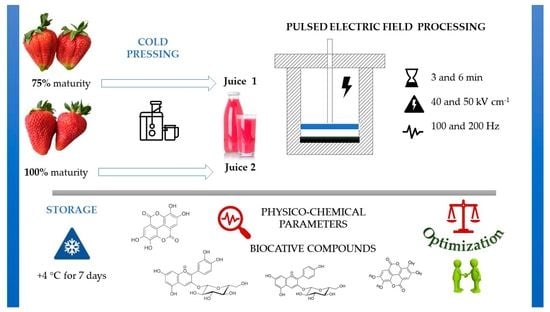
The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Standards
2.2. Strawberry Juice Preparation
2.3. High-Intensity Pulsed Electric Field (HIPEF) Processing of Strawberry Juice Samples
2.4. Characterization of Untreated and HIPEF-Treated Strawberry Juices
2.4.1. Determination of pH and Soluble Solids Content (SSC)
2.4.2. Extraction of Bioactive Compounds
2.4.3. Determination of Total Phenolic Content (TPC)
2.4.4. Determination of Total Monomeric Anthocyanins (ANT)
2.4.5. Determination of Total Hydroxycinnamic Acids (HCA)
2.4.6. Determination of Total Flavonols (FL)
2.4.7. Determination of Condensed Tannins (CT)
2.5. Statistical Analysis
3. Results and Discussion
3.1. Changes of the Physicochemical Parameters in Untreated and HIPEF-Treated Strawberry Juices during Storage
3.2. The Changes in the Stability of Bioactive Compounds of Untreated Strawberry Juices during Storage
3.3. The Influence of HIPEF Processing on the Stability of Bioactive Compounds in Strawberry Juices during Storage
3.4. Comparison of the Stability of Bioactive Compounds in Treated vs. Untreated Strawberry Juices during Storage
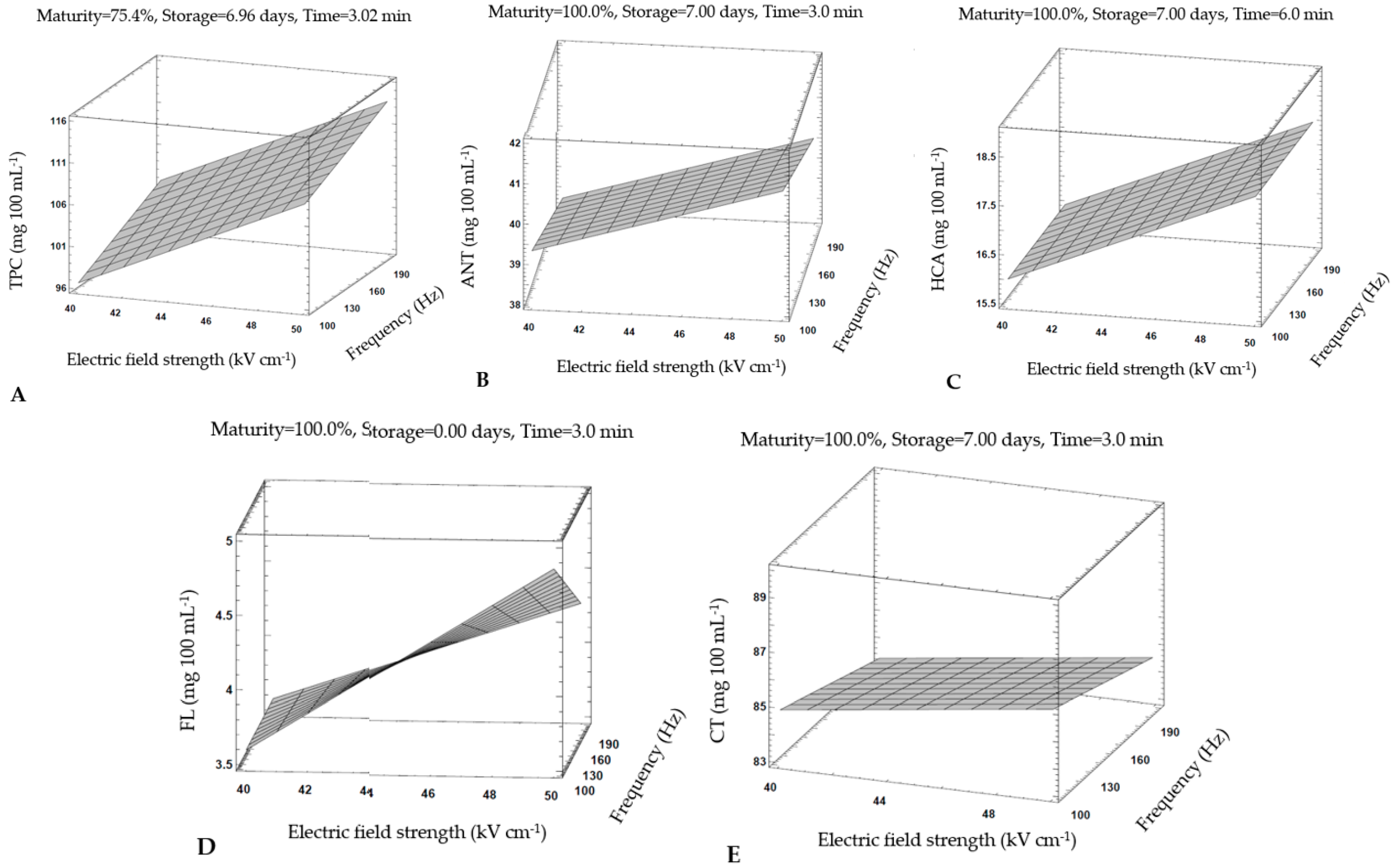
3.5. Optimization of HIPEF Processing Parameters for Strawberry Juice Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2013, 54, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Tomašević, I.; Putnik, P.; Valjak, F.; Pavlić, B.; Šojić, B.; Markovinović, A.B.; Kovačević, D.B. 3d printing as novel tool for fruit-based functional food production. Curr. Opin. Food Sci. 2021, 41, 138–145. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Ramos, P. Chilean strawberry (Fragaria chiloensis): An integrative and comprehensive review. Food Res. Int. 2019, 119, 769–776. [Google Scholar] [CrossRef]
- Milić, A.; Daničić, T.; Horecki, A.T.; Šumić, Z.; Teslić, N.; Kovačević, D.B.; Putnik, P.; Pavlić, B. Sustainable extractions for maximizing content of antioxidant phytochemicals from black and red currants. Foods 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive compounds of strawberry and blueberry and their potential health effects based on human intervention studies: A brief overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šamec, D.; Maretić, M.; Lugarić, I.; Mešić, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2016, 194, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Vlahoviček-Kahlina, K.; Duralija, B.; Maslov Bandić, L.; Nekić, P.; Vinceković, M. Stimulation of plant secondary metabolites synthesis in soilless cultivated strawberries (Fragaria × ananassa duchesne) using zinc-alginate microparticles. Turk. J. Agric. For. 2021, 45, 324–334. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, X.; Chen, H.; Liu, Y.; Xiao, Y.; Chen, H.; Tang, Z.; Li, Q.; Yao, H. Evaluation of a strawberry fermented beverage with potential health benefits. PeerJ 2021, 9, 11974. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villena, J.; Elean, M.; de Giori, G.S.; Saavedra, L.; Hebert, E.M. Immunomodulatory properties of a γ-aminobutyric acid-enriched strawberry juice produced by Levilactobacillus brevis crl 2013. Front. Microbiol. 2020, 11, 610016. [Google Scholar] [CrossRef]
- Leyva-Porras, C.; Saavedra-Leos, M.Z.; López-Martinez, L.A.; Espinosa-Solis, V.; Terán-Figueroa, Y.; Toxqui-Terán, A.; Compeán-Martínez, I. Strawberry juice powders: Effect of spray-drying conditions on the microencapsulation of bioactive components and physicochemical properties. Molecules 2021, 26, 5466. [Google Scholar] [CrossRef]
- Mazur, S.P.; Nes, A.; Wold, A.-B.; Remberg, S.F.; Martinsen, B.K.; Aaby, K. Effects of ripeness and cultivar on chemical composition of strawberry (Fragaria × ananassa duch.) fruits and their suitability for jam production as a stable product at different storage temperatures. Food Chem. 2014, 146, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Markovinović, A.B.; Putnik, P.; Duralija, B.; Krivohlavek, A.; Ivešić, M.; Andačić, I.M.; Bešlić, I.P.; Pavlić, B.; Lorenzo, J.M.; Kovačević, D.B. Chemometric valorization of strawberry (Fragaria x ananassa duch.) cv. ‘Albion’ for the production of functional juice: The impact of physicochemical, toxicological, sensory, and bioactive value. Foods 2022, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, S.; Pokhrel, P.R.; Unluturk, S.; Barbosa-Cánovas, G.V. Changes in quality characteristics of strawberry juice after equivalent high pressure, ultrasound, and pulsed electric fields processes. Food Eng. Rev. 2020, 13, 601–612. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Pulsed electric fields processing effects on quality and health-related constituents of plant-based foods. Trends Food Sci. Technol. 2013, 29, 98–107. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes in quality attributes throughout storage of strawberry juice processed by high-intensity pulsed electric fields or heat treatments. LWT Food Sci. Technol. 2009, 42, 813–818. [Google Scholar] [CrossRef]
- Gabrić, D.; Barba, F.; Roohinejad, S.; Gharibzahedi, S.M.T.; Radojčin, M.; Putnik, P.; Kovačević, D.B. Pulsed electric fields as an alternative to thermal processing for preservation of nutritive and physicochemical properties of beverages: A review. J. Food Process Eng. 2018, 41, e12638. [Google Scholar] [CrossRef]
- Elez-Martínez, P.; Odriozola-Serrano, I.; Oms-Oliu, G.; Soliva-Fortuny, R.; Martín-Belloso, O. Effects of pulsed electric fields processing strategies on health-related compounds of plant-based foods. Food Eng. Rev. 2017, 9, 213–225. [Google Scholar] [CrossRef]
- Asl, P.J.; Rajulapati, V.; Gavahian, M.; Kapusta, I.; Putnik, P.; Khaneghah, A.M.; Marszałek, K. Non-thermal plasma technique for preservation of fresh foods: A review. Food Control 2022, 134, 108560. [Google Scholar] [CrossRef]
- Škegro, M.; Putnik, P.; Kovačević, D.B.; Kovač, A.P.; Salkić, L.; Čanak, I.; Frece, J.; Zavadlav, S.; Ježek, D. Chemometric comparison of high-pressure processing and thermal pasteurization: The nutritive, sensory, and microbial quality of smoothies. Foods 2021, 10, 1167. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the ph differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Wojdyło, A. Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur. Food Res. Technol. 2008, 228, 623–631. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.-J.; Shin, Y. Influence of ripening stage and cultivar on physicochemical properties, sugar and organic acid profiles, and antioxidant compositions of strawberries. Food Sci. Biotechnol. 2019, 28, 1659–1667. [Google Scholar] [CrossRef]
- Ornelas-Paz, J.d.J.; Yahia, E.M.; Ramírez-Bustamante, N.; Pérez-Martínez, J.D.; Escalante-Minakata, M.D.P.; Ibarra-Junquera, V.; Acosta-Muñiz, C.; Guerrero-Prieto, V.; Ochoa-Reyes, E. Physical attributes and chemical composition of organic strawberry fruit (Fragaria x ananassa duch, cv. Albion) at six stages of ripening. Food Chem. 2013, 138, 372–381. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jiang, X.; Liu, H. Detection of the quality of juicy peach during storage by visible/near infrared spectroscopy. Vib. Spectrosc. 2020, 111, 103152. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, W.; Ge, Z.; Zhang, L.; Li, C.; Zhang, B.; Zong, W. Comparison of the effects of dynamic high-pressure microfluidization and conventional homogenization on the quality of peach juice. J. Sci. Food Agric. 2019, 99, 5994–6000. [Google Scholar] [CrossRef]
- Aaby, K.; Grimsbo, I.H.; Hovda, M.B.; Rode, T.M. Effect of high pressure and thermal processing on shelf life and quality of strawberry purée and juice. Food Chem. 2018, 260, 115–123. [Google Scholar] [CrossRef]
- Mtaoua, H.; Sánchez-Vega, R.; Ferchichi, A.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields or thermal treatment on the quality attributes of date juice through storage. J. Food Processing Preserv. 2017, 41, e13052. [Google Scholar] [CrossRef] [Green Version]
- Geveke, D.J.; Aubuchon, I.; Zhang, H.Q.; Boyd, G.; Sites, J.E.; Bigley, A.B.W. Validation of a pulsed electric field process to pasteurize strawberry purée. J. Food Eng. 2015, 166, 384–389. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Changes in the polyphenol profile of tomato juices processed by pulsed electric fields. J. Agric. Food Chem. 2012, 60, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Queralt, A.; Odriozola-Serrano, I.; Oms-Oliu, G.; Lamuela-Raventós, R.M.; Elez-Martínez, P.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields on carotenoids profile of tomato juice made of moderate-intensity pulsed electric field-treated tomatoes. Food Chem. 2013, 141, 3131–3138. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Phenolic acids, flavonoids, vitamin c and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- Pradas, I.; Medina, J.J.; Ortiz, V.; Moreno-Rojas, J.M. ‘Fuentepina’ and ‘amiga’, two new strawberry cultivars: Evaluation of genotype, ripening and seasonal effects on quality characteristics and health-promoting compounds. J. Berry Res. 2015, 5, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Fait, A.; Hanhineva, K.; Beleggia, R.; Dai, N.; Rogachev, I.; Nikiforova, V.J.; Fernie, A.R.; Aharoni, A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiol. 2008, 148, 730–750. [Google Scholar] [CrossRef] [Green Version]
- Aubert, C.; Bruaut, M.; Chalot, G.; Cottet, V. Impact of maturity stage at harvest on the main physicochemical characteristics, the levels of vitamin c, polyphenols and volatiles and the sensory quality of gariguette strawberry. Eur. Food Res. Technol. 2020, 247, 37–49. [Google Scholar] [CrossRef]
- Wei, M.; Wang, H.; Ma, T.; Ge, Q.; Fang, Y.; Sun, X. Comprehensive utilization of thinned unripe fruits from horticultural crops. Foods 2021, 10, 2043. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Roobab, U.; Aadil, R.M.; Madni, G.M.; Bekhit, A.E.-D. The impact of nonthermal technologies on the microbiological quality of juices: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 437–457. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Siddeeg, A.; Manzoor, M.F.; Ahmad, M.H.; Ahmad, N.; Ahmed, Z.; Khan, M.K.I.; Maan, A.A.; Mahr Un, N.; Zeng, X.-A.; Ammar, A.-F. Pulsed electric field-assisted ethanolic extraction of date palm fruits: Bioactive compounds, antioxidant activity and physicochemical properties. Processes 2019, 7, 585. [Google Scholar] [CrossRef] [Green Version]
- Stübler, A.-S.; Lesmes, U.; Juadjur, A.; Heinz, V.; Rauh, C.; Shpigelman, A.; Aganovic, K. Impact of pilot-scale processing (thermal, pef, hpp) on the stability and bioaccessibility of polyphenols and proteins in mixed protein- and polyphenol-rich juice systems. Innov. Food Sci. Emerg. Technol. 2020, 64, 102426. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Impact of high-intensity pulsed electric fields variables on vitamin c, anthocyanins and antioxidant capacity of strawberry juice. LWT Food Sci. Technol. 2009, 42, 93–100. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of fruits and vegetables in refrigerator increases their phenolic acids but decreases the total phenolics, anthocyanins and vitamin c with subsequent loss of their antioxidant capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Hernández-Jover, T.; Martín-Belloso, O. Carotenoid and phenolic profile of tomato juices processed by high intensity pulsed electric fields compared with conventional thermal treatments. Food Chem. 2009, 112, 258–266. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Qualitative analysis for evaluation of herbal drugs. In Quality Control and Evaluation of Herbal Drugs; Elsevier: London, UK, 2019; pp. 79–149. [Google Scholar]
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and ft-ir analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Rosa, M.D. Pulsed electric field (pef) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, X.; Ni, Y.; Wu, J.; Hu, X.; Wang, Z.; Chen, F. Kinetic analysis of the degradations and its color change of cyaniding-3-glucoside exposed to pulsed electric field. Eur. Food Res. Technol. 2007, 224, 597–603. [Google Scholar] [CrossRef]
- Putnik, P.; Pavlić, B.; Šojić, B.; Zavadlav, S.; Žuntar, I.; Kao, L.; Kitonić, D.; Kovačević, D.B. Innovative hurdle technologies for the preservation of functional fruit juices. Foods 2020, 9, 699. [Google Scholar] [CrossRef]
- Poojary, M.M.; Lund, M.N.; Barba, F.J. Pulsed electric field (pef) as an efficient technology for food additives and nutraceuticals development. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier: London, UK, 2020; pp. 65–99. [Google Scholar]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Gimeno-Añó, V.; Martín-Belloso, O. Kinetic study of anthocyanins, vitamin c, and antioxidant capacity in strawberry juices treated by high-intensity pulsed electric fields. J. Agric. Food Chem. 2008, 56, 8387–8393. [Google Scholar] [CrossRef]
- Guderjan, M.; Töpfl, S.; Angersbach, A.; Knorr, D. Impact of pulsed electric field treatment on the recovery and quality of plant oils. J. Food Eng. 2005, 67, 281–287. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Álvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Asavasanti, S.; Ristenpart, W.; Stroeve, P.; Barrett, D.M. Permeabilization of plant tissues by monopolar pulsed electric fields: Effect of frequency. J. Food Sci. 2011, 76, E98–E111. [Google Scholar] [CrossRef] [PubMed]
- Ersus, S.; Oztop, M.H.; McCarthy, M.J.; Barrett, D.M. Disintegration efficiency of pulsed electric field induced effects on onion (Allium cepa L.) tissues as a function of pulse protocol and determination of cell integrity by 1h-nmr relaxometry. J. Food Sci. 2010, 75, E444–E452. [Google Scholar] [CrossRef]

| Sample | Juice | Storage (Days) | Treatment | Electric Field Strength (kV cm−1) | Frequency (Hz) | Pulse Duration (min) |
|---|---|---|---|---|---|---|
| 1 | J1 | 0 | Control | / | / | / |
| 2 | J1 | 0 | HIPEF | 40 | 100 | 3 |
| 3 | J1 | 0 | HIPEF | 200 | 3 | |
| 4 | J1 | 0 | HIPEF | 100 | 6 | |
| 5 | J1 | 0 | HIPEF | 200 | 6 | |
| 6 | J1 | 0 | HIPEF | 50 | 100 | 3 |
| 7 | J1 | 0 | HIPEF | 200 | 3 | |
| 8 | J1 | 0 | HIPEF | 100 | 6 | |
| 9 | J1 | 0 | HIPEF | 200 | 6 | |
| 10 | J2 | 0 | Control | / | / | / |
| 11 | J2 | 0 | HIPEF | 40 | 100 | 3 |
| 12 | J2 | 0 | HIPEF | 200 | 3 | |
| 13 | J2 | 0 | HIPEF | 100 | 6 | |
| 14 | J2 | 0 | HIPEF | 200 | 6 | |
| 15 | J2 | 0 | HIPEF | 50 | 100 | 3 |
| 16 | J2 | 0 | HIPEF | 200 | 3 | |
| 17 | J2 | 0 | HIPEF | 100 | 6 | |
| 18 | J2 | 0 | HIPEF | 200 | 6 | |
| 19 | J1 | 7 | Control | / | / | / |
| 20 | J1 | 7 | HIPEF | 40 | 100 | 3 |
| 21 | J1 | 7 | HIPEF | 200 | 3 | |
| 22 | J1 | 7 | HIPEF | 100 | 6 | |
| 23 | J1 | 7 | HIPEF | 200 | 6 | |
| 24 | J1 | 7 | HIPEF | 50 | 100 | 3 |
| 25 | J1 | 7 | HIPEF | 200 | 3 | |
| 26 | J1 | 7 | HIPEF | 100 | 6 | |
| 27 | J1 | 7 | HIPEF | 200 | 6 | |
| 28 | J2 | 7 | Control | / | / | / |
| 29 | J2 | 7 | HIPEF | 40 | 100 | 3 |
| 30 | J2 | 7 | HIPEF | 200 | 3 | |
| 31 | J2 | 7 | HIPEF | 100 | 6 | |
| 32 | J2 | 7 | HIPEF | 200 | 6 | |
| 33 | J2 | 7 | HIPEF | 50 | 100 | 3 |
| 34 | J2 | 7 | HIPEF | 200 | 3 | |
| 35 | J2 | 7 | HIPEF | 100 | 6 | |
| 36 | J2 | 7 | HIPEF | 200 | 6 |
| Variables | n | SSC (%) | pH |
|---|---|---|---|
| Maturity | p ≤ 0.01 † | p ≤ 0.01 † | |
| 75% (Juice J1) | 4 | 6.15 ± 0.03 b | 3.38 ± 0.01 b |
| 100% (Juice J2) | 4 | 8.03 ± 0.03 a | 3.53 ± 0.01 a |
| Storage | 4 | p ≤ 0.01 † | p = 0.02 † |
| 0 days | 4 | 7.20 ± 0.03 a | 3.48 ± 0.01 a |
| 7 days | 8 | 6.98 ± 0.03 b | 3.43 ± 0.01 a |
| Dataset average | 7.09 ± 0.02 | 3.41 ± 0.01 |
| Variables | n | SSC (%) | pH |
|---|---|---|---|
| Maturity | p ≤ 0.01 † | p ≤ 0.01 † | |
| 75% (Juice J1) | 32 | 5.82 ± 0.01 b | 3.44 ± 0.01 b |
| 100% (Juice J2) | 32 | 7.92 ± 0.01 a | 3.51 ± 0.01 a |
| Storage | p ≤ 0.01 † | p = 0.22 ‡ | |
| 0 days | 32 | 6.97 ± 0.01 a | 3.48 ± 0.01 a |
| 7 days | 32 | 6.78 ± 0.01 b | 3.46 ± 0.01 a |
| Electric field strength | p ≤ 0.01 † | p = 0.41 ‡ | |
| 40 kV cm−1 | 32 | 6.82 ± 0.01 b | 3.46 ± 0.01 a |
| 50 kV cm−1 | 32 | 6.92 ± 0.01 a | 3.48 ± 0.01 a |
| Frequency | p = 0.39 ‡ | p = 0.23 ‡ | |
| 100 Hz | 32 | 6.86 ± 0.01 a | 3.46 ± 0.01 a |
| 200 Hz | 32 | 6.89 ± 0.01 a | 3.48 ± 0.01 a |
| Treatment time | p = 0.31 ‡ | p = 0.21 ‡ | |
| 3 min | 32 | 6.85 ± 0.01 a | 3.48 ± 0.01 a |
| 6 min | 32 | 6.89 ± 0.01 a | 3.46 ± 0.01 a |
| Dataset average | 64 | 6.87 ± 0.01 | 3.47 ± 0.01 |
| Variables | n | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|---|
| Maturity | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 75% (Juice J1) | 4 | 85.19 ± 1.77 a | 16.87 ± 0.20 b | 9.76 ± 0.44 b | 1.78 ± 0.10 b | 56.28 ± 0.58 b |
| 100% (Juice J2) | 4 | 74.39 ± 1.77 b | 36.80 ± 0.20 a | 12.71 ± 0.44 a | 2.29 ± 0.10 a | 64.70 ± 0.58 a |
| Storage | p ≤ 0.01 † | p ≤ 0.01† | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 0 days | 4 | 63.55 ± 1.77 b | 23.82 ± 0.20 b | 8.76 ± 0.44 b | 2.94 ± 0.10 a | 41.20 ± 0.58 b |
| 7 days | 4 | 96.02 ± 1.77 a | 29.85 ± 0.20 a | 13.71 ± 0.44 a | 1.13 ± 0.10 b | 79.78 ± 0.58 a |
| Dataset average | 8 | 79.78 ± 1.25 | 26.83 ± 0.14 | 11.24 ± 0.31 | 2.03 ± 0.07 | 60.49 ± 0.40 |
| Variables | n | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|---|
| Maturity | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 75% (Juice J1) | 32 | 96.91 ± 1.52 a | 19.01 ± 0.18 b | 10.62 ± 0.12 b | 1.81 ± 0.04 b | 64.99 ± 0.22 b |
| 100% (Juice J2) | 32 | 82.08 ± 1.52 b | 37.97 ± 0.18 a | 14.10 ± 0.12 a | 2.48 ± 0.04 a | 69.99 ± 0.22 a |
| Storage | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 0 days | 32 | 83.11 ± 1.52 b | 27.35 ± 0.18 b | 10.14 ± 0.12 b | 3.29 ± 0.04 a | 54.79 ± 0.22 b |
| 7 days | 32 | 95.88 ± 1.52 a | 29.63 ± 0.18 a | 14.58 ± 0.12 a | 1.00 ± 0.04 b | 80.18 ± 0.22 a |
| Electric field strength | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 40 kV cm−1 | 32 | 85.89 ± 1.52 b | 27.73 ± 0.18 b | 11.59 ± 0.12 b | 1.91 ± 0.04 b | 63.98 ± 0.22 b |
| 50 kV cm−1 | 32 | 93.11 ± 1.52 a | 29.26 ± 0.18 a | 13.12 ± 0.12 a | 2.38 ± 0.04 a | 70.99 ± 0.22 a |
| Frequency | p = 0.27 ‡ | p = 0.03 † | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † | |
| 100 Hz | 32 | 90.69 ± 1.52 a | 28.79 ± 0.18 a | 12.60 ± 0.12 a | 2.29 ± 0.04 a | 68.57 ± 0.22 a |
| 200 Hz | 32 | 88.31 ± 1.52 a | 28.19 ± 0.18 a | 12.12 ± 0.12 b | 2.00 ± 0.04 b | 66.40 ± 0.22 b |
| Time | p = 0.99 ‡ | p = 0.40 ‡ | p = 0.30 ‡ | p ≤ 0.01 † | p = 0.05 † | |
| 3 min | 32 | 89.49 ± 1.52 a | 28.38 ± 0.18 a | 12.45 ± 0.12 a | 2.29 ± 0.04 a | 67.78 ± 0.22 a |
| 6 min | 32 | 89.5 ± 1.52 a | 28.60 ± 0.18 a | 12.27 ± 0.12 a | 2.00 ± 0.04 b | 67.19 ± 0.22 b |
| Dataset average | 64 | 89.49 ± 0.50 | 28.60 ± 0.13 | 12.36 ± 0.09 | 2.15 ± 0.03 | 67.49 ± 0.14 |
| Variables | T1 (°C) | T2 (°C) | ΔT (°C) | Voltage (kV) | Current (mA) | Power (W) |
|---|---|---|---|---|---|---|
| Electric field strength | p = 0.30 ‡ | p = 0.21 ‡ | p = 0.80 ‡ | p ≤ 0.01 † | p ≤ 0.01 † | p ≤ 0.01 † |
| 40 kV cm−1 | 20.11 ± 1.04 a | 22.06 ± 0.57 a | 1.95 ± 1.42 a | 39.89 ± 0.02 b | 3.00 ± 0.02 b | 119.75 ± 0.72 b |
| 50 kV cm−1 | 21.75 ± 1.04 a | 23.16 ± 0.57 a | 1.41 ± 1.42 a | 49.93 ± 0.02 a | 3.84 ± 0.02 a | 190.00 ± 0.72 a |
| Frequency | p = 0.32 ‡ | p = 0.44 ‡ | p = 0.30 ‡ | p = 0.58 ‡ | p ≤ 0.01 † | p ≤ 0.01 † |
| 100 Hz | 20.15 ± 1.04 a | 22.94 ± 0.57 a | 2.79 ± 1.42 a | 44.90 ± 0.02 a | 2.83 ± 0.02 b | 128.38 ± 0.72 b |
| 200 Hz | 21.71 ± 1.04 a | 22.29 ± 0.57 a | 0.58 ± 1.42 a | 44.91 ± 0.02 a | 4.01 ± 0.02 a | 181.38 ± 0.72 a |
| Treatment time | p = 0.50 ‡ | p = 0.49 ‡ | p = 0.82 ‡ | p = 0.12 ‡ | p = 0.58 ‡ | p = 0.25 ‡ |
| 3 min | 20.41 ± 1.04 a | 22.33 ± 0.57 a | 1.91 ± 1.42 a | 44.89 ± 0.02 a | 3.41 ± 0.02 a | 154.25 ± 0.72 a |
| 6 min | 21.45 ± 1.04 a | 22.90 ± 0.57 a | 1.45 ± 1.42 a | 44.93 ± 0.02 a | 3.43 ± 0.02 a | 155.50 ± 0.72 a |
| Dataset average | 20.93 ± 0.74 | 22.93 ± 0.40 | 1.68 ± 1.00 | 44.91 ± 0.11 | 3.42 ± 0.01 | 154.88 ± 0.51 |
| Analytical Variable | TPC | ANT | HCA | FL | CT |
|---|---|---|---|---|---|
| Content (mg 100 mL−1) | 113.75 | 41.04 | 18.00 | 4.85 | 86.07 |
| Maturity (%) | 75.40 | 100.00 | 100.00 | 100.00 | 100.00 |
| Storage (days) | 6.96 | 7.00 | 7.00 | 0.00 | 7.00 |
| Field (kV cm−1) | 49.90 | 50.00 | 50.00 | 50.00 | 50.00 |
| Frequency (Hz) | 199.74 | 100.00 | 100.00 | 100.00 | 100.00 |
| Time (min) | 3.02 | 3.00 | 6.00 | 3.00 | 3.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bebek Markovinović, A.; Putnik, P.; Stulić, V.; Batur, L.; Duralija, B.; Pavlić, B.; Vukušić Pavičić, T.; Herceg, Z.; Bursać Kovačević, D. The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness. Foods 2022, 11, 1997. https://doi.org/10.3390/foods11141997
Bebek Markovinović A, Putnik P, Stulić V, Batur L, Duralija B, Pavlić B, Vukušić Pavičić T, Herceg Z, Bursać Kovačević D. The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness. Foods. 2022; 11(14):1997. https://doi.org/10.3390/foods11141997
Chicago/Turabian StyleBebek Markovinović, Anica, Predrag Putnik, Višnja Stulić, Luka Batur, Boris Duralija, Branimir Pavlić, Tomislava Vukušić Pavičić, Zoran Herceg, and Danijela Bursać Kovačević. 2022. "The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness" Foods 11, no. 14: 1997. https://doi.org/10.3390/foods11141997
APA StyleBebek Markovinović, A., Putnik, P., Stulić, V., Batur, L., Duralija, B., Pavlić, B., Vukušić Pavičić, T., Herceg, Z., & Bursać Kovačević, D. (2022). The Application and Optimization of HIPEF Technology in the Processing of Juice from Strawberries Harvested at Two Stages of Ripeness. Foods, 11(14), 1997. https://doi.org/10.3390/foods11141997