuara, E. Oleoresins from Capsicum spp.: Extraction Methods and Bioactivity. Food Bioprocess Technol. 2017, 10, 51–76. [Google Scholar] [CrossRef]
- Akbas, E.; Soyler, U.B.; Oztop, M.H. Physicochemical and Antimicrobial Properties of Oleoresin Capsicum Nanoemulsions Formulated with Lecithin and Sucrose Monopalmitate. Appl. Biochem. Biotechnol. 2019, 188, 54–71. [Google Scholar] [CrossRef]
- Nadeem, M.; Anjum, F.M.; Khan, M.R.; Saeed, M.; Riaz, A. Antioxidant Potential of Bell Pepper (Capsicum Annum L.)—A Review. Pak. J. Food Sci. 2011, 21, 45–51. [Google Scholar]
- Nurjanah, S.; Sudaryanto, Z.; Widyasanti, A.; Pratiwi, H. Antibacterial Activity of Capsicum Annuum L. Oleoresin. Acta Hortic. 2016, 1125, 189–194. [Google Scholar] [CrossRef]
- Sharma, P.K.; Fuloria, S.; Alam, S.; Sri, M.V.; Singh, A.; Sharma, V.K.; Kumar, N.; Subramaniyan, V.; Fuloria, N.K. Chemical Composition and Antimicrobial Activity of Oleoresin of Capsicum Annuum Fruits. Mindanao J. Sci. Technol. 2021, 19, 29–43. [Google Scholar]
- Nadi, M.S.; Fikri, F.; Purnama, T.E. Determination of Capsaicin Levels in Capsicum Annum Linn Ethanolic Extract Using Thin Layer Chromatography Analysis. Syst. Rev. Pharm. 2020, 11, 661–664. [Google Scholar]
- Al Othman, Z.A.; Ahmed, Y.B.H.; Habila, M.A.; Ghafar, A.A. Determination of Capsaicin and Dihydrocapsaicin in Capsicum Fruit Samples Using High Performance Liquid Chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, J.; Zheng, Q.; Wang, M.; Deng, W.; Li, Q.; Firempong, C.K.; Wang, S.; Tong, S.; Xu, X.; et al. In Vitro and In Vivo Evaluation of Capsaicin-Loaded Microemulsion for Enhanced Oral Bioavailability. J. Sci. Food Agric. 2015, 95, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moriel, C.; Quintero-Ramos, A.; Camacho-Dávila, A.; Ruíz-Gutiérrez, M.; Talamás-Abbud, R.; Olivas-Vargas, R.; Barnard, J. Optimization of Chipotle Pepper Smoking Process Using Response Surface Methodology. J. Food Qual. 2012, 35, 21–33. [Google Scholar] [CrossRef]
- Natividad-Torres, E.A.; Guevara-Aguilar, A.; Sánchez, E.; Sida-Arreola, J.P.; Muñoz-Márquez, E.; Chávez-Mendoza, C. Processing Effect on the Bioactive Compounds Content of Mexican Jalapeño Peppers for Chipotle (Capsicum Annum L.). Acta Agrícola Pecu. 2021, 7, 1339–1349. [Google Scholar] [CrossRef]
- Moreno-Escamilla, J.O.; de la Rosa, L.A.; López-Díaz, J.A.; Rodrigo-García, J.; Núñez-Gastélum, J.A.; Alvarez-Parrilla, E. Effect of the Smoking Process and Firewood Type in the Phytochemical Content and Antioxidant Capacity of Red Jalapeño Pepper during Its Transformation to Chipotle Pepper. Food Res. Int. 2015, 76, 654–660. [Google Scholar] [CrossRef]
- Vergara, H.; Cózar, A.; Rubio, N. Effect of Adding of Different Forms of Oregano (Origanum vulgare) on Lamb Meat Burgers Quality during the Storage Time. CyTA-J. Food 2020, 18, 535–542. [Google Scholar] [CrossRef]
- Rojas, M.C.; Brewer, M.S. Effect of Natural Antioxidants on Oxidative Stability of Cooked, Refrigerated Beef and Pork. J. Food Sci. 2007, 72, S282–S288. [Google Scholar] [CrossRef]
- Velasco, V.; Williams, P. Improving Meat Quality through Natural Antioxidants. Chil. J. Agric. Res. 2011, 71, 313–322. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.E.; Morsy, N.F.S. Keeping Quality of Frozen Beef Patties by Marjoram and Clove Essential Oils. J. Food Process. Preserv. 2015, 39, 956–965. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Hosseini, S.M.H.; Taghavifard, M.H.; Eskandari, M.H.; Golmakani, M.-T.; Shad, E. Lipid Oxidation, Color Changes, and Microbiological Quality of Frozen Beef Burgers Incorporated with Shirazi Thyme, Cinnamon, and Rosemary Extracts. J. Food Qual. 2017, 2017, 6350156. [Google Scholar] [CrossRef]
- Dzudie, T.; Kouebou, C.P.; Essia-Ngang, J.J.; Mbofung, C.M.F. Lipid Sources and Essential Oils Effects on Quality and Stability of Beef Patties. J. Food Eng. 2004, 65, 67–72. [Google Scholar] [CrossRef]
- Colindres, P.; Susan Brewer, M. Oxidative Stability of Cooked, Frozen, Reheated Beef Patties: Effect of Antioxidants. J. Sci. Food Agric. 2011, 91, 963–968. [Google Scholar] [CrossRef]
- Barber, L.I.; Obinna- Echem, P.C.; Hart, B.E. Proximate Composition, Thiobarbituric Acid and Sensory Properties of Chicken-Breadfruit Patties with Piper Guineense and Monodora Myristica Oleoresins. Am. J. Food Sci. Technol. 2020, 8, 56–62. [Google Scholar]
- Vargas, F.C.; Arantes-Pereira, L.; da Costa, P.A.; de Melo, M.P.; do Sobral, P.J.A. Rosemary and Pitanga Aqueous Leaf Extracts on Beef Patties Stability under Cold Storage. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Hassanzad Azar, H.; Hejazi, J. The Use of Herbal Extracts and Essential Oils as a Potential Antimicrobial in Meat and Meat Products; a Review. J. Hum. Environ. Health Promot. 2016, 1, 63–74. [Google Scholar] [CrossRef]
- Yoon, J.; Bae, S.M.; Gwak, S.H.; Jeong, J.Y. Use of Green Tea Extract and Rosemary Extract in Naturally Cured Pork Sausages with White Kimchi Powder. Food Sci. Anim. Resour. 2021, 41, 840–854. [Google Scholar] [CrossRef]
- Alsaiqali, M.; El-Shibiny, A.A.; Adel, M.; Abdel-Samie, M.; Ghoneim, S. Use of Some Essential Oils as Antimicrobial Agents to Control Pathogenic Bacteria in Beef Burger. World J. Dairy Food Sci. 2016, 11, 109–120. [Google Scholar]
- Al-Hijazeen, M. The Combination Effect of Adding Rosemary Extract and Oregano Essential Oil on Ground Chicken Meat Quality. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- García-Díez, J.; Alheiro, J.; Pinto, A.L.; Falco, V.; Fraqueza, M.J.; Patarata, L. Synergistic Activity of Essential Oils from Herbs and Spices Used on Meat Products against Food Borne Pathogens. Nat. Prod. Commun. 2017, 12, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, G. Oleorresinas de Capsicum En La Industria Alimentaria. Rev.Sallista Investig. 2007, 3, 43–47. [Google Scholar]
- Ogaz, J.R. Escalamiento a Nivel de Planta Piloto Del Proceso de Extracción de Oleorresinas a Partir de Chile Jalapeño (Capsicum annuum); Universidad Autónoma de Chihuahua: Chihuahua, Mexico, 2017. [Google Scholar]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1992. [Google Scholar]
- Honikel, K.O. Reference Methods for the Assessment of Physical Characteristics of Meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Ramírez-Navas, J.S.; Rodríguez de Stouvenel, A. Characterization of Colombian Quesillo Cheese by Spectrocolorimetry. Vitae 2012, 19, 178–185. [Google Scholar]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Tocopherol Contents and Lipid Oxidation in Pork Muscle and Adipose Tissue during Storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- NOM. Norma Oficial Mexicana NOM-114-SSA1-1994, Bienes y Servicios. Método Para La Determinación de Salmonella En Alimentos; Gobierno de Mexico. 1995. Available online: file:///C:/Users/MDPI/Downloads/wo69538-1.pdf (accessed on 29 June 2022).
- NOM. Norma Oficial Mexicana NOM-243-SSA1-2010, Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba; Gobierno de Mexico. 2010. Available online: https://dof.gob.mx/normasOficiales/4156/salud2a/salud2a.htm (accessed on 29 June 2022).
- Renye, J.A.; Somkuti, G.A.; Vallejo-Cordoba, B.; van Hekken, D.L.; Gonzalez-Cordova, A.F. Characterization of the Microflora Isolated from Queso Fresco Made from Raw and Pasteurized Milk. J. Food Saf. 2008, 28, 59–75. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J. Sensory Evaluation Practices, 3rd ed.; Elsevier Academic Press: Cambridge, MA, USA, 2004; Volume 1. [Google Scholar]
- Meilgaard, M.; Vance Civille, G.; Thomas Carr, B. Sensory Evaluation Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar] [CrossRef]
- Anzaldúa Morales, A. La Evaluación Sensorial de Los Alimentos En Teoría y La Práctica, 1st ed.; Acribia: Zaragoza, Spain, 1994. [Google Scholar]
- SAS Institute Inc. SAS/STAT User’s Guide: Statistic Version 9. North Carolina 2002. Available online: https://support.sas.com/documentation/cdl/en/statug/63962/HTML/default/viewer.htm#titlepage.htm (accessed on 29 June 2022).
- OECD/FAO. OECD-FAO Agricultural Outlook, OECD Agricultura Statistics; OECD-FAO: Rome, Italy, 2019. [Google Scholar]
- Valero Gaspar, T.; del Pozo de la Calle, S.; Ruíz Moreno, E.; Ávila Torres, J.M.; Valera Moreiras, G. Guía Nutricional de La Carne; Nutricional de la Carne, F., Ed.; Federacion Madrileña de Detallistas de la Carne: Madrid, Spain, 2010. [Google Scholar]
- Lemay, M.-J.; Choquette, J.; Delaquis, P.J.; Gariépy, C.; Rodrigue, N.; Saucier, L. Antimicrobial Effect of Natural Preservatives in a Cooked and Acidified Chicken Meat Model. Int. J. Food Microbiol. 2002, 78, 217–226. [Google Scholar] [CrossRef]
- Mukhtar, S.; Zahoor, T.; Randhawa, M.A.; Iqbal, R.; Shabbir, A.; Liaqat, A.; Ahsan, S. Synergistic Effect of Chitosan and Clove Oil on Raw Poultry Meat. J. Food Process. Technol. 2018, 9, 1–5. [Google Scholar] [CrossRef]
- Zuasnabar, Y.; García, O.; Díaz, M. Elaboración de Hamburguesas de Carne Vacuna Libre de Gluten. Master’s Thesis, Universidad Nacional del Centro de la Provincia de Buenos Aires, Tandil, Argentina, 2016. [Google Scholar]
- Kirkpinar, F.; Ünlü, H.B.; Serdaroğlu, M.; Turp, G.Y. Effects of Dietary Oregano and Garlic Essential Oils on Carcass Characteristics, Meat Composition, Colour, PH and Sensory Quality of Broiler Meat. Br. Poult. Sci. 2014, 55, 157–166. [Google Scholar] [CrossRef]
- Wyszecki, G.; Stiles, W. Color Science. Concepts and Methods, Quantitative Data and Formulae, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Hernández, B.; Sáenz, C.; Alberdi, C.; Diñeiro, J.M. CIELAB Color Coordinates versus Relative Proportions of Myoglobin Redox Forms in the Description of Fresh Meat Appearance. J. Food Sci. Technol. 2016, 53, 4159–4167. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Assessment of Carotenoids, Capsaicinoids and Ascorbic Acid Composition of Some Selected Pepper Cultivars (Capsicum annuum L.) Grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Seideman, S.C.; Cross, H.R.; Smith, G.C.; Durland, P.R. Factos Associated with Fresh Meat Color: A Review. J. Food Qual. 1984, 6, 211–237. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, Z.; Bi, J.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Degradation Kinetics of Total Phenolic Compounds, Capsaicinoids and Antioxidant Activity in Red Pepper during Hot Air and Infrared Drying Process. Int. J. Food Sci. Technol. 2016, 51, 842–853. [Google Scholar] [CrossRef]
- Carvalho, A.; Mattietto, R.; Oliveira, A.; Almeida, R.; Moresco, K.; Souza, T. Bioactive Compounds and Antioxidant Activity of Pepper (Capsicum sp.) Genotypes. J. Food Sci. Technol. 2015, 52, 7457–7464. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Dolatowski, Z.J.; Okoń, A. The Effect of Water Plant Extracts Addition on the Oxidative Stability of Meat Products. Acta Sci. Pol. Technol. Aliment. 2011, 10, 175–188. [Google Scholar]
- Daood, H.G.; Vinkler, M.; Markus, F.; Hebshi, E.A.; Biacs, P.A. Antioxidant Vitamin Content of Spice Red Pepper (Paprika) as Affected by Technological and Varietal Factors. Food Chem. 1996, 55, 365–372. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Gómez-Ladrón de Guevara, R.; Mínguez-Mosquera, M.I. Carotenoid Biosynthesis Changes in Five Red Pepper (Capsicum Annuum L.) Cultivars during Ripening. Cultivar Selection for Breeding. J. Agric. Food Chem. 2000, 48, 3857–3864. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of Rosemary Extract, Chitosan and α-Tocopherol on Lipid Oxidation and Colour Stability during Frozen Storage of Beef Burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef]
- Chipault, J.H.; Mizuno, G.R.; Hawkins, J.M.; Lundberg, W.O. The Antioxidant Properties of Natural Spices. J. Food Sci. 1952, 17, 46–55. [Google Scholar] [CrossRef]
- Jordán, M.J.; Castillo, J.; Bañón, S.; Martínez-Conesa, C.; Sotomayor, J.A. Relevance of the Carnosic Acid/Carnosol Ratio for the Level of Rosemary Diterpene Transfer and for Improving Lamb Meat Antioxidant Status. Food Chem. 2014, 151, 212–218. [Google Scholar] [CrossRef]
- Georgantelis, D.; Ambrosiadis, I.; Katikou, P.; Blekas, G.; Georgakis, S.A. Effect of Rosemary Extract, Chitosan and α-Tocopherol on Microbiological Parameters and Lipid Oxidation of Fresh Pork Sausages Stored at 4 °C. Meat Sci. 2007, 76, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo-Cruz, L.; Azuara-Nieto, E.; Hernández-Álvarez, A.J.; González-González, C.R.; Melgar-Lalanne, G. Evaluation of the Oxidative Stability of Chipotle Chili (Capsicum Annuum L.) Oleoresins in Avocado Oil. Grasas Aceites 2018, 69, 240. [Google Scholar] [CrossRef]
- Bhandari, P.R. Garlic (Allium Sativum L.): A Review of Potential Therapeutic Applications. Int. J. Green Pharm. 2012, 6, 118–129. [Google Scholar] [CrossRef]
- Salem, A.M.; Amin, R.A.; Afifi, G.S. Studies on Antimicrobial and Antioxidant Efficiency of Some Oils in Minced Beef. J. Am. Sci. 2010, 6, 691–700. [Google Scholar]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and Antibacte Rial Properties of Alginate-Based Edible Film Incorporated with Garlic Oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Joković, N.; Jokanović, M.; Ivić, M.; Šojić, B.; Škaljac, S.; Stojanović, P.; Mihajilov-Krstev, T. Inhibition of Salmonella Enteritidis Growth and Storage Stability in Chicken Meat Treated with Basil and Rosemary Essential Oils Alone or in Combination. Food Control 2018, 90, 332–343. [Google Scholar] [CrossRef]
- Realini, C.E.; Marcos, B. Active and Intelligent Packaging Systems for a Modern Society. Meat Sci. 2014, 98, 404–419. [Google Scholar] [CrossRef]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The Phenolic Hydroxyl Group of Carvacrol Is Essential for Action against the Food-Borne Pathogen Bacillus Cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Bonesi, M.; Serio, A.; Chaves-López, C.; Falco, T.; Paparella, A.; Menichini, F.; Tundis, R. Application of Nine Air-Dried Capsicum Annum Cultivars as Food Preservative: Micronutrient Content, Antioxidant Activity, and Foodborne Pathogens Inhibitory Effects. Int. J. Food Prop. 2017, 20, 899–910. [Google Scholar] [CrossRef]
- do Nascimento, N.F.F.; do Rêgo, E.R.; Nascimento, M.F.; Bruckner, C.H.; Finger, F.L.; do Rêgo, M.M. Combining Ability for Yield and Fruit Quality in the Pepper Capsicum Annuum. Genet. Mol. Res. 2014, 13, 3237–3249. [Google Scholar] [CrossRef] [PubMed]
- Kollia, E.; Proestos, C.; Zoumpoulakis, P.; Markaki, P. Capsaicin, an Inhibitor of Ochratoxin A Production by Aspergillus Section Nigri Strains in Grapes (Vitis Vinifera L.). Food Addit. Contam. Part A 2019, 36, 1709–1721. [Google Scholar] [CrossRef]
- Jridi, M.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Elhatmi, M.; Taktak, M.A.; Nasri, M.; Zouari, N. Effect of Rosemary Leaves and Essential Oil on Turkey Sausage Quality. Acta Aliment 2015, 44, 534–541. [Google Scholar] [CrossRef]
- Ntzimani, A.G.; Giatrakou, V.I.; Savvaidis, I.N. Combined Natural Antimicrobial Treatments (EDTA, Lysozyme, Rosemary and Oregano Oil) on Semi Cooked Coated Chicken Meat Stored in Vacuum Packages at 4 °C: Microbiological and Sensory Evaluation. Innov. Food Sci. Emerg. Technol. 2010, 11, 187–196. [Google Scholar] [CrossRef]
- Abubakar, E.M.M. Efficacy of Crude Extracts of Garlic (Allium Sativum Linn.) against Nosocomial Escherichia Coli, Staphylococcus Aureus, Streptococcus Pneumoniea and Pseudomonas Aeruginosa. J. Med. Plants Res. 2009, 3, 179–185. [Google Scholar]
- Cárdenas-Castro, A.P.; Perales-Vázquez, G.d.C.; de la Rosa, L.A.; Zamora-Gasga, V.M.; Ruiz-Valdiviezo, V.M.; Alvarez-Parrilla, E.; Sáyago-Ayerdi, S.G. Sauces: An Undiscovered Healthy Complement in Mexican Cuisine. Int. J. Gastron. Food Sci. 2019, 17, 100154. [Google Scholar] [CrossRef]
- de Lima Júnior, D.M.; do Nascimento Rangel, A.H.; Urbano, S.A.; Moreno, G.M.B. Lipid Oxidation and Lamb Meat Quality. Acta Vet. Bras. 2013, 7, 14–28. [Google Scholar] [CrossRef][Green Version]
- Dias Borges, L.; Veiga de Camargo, E.; Cassol Pires, C.; Lisboa Mello, G.; Silveira Campos, P.N.; Giongó, C.; Silveira Nalério, É. Características Sensoriais Da Carne de Cordeiros Suplementados Com Óleos EssenciaisNo Title. In 52a Reunion Anual da Sociedade Brasileira de Zootecnia; Sociedade Brasileira de Zootecnia: Belo Horizonte, Brazil, 2015; pp. 1–3. [Google Scholar]
- Mathenjwa, S.A.; Hugo, C.J.; Bothma, C.; Hugo, A. Effect of Alternative Preservatives on the Microbial Quality, Lipid Stability and Sensory Evaluation of Boerewors. Meat Sci. 2012, 91, 165–172. [Google Scholar] [CrossRef]
- Saad, S.M.; Shaltout, F.A.; Abou Elroos, N.A.; El-nahas, S.B. Antimicrobial Effect of Some Essential Oils on Some Pathogenic Bacteria in Minced Meat. J. Food Sci. Nutr. Res. 2019, 2, 13–21. [Google Scholar] [CrossRef]
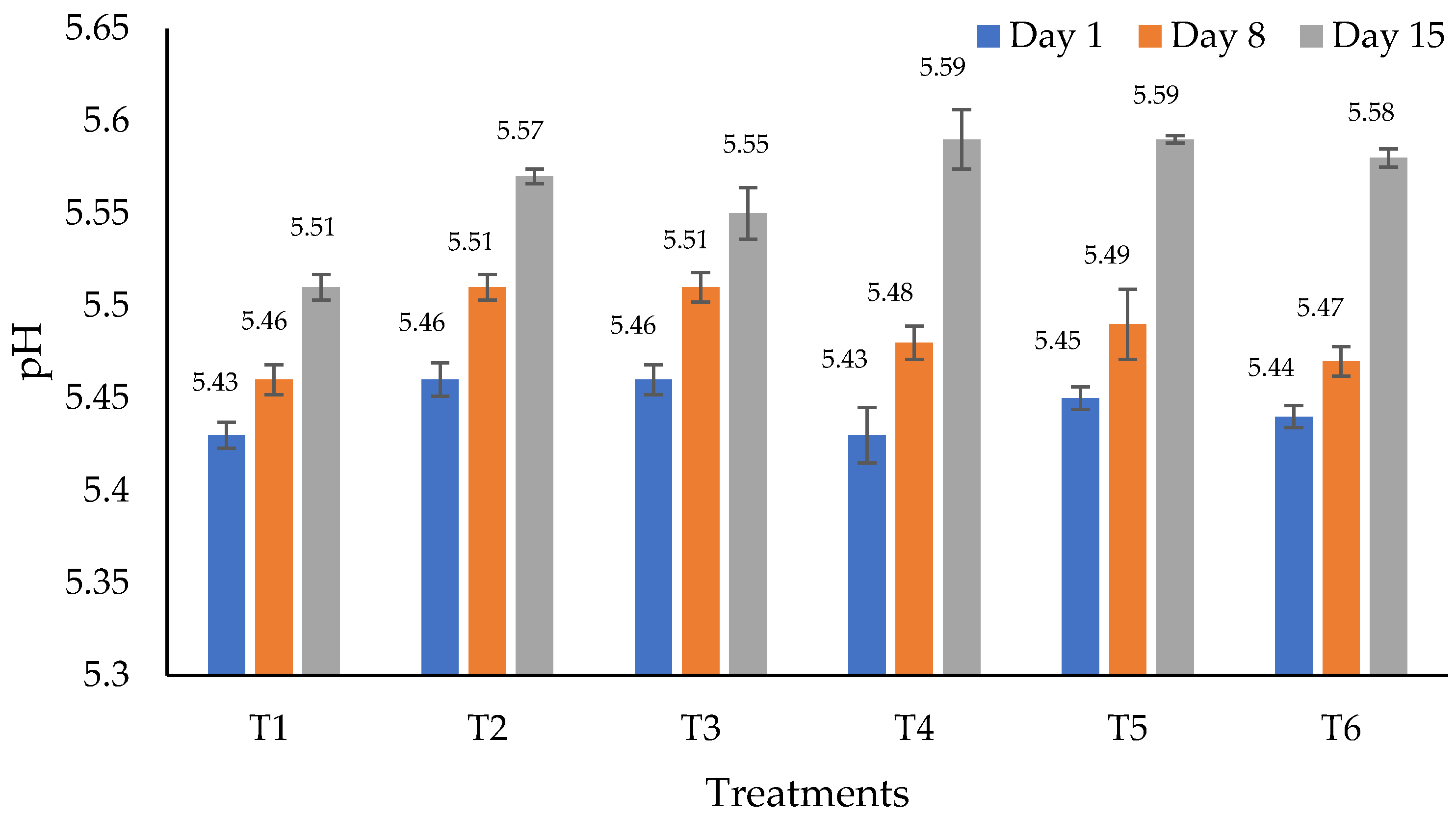
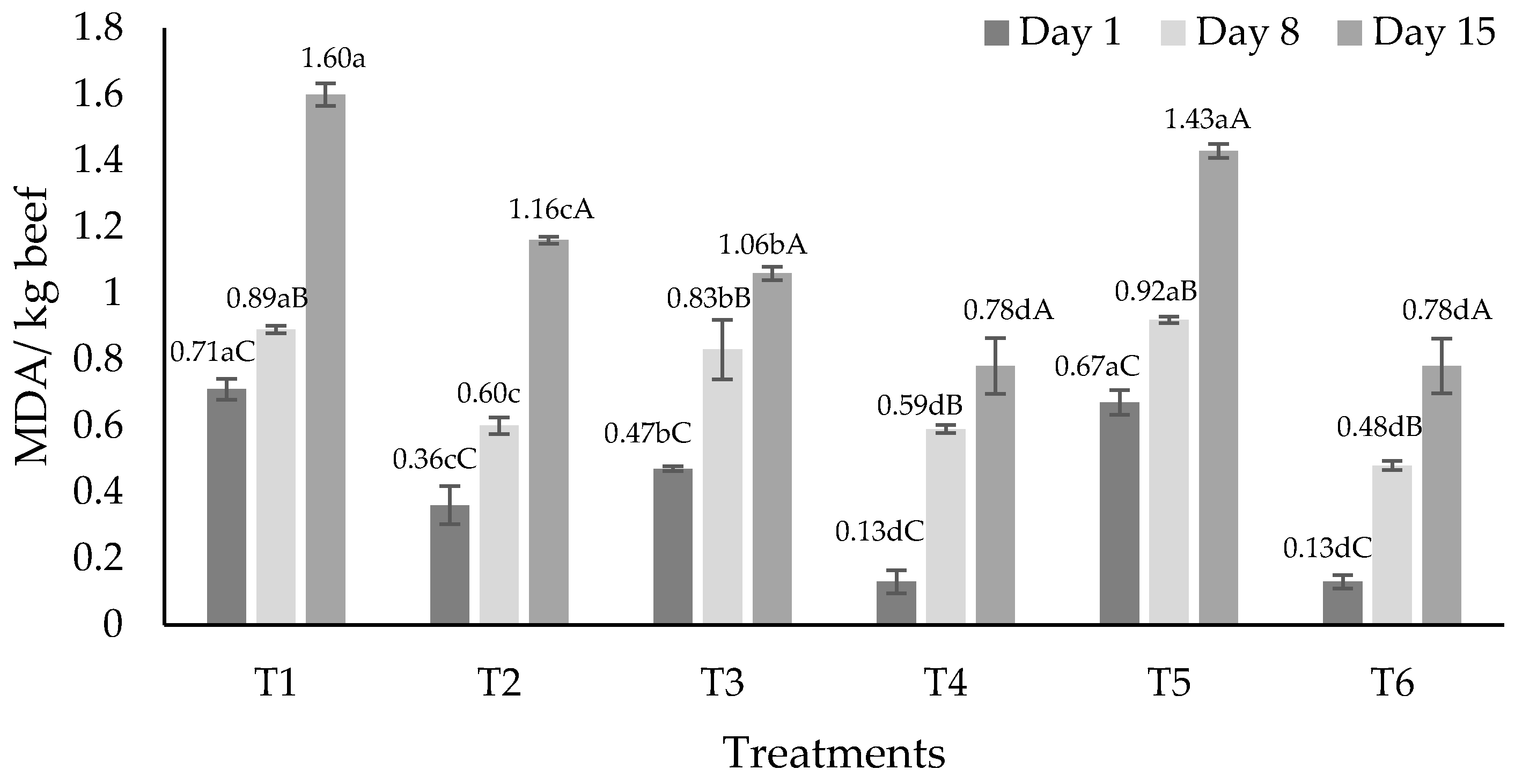

| Treatments 1 | Fat | Protein | Moisture | Ashes |
|---|---|---|---|---|
| T1 | 11.60 ± 0.21 | 21.74 ± 1.32 | 70.69 ± 1.52 | 2.44 ± 0.14 |
| T2 | 11.06 ± 0.92 | 21.80 ± 0.89 | 70.62 ± 1.77 | 2.30 ± 0.34 |
| T3 | 10.70 ± 1.19 | 21.29 ± 1.00 | 71.26 ± 1.00 | 2.36 ± 0.29 |
| T4 | 11.11 ± 0.65 | 21.55 ± 0.96 | 71.62 ± 1.06 | 2.30 ± 0.17 |
| T5 | 10.63 ± 0.50 | 20.63 ± 0.87 | 70.70 ± 1.35 | 2.29 ± 0.29 |
| T6 | 11.11 ± 1.07 | 20.80 ± 0.99 | 71.69 ± 1.23 | 2.22 ± 0.35 |
| Parameter | Time (d) | Treatments 1 | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| L* | 1 | 31.73 ± 1.19 a,B | 30.52 ± 1.43 a,B | 31.27 ± 1.43 a,B | 31.55 ± 1.75 a,B | 30.27 ± 1.36 a,B | 30.70 ± 1.14 a,B |
| 8 | 32.34 ± 1.40 a,A | 32.57 ± 0.78 a,A | 32.00 ± 1.32 a,A | 31.68 ± 1.32 a,A | 31.98 ± 2.75 a,A | 32.13 ± 1.87 a,A | |
| 15 | 31.47 ± 1.04 a,A | 31.39 ± 1.14 a,A | 32.88 ± 0.78 a,A | 31.56 ± 0.96 a,A | 31.42 ± 0.86 a,A | 33.50 ± 0.77 a,A | |
| a* | 1 | 12.17 ± 2.90 b,A | 11.62 ± 2.37 b,A | 10.55 ± 1.25 b,A | 13.29 ± 1.15 a,A | 11.37 ± 1.34 b,A | 11.53 ± 3.38 b,A |
| 8 | 8.33 ± 1.34 b,B | 8.76 ± 1.08 b,B | 8.05 ± 1.59 b,B | 10.89 ± 1.32 a,B | 9.10 ± 1.32 b,B | 10.28 ± 1.02 ab,B | |
| 15 | 10.60 ± 1.02 b,A | 11.42 ± 0.81 b,A | 11.06 ± 0.80 b,A | 12.30 ± 0.73 a,A | 10.73 ± 0.52 b,A | 11.80 ± 1.16 ab,A | |
| b* | 1 | 6.95 ± 0.56 a,B | 6.64 ± 0.59 a,B | 6.72 ± 0.56 a,B | 8.35 ± 1.04 a,B | 7.40 ± 0.60 a,B | 7.59 ± 0.74 a,B |
| 8 | 10.66 ± 1.19 a,A | 10.82 ± 0.84 a,A | 10.52 ± 2.56 a,A | 10.60 ± 2.56 a,A | 11.11 ± 0.98 a,A | 11.60 ± 0.99 a,A | |
| 15 | 12.13 ± 0.95 a,A | 11.88 ± 1.00 a,A | 11.28 ± 1.32 a,A | 8.96 ± 1.66 a,A | 9.26 ± 0.41 a,A | 11.40 ± 0.76 a,A | |
| ΔE* | 1 | - | 1.74 ± 0.57 b,A | 2.06 ± 0.87 b,A | 3.65 ± 1.16 a,A | 3.22 ± 1.16 a,A | 3.66 ± 1.55 a,A |
| 8 | - | 1.80 ± 0.76 b,A | 2.28 ± 0.98 b,A | 3.72 ± 1.06 a,A | 3.06 ± 1.45 a,A | 3.42 ± 2.04 a,A | |
| 15 | - | 2.49 ± 1.14 b,A | 2.23 ± 0.91 b,A | 3.65 ± 1.45 a,A | 3.67 ± 0.48 a,A | 3.30 ± 1.47 a,A | |
| Microorg | Time (d) | Treatment 1 | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | ||
| TAC | 1 | 2.59 ± 0.90 a,B | 1.82 ± 0.17 b,B | 1.60 ± 0.14 b,B | 1.82 ± 0.02 b,B | 1.75 ± 0.24 b,B | 1.58 ± 0.22 b,B |
| 8 | 3.14 ± 0.02 a,B | 2.37 ± 0.28 b,B | 2.77 ± 0.11 b,B | 3.30 ± 0.47 b,B | 2.43 ± 0.12 b,B | 2.60 ± 0.17 b,B | |
| 15 | 5.25 ± 0.14 a,A | 4.48 ± 0.00 b,A | 4.7 ± 0.06 b,A | 4.8 ± 0.10 b,A | 4.52 ± 0.39 b,A | 4.35 ± 0.26 b,A | |
| S. aureus | 1 | 1.26 ± 0.21 a,A | 1.31 ± 0.19 a,A | 1.08 ± 0.09 a,A | 0.85 ± 0.58 a,A | 1.01 ± 0.68 a,A | 0.60 ± 0.58 a,A |
| 8 | 1.37 ± 0.27 a,B | 1.50 ± 0.01 a,B | 1.38 ± 0.09 a,B | 1.35 ± 0.11 a,B | 1.32 ± 0.09 a,B | 1.37 ± 0.25 a,B | |
| 15 | 1.02 ± 0.35 a,C | ND | ND | 0.79 ± 0.56 a,C | ND | ND | |
| Salmonella spp. | 1 | ND | ND | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND | ND | ND | |
| 15 | ND | ND | ND | ND | ND | ND | |
| Pseudomonas spp. | 1 | 1.16 ± 0.13 b,B | 1.06 ± 0.61 b,B | 0.37 ± 0.40 a,B | 1.10 ± 0.63 b,B | 1.00 ± 0.58 b,B | 1.00 ± 0.58 b,B |
| 8 | 2.51 ± 0.08 a,B | 1.56 ± 0.03 b,B | 2.05 ± 0.23 b,A | 2.44 ± 0.33 b,B | 2.25 ± 0.49 b,B | 2.43 ± 0.36 b,B | |
| 15 | 3.25 ± 0.02 a,A | 2.17 ± 0.06 b,A | 2.68 ± 0.14 b,A | 2.51 ± 0.01 b,A | 2.28 ± 0.08 b,A | 2.58 ± 0.09 b,A | |
| B. therm | 1 | ND | ND | ND | ND | ND | ND |
| 8 | 1.99 ± 0.11 a,B | ND | 1.54 ± 0.82 b,B | ND | ND | ND | |
| 15 | 3.24 ± 0.13 a,A | 2.5 ± 0.08 b,A | ND | 0.27 ± 0.13 c,A | 0.50 ± 0.08 c,A | ND | |
| M&Y | 1 | ND | ND | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND | ND | ND | |
| 15 | 1.24 ± 0.03 a,A | 0.52 ± 0.20 b,A | 0.30 ± 0.24 b,A | 0.22 ± 0.28 b,A | 1.07 ± 0.18 a,b,A | 0.82 ± 0.11 a,b,A | |
| LAB | 1 | 0.6 ± 0.58 a,B | ND | ND | 0.60 ± 0.58 a,B | ND | ND |
| 8 | 0.92 ± 0.78 a,B | 0.60 ± 0.58 a,B | ND | ND | 0.6 ± 0.58 a,B | ND | |
| 15 | 1.6 ± 1.02 a,A | 0.99 ± 0.67 a,b,A | 0.87 ± 0.30 b,A | 1.15 ± 0.35 a,b,A | 0.85 ± 0.58 b,A | 1.22 ± 0.20 a,b,A | |
| Total coliforms | 1 | ND | ND | ND | ND | ND | ND |
| 8 | ND | ND | ND | ND | ND | ND | |
| 15 | 0.77 ± 0.70 a,A | ND | 0.43 ± 0.73 a,A | ND | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivas-Méndez, P.; Chávez-Martínez, A.; Santellano-Estrada, E.; Guerrero Asorey, L.; Sánchez-Vega, R.; Rentería-Monterrubio, A.L.; Chávez-Flores, D.; Tirado-Gallegos, J.M.; Méndez-Zamora, G. Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods 2022, 11, 2018. https://doi.org/10.3390/foods11142018
Olivas-Méndez P, Chávez-Martínez A, Santellano-Estrada E, Guerrero Asorey L, Sánchez-Vega R, Rentería-Monterrubio AL, Chávez-Flores D, Tirado-Gallegos JM, Méndez-Zamora G. Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods. 2022; 11(14):2018. https://doi.org/10.3390/foods11142018
Chicago/Turabian StyleOlivas-Méndez, Paulina, América Chávez-Martínez, Eduardo Santellano-Estrada, Luis Guerrero Asorey, Rogelio Sánchez-Vega, Ana Luisa Rentería-Monterrubio, David Chávez-Flores, Juan Manuel Tirado-Gallegos, and Gerardo Méndez-Zamora. 2022. "Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers" Foods 11, no. 14: 2018. https://doi.org/10.3390/foods11142018
APA StyleOlivas-Méndez, P., Chávez-Martínez, A., Santellano-Estrada, E., Guerrero Asorey, L., Sánchez-Vega, R., Rentería-Monterrubio, A. L., Chávez-Flores, D., Tirado-Gallegos, J. M., & Méndez-Zamora, G. (2022). Antioxidant and Antimicrobial Activity of Rosemary (Rosmarinus officinalis) and Garlic (Allium sativum) Essential Oils and Chipotle Pepper Oleoresin (Capsicum annum) on Beef Hamburgers. Foods, 11(14), 2018. https://doi.org/10.3390/foods11142018







