The Effectiveness of Clove Extract on Oxidization-Induced Changes of Structure and Gelation in Porcine Myofibrillar Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CE
2.3. Preparation of MPs
2.4. Oxidation of MPs Samples and Experimental Design
2.5. UV Scanning Spectra
2.6. Secondary Structure of MPs
2.7. Fourier Transform Infrared (FTIR) Spectra
2.8. Particle Size
2.9. Protein Turbidity
2.10. Determination of Protein Gel Strength
2.11. Scanning Electron Microscopy
2.12. Statistical Analysis
3. Results and Discussion
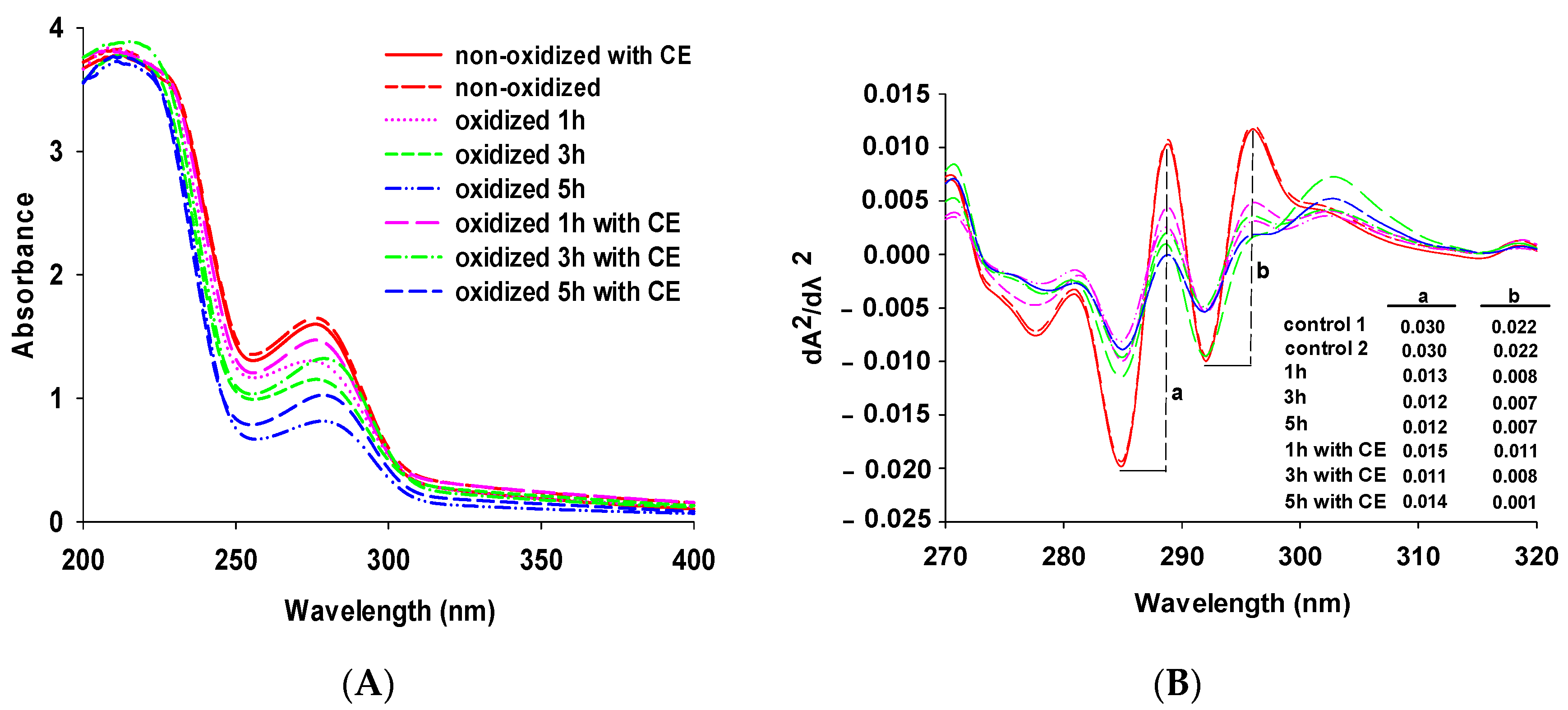
3.1. Changes in UV Spectra
3.2. Changes in CD Spectra
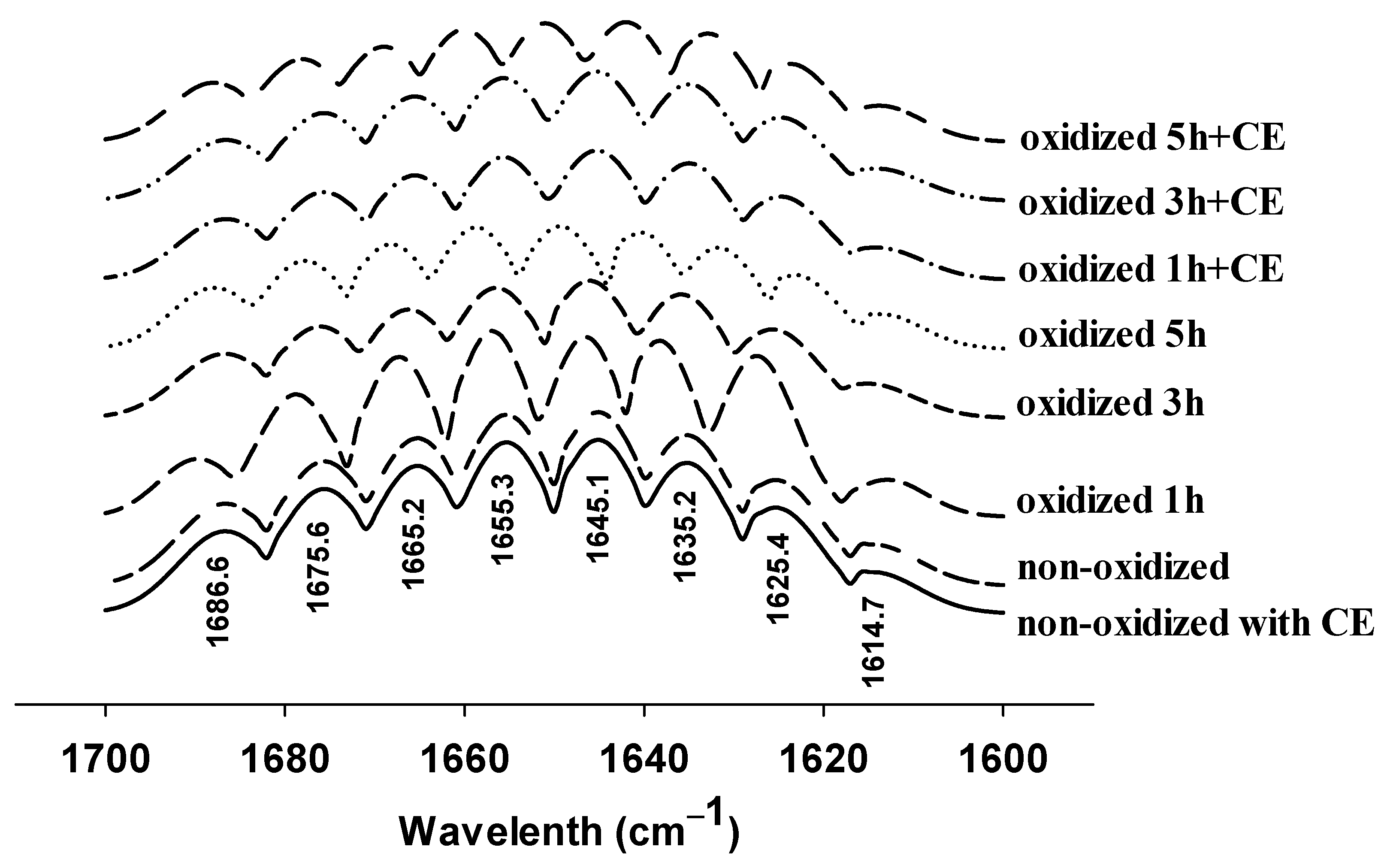
3.3. Changes in FTIR Spectra
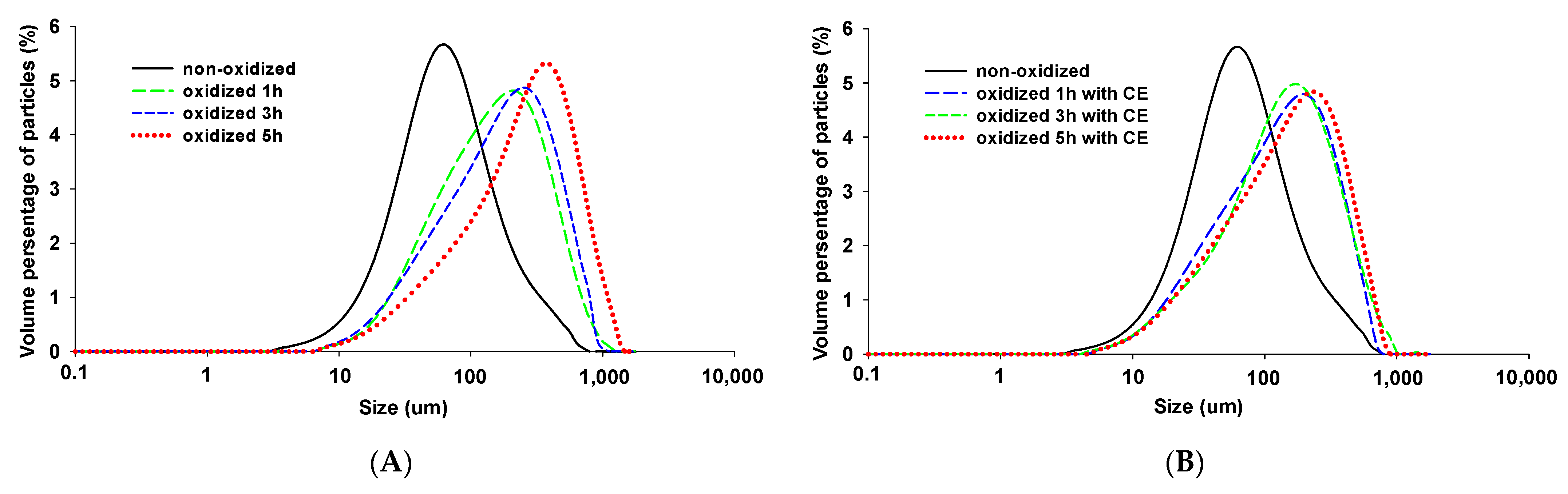
3.4. Particle Size
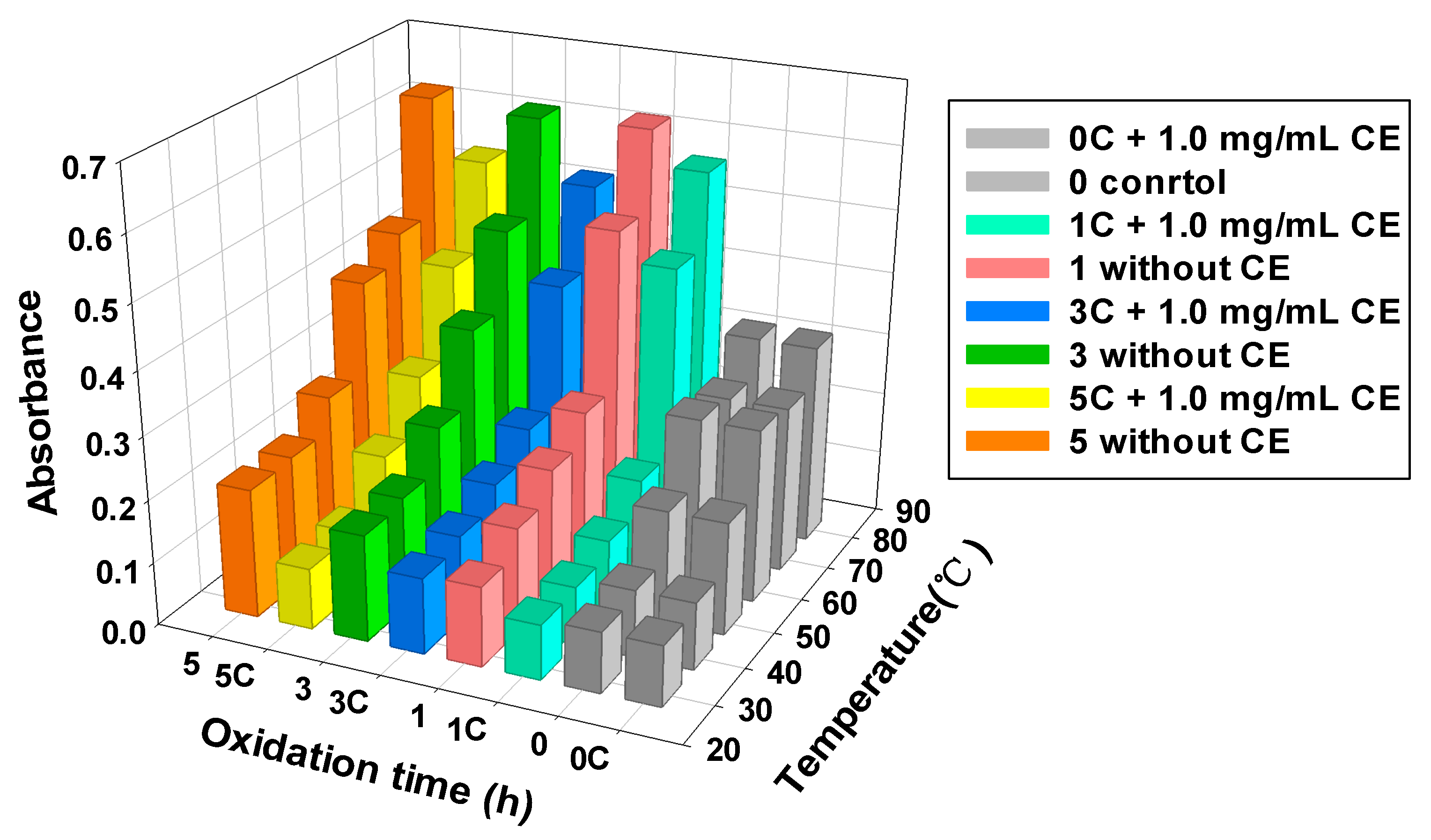
3.5. MPs Turbidity
3.6. Gel Strength
3.7. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Morzel, M.; Gatellier, P.; Sayd, T.; Renerre, M.; Laville, E. Chemical oxidation decreases proteolytic susceptibility of skeletal muscle myofibrillar proteins. Meat Sci. 2006, 73, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Park, D.; Xiong, Y.L.; Alderton, A.L. Concentration effects of hydroxyl radical oxidizing systems on biochemical properties of porcine muscle myofibrillar protein. Food Chem. 2006, 101, 1239–1246. [Google Scholar] [CrossRef]
- Chen, H.; Diao, J.; Li, Y.; Chen, Q.; Kong, B. The effectiveness of clove extracts in the inhibition of hydroxyl radical oxidation-induced structural and rheological changes in porcine myofibrillar protein. Meat Sci. 2016, 111, 60–66. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, D.; Liu, Y. Comparison of protein differences between high- and low-quality goat and bovine parts based on iTRAQ technology. Food Chem. 2019, 289, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Huang, F.; Huang, M.; Zhou, G. Influence of oxidation on myofibrillar proteins degradation from bovine via μ-calpain. Food Chem. 2012, 134, 106–112. [Google Scholar] [CrossRef]
- Estévez, M.; Heinonen, M. Effect of Phenolic Compounds on the Formation of α-Aminoadipic and γ-Glutamic Semialdehydes from Myofibrillar Proteins Oxidized by Copper, Iron, and Myoglobin. J. Agric. Food Chem. 2010, 58, 4448–4455. [Google Scholar] [CrossRef]
- Santé-Lhoutellier, V.; Engel, E.; Aubry, L.; Gatellier, P. Effect of animal (lamb) diet and meat storage on myofibrillar protein oxidation and in vitro digestibility. Meat Sci. 2008, 79, 777–783. [Google Scholar] [CrossRef]
- Kong, B.; Zhang, H.; Xiong, Y.L. Antioxidant activity of spice extracts in a liposome system and in cooked pork patties and the possible mode of action. Meat Sci. 2010, 85, 772–778. [Google Scholar] [CrossRef]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Zhang, Y.; Yan, L.; Guo, Y.; Niu, L. Phenolic compounds and antioxidant activity of bulb extracts of six lilium species native to china. Molecules 2012, 17, 9361–9378. [Google Scholar] [CrossRef] [PubMed]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cassiana Frohlich, P.; Andressa Santos, K.; Din Mahmud Hasan, S.; Antônio da Silva, E. Evaluation of the ethanolic ultrasound-assisted extraction from clove (Syzygium aromaticum) leaves and chemical characterization of the extracts. Food Chem. 2022, 373, 131351. [Google Scholar] [CrossRef] [PubMed]
- Tomova, T.; Petkov, V.; Slavova, I.; Stoyanov, P.; Argirova, M. Naturally present metal ions in plants could interfere with common antioxidant assays. MethodsX 2020, 7, 100995. [Google Scholar] [CrossRef]
- El-Maati, M.F.A.; Mahgoub, S.A.; Labib, S.M.; Al-Gaby, A.M.; Ramadan, M.F. Phenolic extracts of clove (Syzygium aromaticum) with novel antioxidant and antibacterial activities. Eur. J. Integr. Med. 2016, 8, 494–504. [Google Scholar] [CrossRef]
- Tan, P.X.; Ye, T.; Liu, X.X.; He, J.H. Research advances in antioxidant composition of botanical extracts and their action mechanisms. Food Sci. 2010, 31, 288–292. [Google Scholar]
- Zahid, M.A.; Choi, J.Y.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Effects of clove extract on oxidative stability and sensory attributes in cooked beef patties at refrigerated storage. Meat Sci. 2020, 161, 107972. [Google Scholar] [CrossRef]
- Bensid, A.; Ucar, Y.; Bendeddouche, B.; Özogul, F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chem. 2014, 145, 681–686. [Google Scholar] [CrossRef]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Krishnan, K.R.; Babuskin, S.; Babu, P.A.S.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial and antioxidant effects of five spice and herb extracts as natural preservatives of raw pork. J. Sci. Food Agric. 2009, 89, 1879–1885. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, X.; Li, X.; Wu, J.; Guo, X. The application of clove extract protects chinese-style sausages against oxidation and quality deterioration. Korean J. Food Sci. Anim. Resour. 2017, 37, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Armenteros, M.; Morcuende, D.; Ventanas, J.; Estévez, M. The application of natural antioxidants via brine injection protects Iberian cooked hams against lipid and protein oxidation. Meat Sci. 2016, 116, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.S.; Niu, B.H.; Liu, H.; Li, Y.Q.; Zhang, R.H.; Kong, B.H. Antioxidant effect of four spice extracts on porcine myofibrillar protein. Food Sci. 2019, 4, 95–101. [Google Scholar]
- Zhang, H.; Kong, B.; Xiong, Y.L.; Sun, X. Antimicrobial activities of spice extracts against pathogenic and spoilage bacteria in modified atmosphere packaged fresh pork and vacuum packaged ham slices stored at 4 °C. Meat Sci. 2009, 81, 686–692. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Wang, B.; Kong, B.; Li, F.; Liu, Q.; Zhang, H.; Xia, X. Changes in the thermal stability and structure of protein from porcine longissimus dorsi induced by different thawing methods. Food Chem. 2020, 316, 126375. [Google Scholar] [CrossRef]
- Chen, X.; Zou, Y.; Han, M.; Pan, L.; Xing, T.; Xu, X.; Zhou, G. Solubilisation of myosin in a solution of low ionic strength L-histidine: Significance of the imidazole ring. Food Chem. 2016, 196, 42–49. [Google Scholar] [CrossRef]
- Li, X.X.; Fan, M.C.; Huang, Q.L.; Zhao, S.M.; Xiong, S.B.; Yin, T.; Zhang, B.J. Effect of micro- and nano-starch on the gel properties, microstructure and water mobility of myofibrillar protein from grass carp. Food Chem. 2022, 366, 130579. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Zhao, M.; Yang, B.; Cui, C. Physicochemical changes of myofibrillar proteins during processing of Cantonese sausage in relation to their aggregation behaviour and in vitro digestibility. Food Chem. 2011, 129, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Xiong, Y.L. Thermosonication-induced structural changes and solution properties of mung bean protein. Ultrason. Sonochem. 2020, 62, 104908. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lian, H.; Jia, H.; Li, S.; Hao, R.; Wang, Y.; Zhang, X.; Dong, X. Ultrasound treatment modified the functional mode of gallic acid on properties of fish myofibrillar protein. Food Chem. 2020, 320, 126637. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Lin, S.; Zhang, F.; Zheng, D.; Liu, D. Improved effect of flaxseed gum on the weakened gelling properties of myofibrillar protein induced by catechin. Food Chem. 2021, 372, 131136. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kong, B.; Xiong, Y.; Ren, Y. Decreased gelling and emulsifying properties of myofibrillar protein from repeatedly frozen-thawed porcine longissimus muscle are due to protein denaturation and susceptibility to aggregation. Meat Sci. 2010, 85, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar] [CrossRef]
- Peng, Z.; Zhu, M.; Zhang, J.; Zhao, S.; He, H.; Kang, Z.; Ma, H.; Xu, B. Physicochemical and structural changes in myofibrillar proteins from porcine longissimus dorsi subjected to microwave combined with air convection thawing treatment. Food Chem. 2021, 343, 128412. [Google Scholar] [CrossRef]
- Chen, G.; Wang, S.; Feng, B.; Jiang, B.; Miao, M. Interaction between soybean protein and tea polyphenols under high pressure. Food Chem. 2019, 277, 632–638. [Google Scholar] [CrossRef]
- Padrós, E.; Morros, A.; Mañosa, J.; Dunach, M. The state of tyrosine and phenylalanine residues in proteins analyzed by fourth-derivative spectrophotometry. Eur. J. Biochem. 1982, 127, 117–122. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R. The effect of pulsed electric fields on the inactivation and structure of lysozyme. Food Chem. 2008, 110, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Cao, A.; Wang, J.; Cai, L.; Regenstein, J.; Ruan, Y.; Li, X. Effect of magnetic nanoparticles plus microwave or far-infrared thawing on protein conformation changes and moisture migration of red seabream (Pagrus major) fillets. Food Chem. 2018, 266, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Keerati-U-Rai, M.; Corredig, M. Heat-induced changes in oil-in-water emulsions stabilized with soy protein isolate. Food Hydrocoll. 2009, 23, 2141–2148. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, F.; Sun, D.W.; Zhao, M. Effect of oxidation on the emulsifying properties of myofibrillar proteins. Food Bioprocess Technol. 2013, 6, 1703–1712. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, M.; Sun, W. Effect of pH on the interaction of porcine myofibrillar proteins with pyrazine compounds. Food Chem. 2019, 287, 93–99. [Google Scholar] [CrossRef]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Ishioroshi, M.; Samejima, K.; Yasui, T. Further studies on the roles of the head and tall regions of the myosin molecule in heat-induced gelation. J. Food Sci. 1982, 47, 114–120. [Google Scholar] [CrossRef]
- Ooizumi, T.; Xiong, Y.L. Identification of cross-linking site(s) of myosin heavy chains in oxidatively stressed chicken myofibrils. J. Food Sci. 2006, 71, C196–C199. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.D.S.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wang, J.Z.; Zhang, C.H.; Sun, H.M.; Wang, C.Q.; Xie, X.L. Effects of oxidation on water distribution and physicochemical properties of porcine myofibrillar protein gel. Food Biophys. 2014, 9, 169–178. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; Xiong, Y.L. Physicochemical changes of myosin and gelling properties of washed tilapia mince as influenced by oxidative stress and microbial transglutaminase. J. Food Sci. Technol. 2014, 52, 3824–3836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Wu, H.; Liu, M.; Liu, Z.; Xu, H.; Lai, F. Analysis of binding interaction between (−)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 164–168. [Google Scholar] [CrossRef]
- Sun, W.; Cui, C.; Zhao, M.; Zhao, Q.; Yang, B. Effects of composition and oxidation of proteins on their solubility, aggregation and proteolytic susceptibility during processing of Cantonese sausage. Food Chem. 2011, 124, 336–341. [Google Scholar] [CrossRef]
- Schmitt, C.; Bovay, C.; Rouvet, M.; Shojaei-Rami, A.S.; Kolodziejczyk, E. Whey protein soluble aggregates from heating with NaCl: Physicochemical, interfacial, and foaming properties. Langmuir 2007, 23, 4155–4166. [Google Scholar] [CrossRef]
- Ekezie, F.C.; Cheng, J.; Sun, D.W. Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei). Food Chem. 2019, 276, 147–156. [Google Scholar] [CrossRef]
- Lefevre, F.; Fauconneau, B.; Ouali, A.; Culioli, J. Thermal gelation of brown trout myofibrils from white and red muscles: Effect of pH and ionic strength. J. Sci. Food Agric. 2002, 82, 452–463. [Google Scholar] [CrossRef]
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Double-crosslinked effect of TGase and EGCG on myofibrillar proteins gel based on physicochemical properties and molecular docking. Food Chem. 2021, 345, 128655. [Google Scholar] [CrossRef]
- Xu, Q.D.; Yu, Z.L.; Zeng, W.C. Structural and functional modifications of myofibrillar protein by natural phenolic compounds and their application in pork meatball. Food Res. Int. 2021, 148, 110593. [Google Scholar] [CrossRef]



| Treatment | Oxidation Time (h) | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) | Aromatic Ring Vibration of Tyrosine Residues (%) |
|---|---|---|---|---|---|---|
| MPs samples without CE | 0 | 17.21 ± 0.23 a | 46.44 ± 1.11 a | 14.81 ± 0.61 b | 17.41 ± 0.40 b | 4.11 ± 0.19 a |
| 1 | 16.23 ± 0.25 ab | 47.91 ± 0.61 a | 14.82 ± 0.50 b | 17.64 ± 0.61 b | 3.42 ± 0.29 b | |
| 3 | 15.48 ± 0.21 bc | 47.31 ± 0.45 a | 14.94 ± 0.42 b | 17.63 ± 0.21 b | 4.44 ± 0.11 a | |
| 5 | 14.66 ± 0.39 c | 39.34 ± 0.33 b | 12.64 ± 0.50 c | 29.00 ± 0.42 a | 4.27 ± 0.07 a | |
| MPs samples with CE | 0 | 17.19 ± 0.25 a | 46.31 ± 1.19 a | 14.77 ± 0.47 b | 17.40 ± 0.43 b | 4.08 ± 0.26 a |
| 1 | 16.66 ± 0.35 a | 47.25 ± 0.82 a | 14.16 ± 0.38 bc | 17.53 ± 0.50 b | 4.39 ± 0.31 a | |
| 3 | 16.65 ± 0.35 a | 47.26 ± 0.94 a | 14.15 ± 0.37 bc | 17.52 ± 0.50 b | 4.40 ± 0.11 a | |
| 5 | 14.59 ± 0.55 c | 40.49 ± 1.06 b | 25.59 ± 0.99 a | 14.78 ± 0.29 c | 4.45 ± 0.22 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Pan, D.; Dong, Y.; Diao, J.; Chen, H. The Effectiveness of Clove Extract on Oxidization-Induced Changes of Structure and Gelation in Porcine Myofibrillar Protein. Foods 2022, 11, 1970. https://doi.org/10.3390/foods11131970
Ma J, Pan D, Dong Y, Diao J, Chen H. The Effectiveness of Clove Extract on Oxidization-Induced Changes of Structure and Gelation in Porcine Myofibrillar Protein. Foods. 2022; 11(13):1970. https://doi.org/10.3390/foods11131970
Chicago/Turabian StyleMa, Jinming, Deyin Pan, Ying Dong, Jingjing Diao, and Hongsheng Chen. 2022. "The Effectiveness of Clove Extract on Oxidization-Induced Changes of Structure and Gelation in Porcine Myofibrillar Protein" Foods 11, no. 13: 1970. https://doi.org/10.3390/foods11131970
APA StyleMa, J., Pan, D., Dong, Y., Diao, J., & Chen, H. (2022). The Effectiveness of Clove Extract on Oxidization-Induced Changes of Structure and Gelation in Porcine Myofibrillar Protein. Foods, 11(13), 1970. https://doi.org/10.3390/foods11131970







