The Adhesion and Spoilage of Shewanella putrefaciens in Tilapia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Strains
2.2. Determination of the Adhesion Ability of S. putrefaciens In Vitro
2.2.1. Bacteria Labeled with Fluorescein
2.2.2. Determination of Adhesion In Vitro
2.3. Determination of Spoilage Ability
2.3.1. Preparation and Inoculation of Sterile Fish Fillets
2.3.2. Total Bacteria in Fish Fillets
2.3.3. Determination of Total Volatile Basic Nitrogen (TVB-N)
2.3.4. The Yield Factor YTVB-N/CFU of TVB-N
2.4. In Vivo Test
2.4.1. Feeding Experiment of Tilapia
2.4.2. Sample Collection and Processing
2.5. Determination of Volatile Flavor Compounds by GC-MS
2.6. Detection of Specific Bacteria in the Intestines and Flesh by Quantitative Real-Time PCR (qPCR)
2.7. Analysis of Changes of Intestinal Microflora in Tilapia by High-Throughput Sequencing
2.8. Statistical Analysis
3. Results
3.1. Adhesion and Spoilage of S. putrefaciens In Vitro
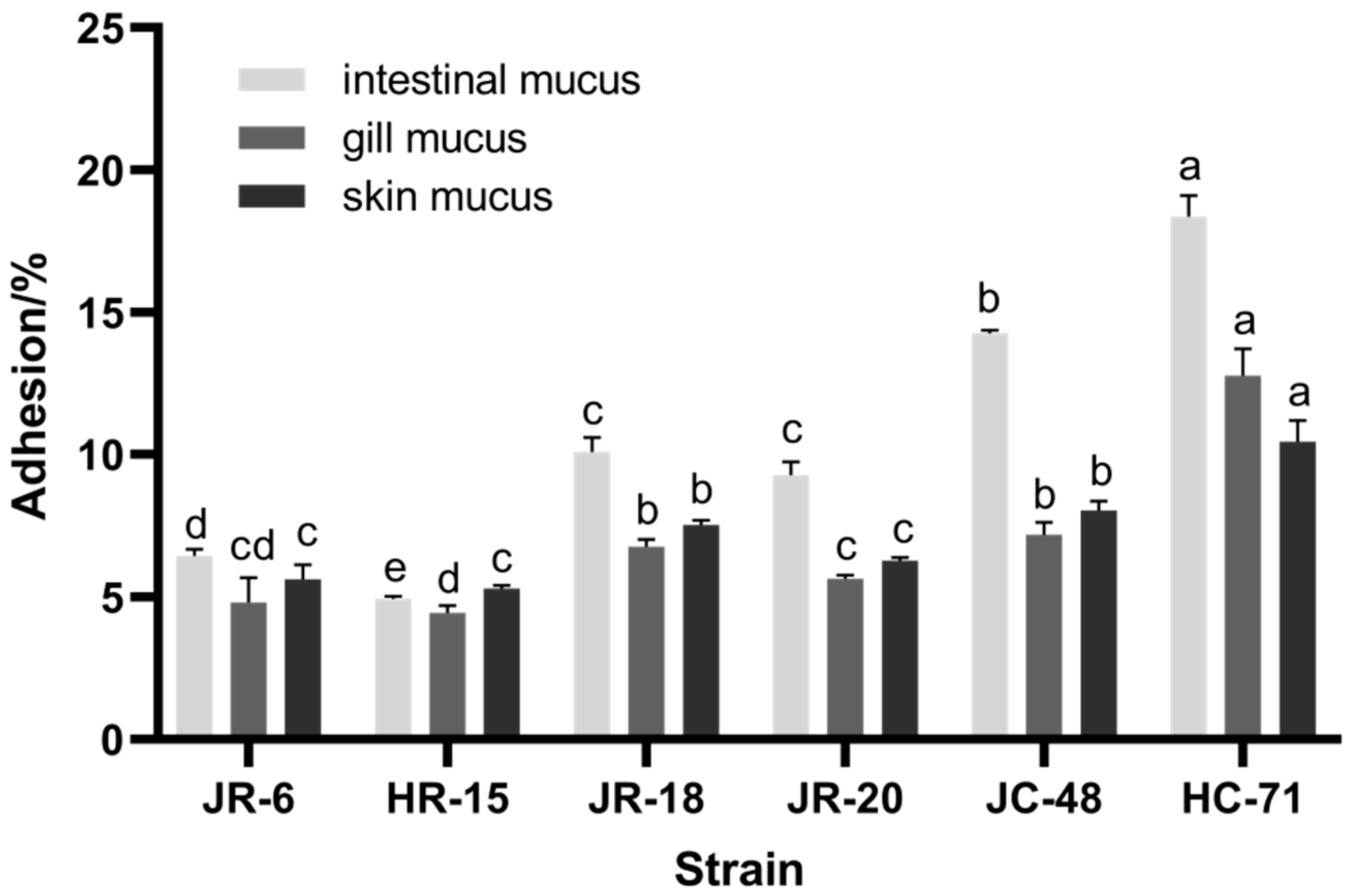
3.1.1. Adhesion Rate of S. putrefaciens in Different Mucus
3.1.2. Analysis of Spoilage Ability In Vitro
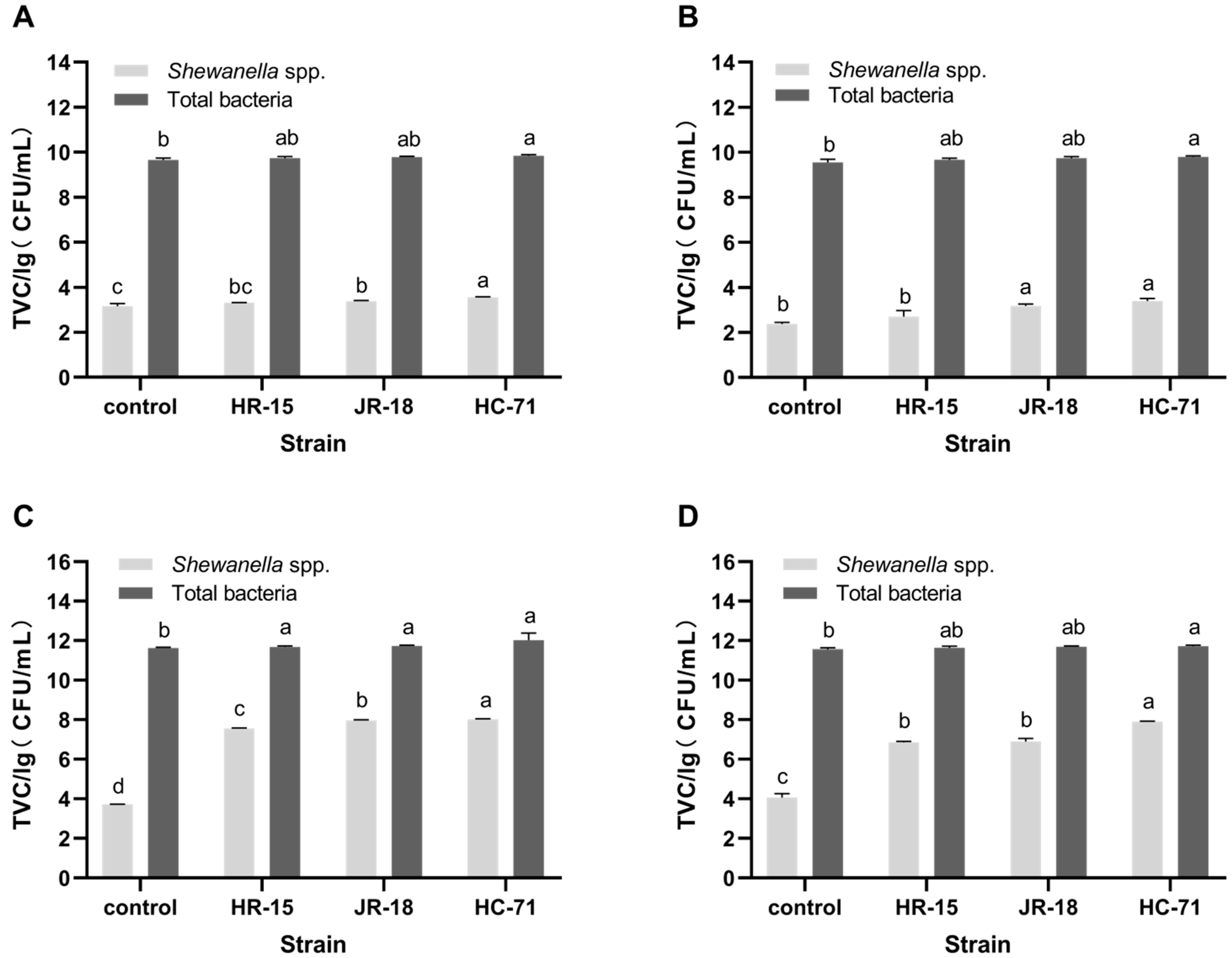
3.2. Adhesion and Spoilage of S. putrefaciens In Vivo
3.3. Effects of S. putrefaciens on the Structural Diversity of Tilapia Flora and Fish Flavor
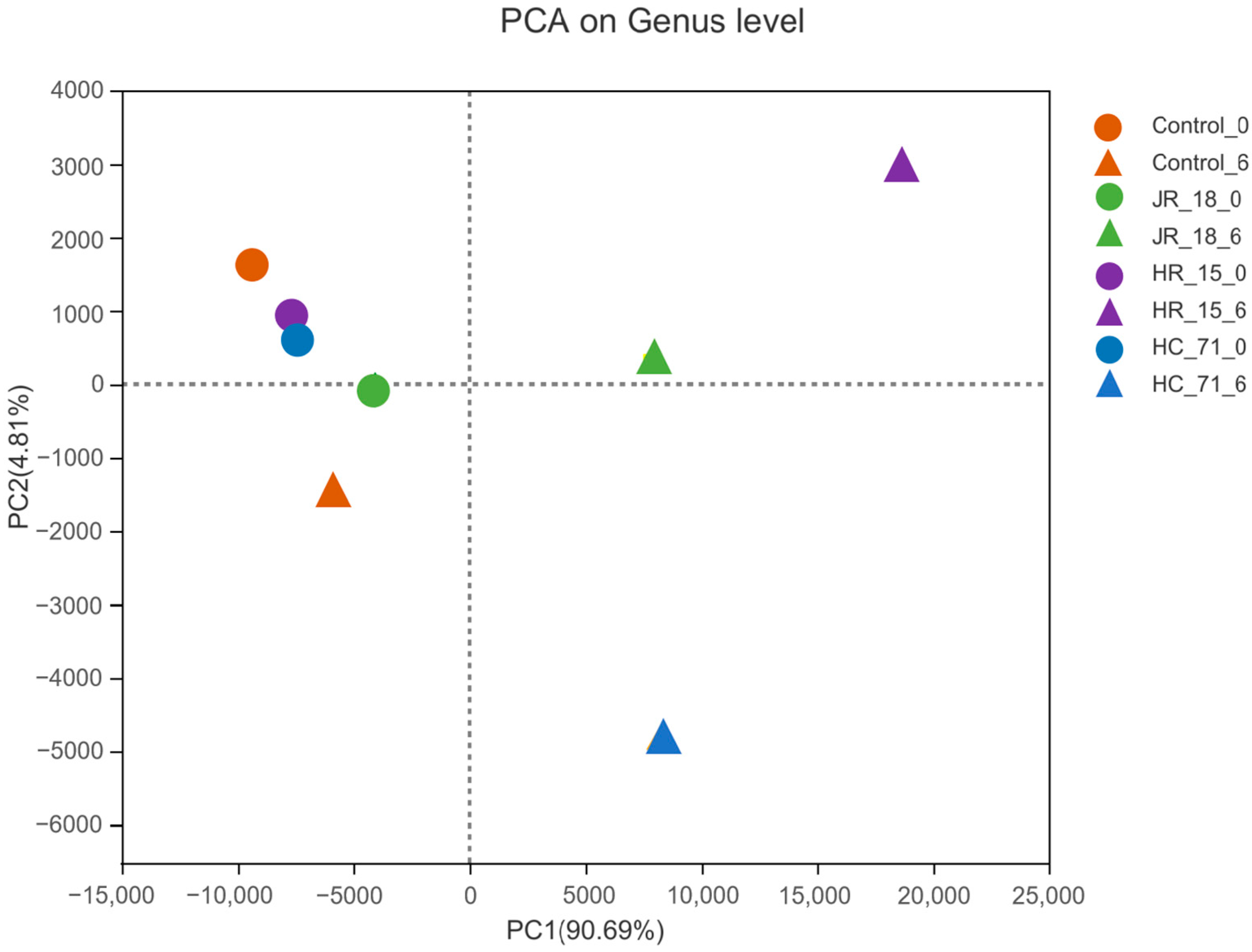
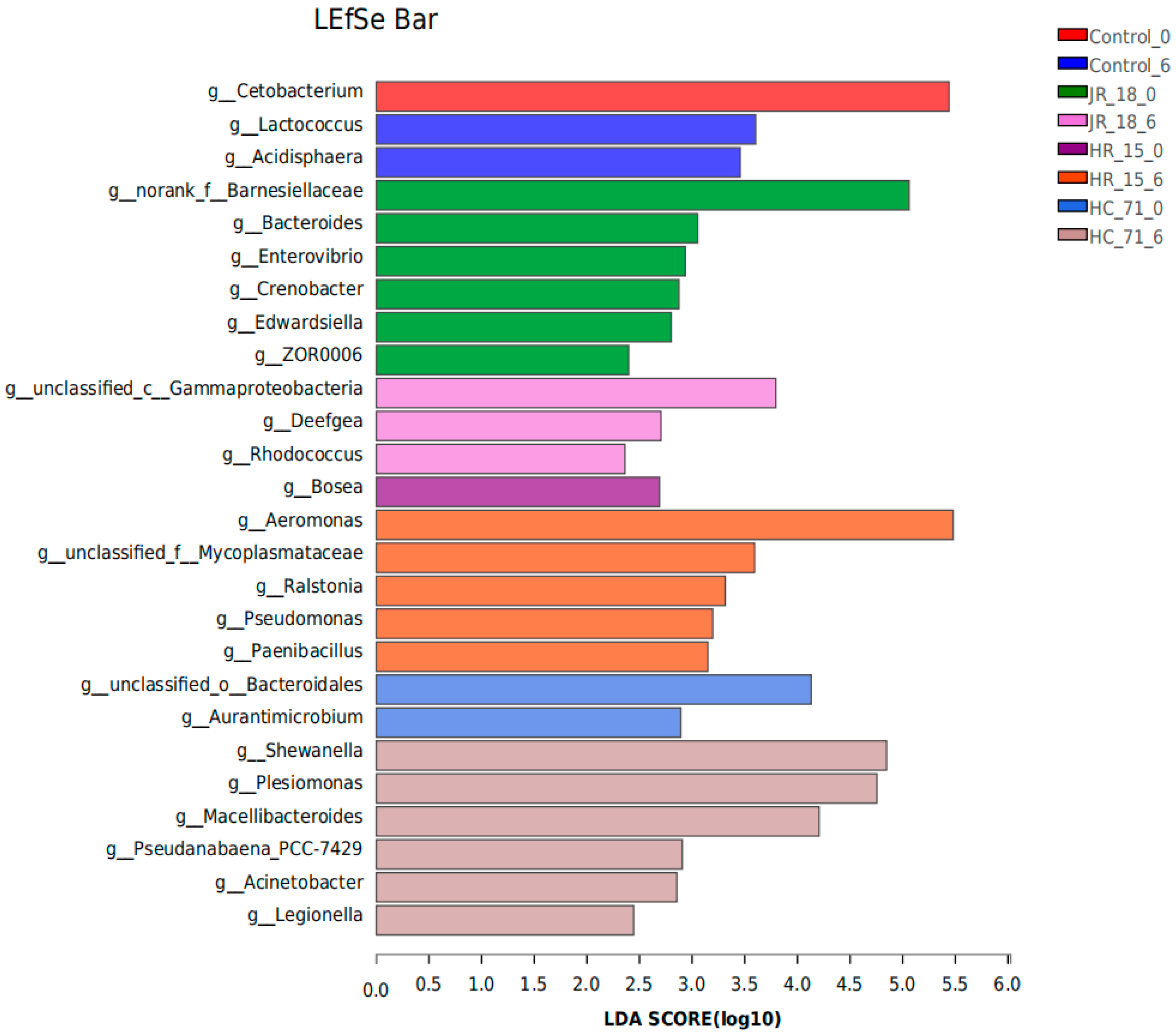
3.3.1. Intestinal Flora Composition Based on High-Throughput Sequencing Results
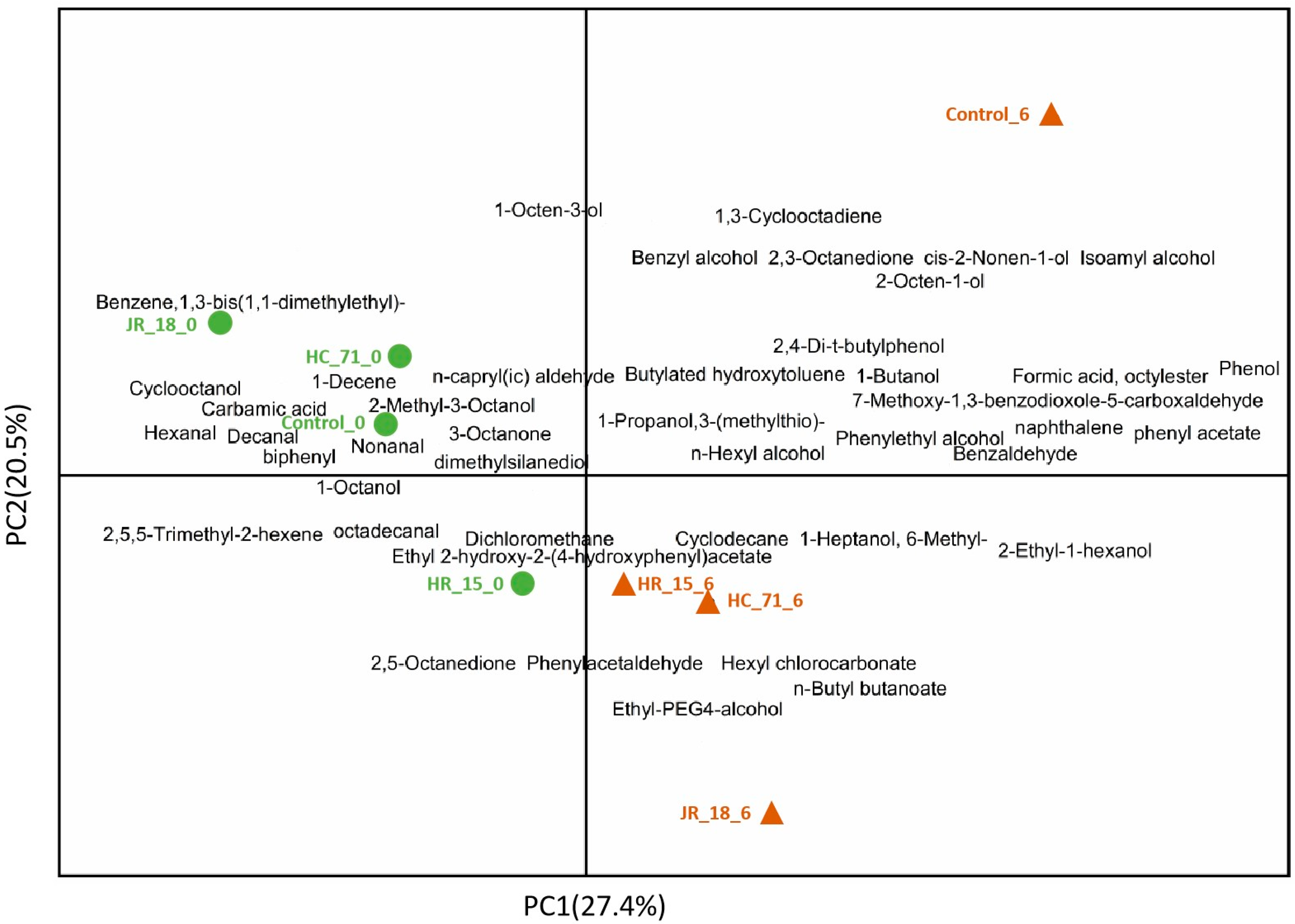
3.3.2. Volatile Components in Putrefied Fish
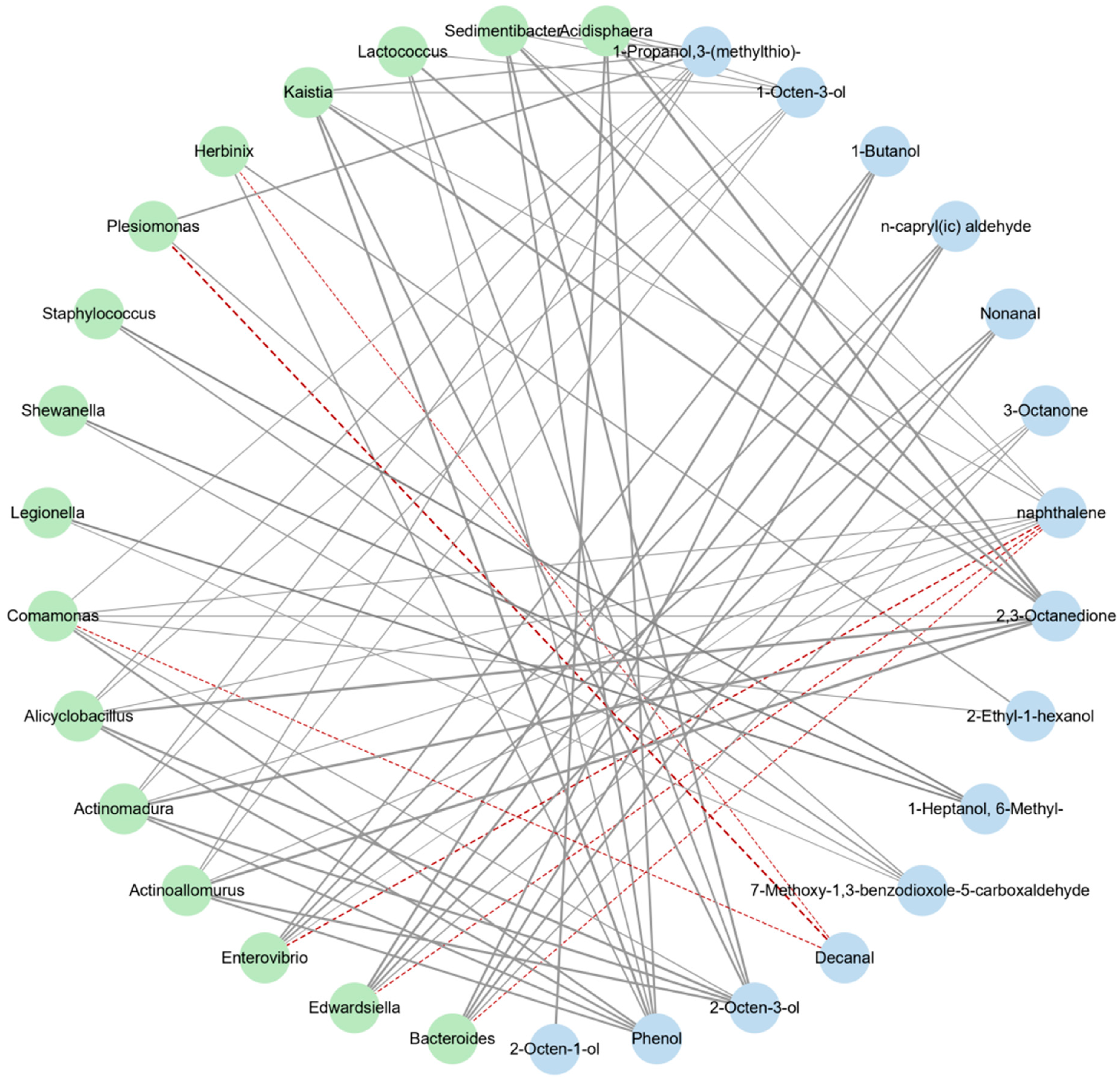
3.3.3. Correlation Analysis between Intestinal Flora and Volatile Flavor Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghaly, A.E.; Dave, D.; Budge, S.; Brooks, M.S. Fish Spoilage Mechanisms and Preservation Techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, L.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate un-inoculated and inoculated with gilt-head sea bream spoilage bacteria. LWT Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, A.; Feng, L.; Gao, H. Quorum sensing signals affect spoilage of refrigerated large yellow croaker (Pseudosciaena crocea) by Shewanella baltica. Int. J. Food Microbiol. 2016, 217, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lan, W.; Zang, Y.; Tang, S.; Liu, D.; Xu, X.; Zhou, D.; Xie, J. Effects of Dominant Spoilage Bacteria on Quality Change in Obscure Pufferfish (Takifugu obscurus) during Cold Storage and Analysis of Their Spoilage Ability. Food Sci. 2022, 43, 191–197. [Google Scholar]
- Lakshmanan, R.; Shakila, R.J.; Jeyasekaran, G. Survival of amine-forming bacteria during the ice storage of fish and shrimp. Food Microbiol. 2002, 19, 617–625. [Google Scholar] [CrossRef]
- Alfaro, B.; Hernandez, I. Evolution of the indigenous microbiota in modified atmosphere packaged Atlantic horse mackerel (Trachurus trachurus) identified by conventional and molecular methods. Int. J. Food Microbiol. 2013, 167, 117–123. [Google Scholar] [CrossRef]
- Alfaro, B.; Hernández, I.; Le Marc, Y.; Pin, C. Modelling the effect of the temperature and carbon dioxide on the growth of spoilage bacteria in packed fish products. Food Control 2013, 29, 429–437. [Google Scholar] [CrossRef]
- Lou, X.; Zhai, D.; Yang, H. Changes of metabolite profiles of fish models inoculated with Shewanella baltica during spoilage. Food Control 2020, 123, 107697. [Google Scholar] [CrossRef]
- Debevere, J.; Devlieghere, F.; van Sprundel, P.; De Meulenaer, B. Influence of acetate and CO2 on the TMAO-reduction reaction by Shewanella baltica. Int. J. Food Microbiol. 2001, 68, 115–123. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Bian, D.; Ruan, C.; Shi, X.; Ni, L. Adhesive Properties and Biofilm Characteristics of Pseudosciaena crocea Spoilage Bacteria. Food Sci. 2019, 40, 84–90. [Google Scholar]
- Zhang, W.; Bian, D.; Wang, F.; Zhang, C.; Ni, L. The Ability of Spoilage and Adhesion of Spoilage Bacteria Isolated from Large Yellow Croaker (Pseudosciaena crocea). J. Chin. Inst. Food Sci. Technol. 2019, 19, 117–124. [Google Scholar]
- Zhang, W.; Tong, Q.X.; You, J.H.; Lv, X.C.; Liu, Z.B.; Ni, L. The Application of Bacillus subtilis for Adhesion Inhibition of Pseudomonas and Preservation of Fresh Fish. Foods 2021, 10, 3093. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.H.; Larsen, J.L.; Olsen, J.E. Chemotaxis of Vibrio anguillarum to fish mucus: Role of the origin of the fish mucus, the fish species and the serogroup of the pathogen. FEMS Microbiol. Ecol. 2001, 38, 77–80. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, Q.; Wang, K.; Zhuang, Z.; Wang, X. Portal of entry for pathogenic Vibrio alginolyticus into large yellow croaker Pseudosciaena crocea, and characteristics of bacterial adhesion to mucus. Dis. Aquat. Org. 2008, 80, 181–188. [Google Scholar] [CrossRef]
- Dalgaard, P.; Gram, L.; Huss, H.H. Spoilage and shelf-life of cod fillets packed in vacuum or modified atmospheres. Int. J. Food Microbiol. 1993, 19, 283–294. [Google Scholar] [CrossRef]
- Huina, D.; Yuanming, G.; Shaoping, F.; Dawei, Z. Application of Biotechnology in Specific Spoilage Organisms of Aquatic Products. Front. Bioeng. Biotechnol. 2022, 10, 895283. [Google Scholar]
- Macé, S.; Joffraud, J.J.; Cardinal, M.; Malcheva, M.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X. Evaluation of the spoilage potential of bacteria isolated from spoiled raw salmon (Salmo salar) fillets stored under modified atmosphere packaging. Int. J. Food Microbiol. 2013, 160, 227–238. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Parlapani, F.F. Specific Spoilage Organisms (SSOs) in Fish-ScienceDirect. Microbiol. Qual. Food 2017, 61–98. [Google Scholar]
- Liu, A.; Xie, J.; Qian, Y. Spoilage Potential of Dominant Spoilage Bacteria from Chilled Tuna (Thunnus obesus). Food Sci. 2018, 39, 7–14. [Google Scholar]
- Yang, X.; Zhang, J.; Cheng, Y. The evaluation of the three edible tissues of dead adult Chinese mitten crabs (Eriocheir sinensis) freshness in harvest season, based on the analysis of TVBN and biogenic amine. SpringerPlus 2016, 5, 1906. [Google Scholar] [CrossRef]
- Shahryari, A.; Nikaeen, M.; Khiadani, M.; Nabavi, F.; Hatamzadeh, M.; Hassanzadeh, A. Applicability of universal Bacteroidales genetic marker for microbial monitoring of drinking water sources in comparison to conventional indicators. Environ. Monit. Assess. 2014, 186, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Xue, C.; Xu, J.; Sheng, W.; Xue, Y.; Li, Z. Analysis of volatile compounds in bighead carp by microwave. Distillation and solid phase microextraction coupled with gas chromatography-mass spectrometry and olfactometry. Se Pu 2007, 25, 267–271. [Google Scholar] [PubMed]
- Zhou, X.; Chong, Y.; Ding, Y.; Gu, S.; Liu, L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016, 207, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Varlet, V.; Knockaert, C.; Prost, C.; Serot, T. Comparison of Odor-Active Volatile Compounds of Fresh and Smoked Salmon. J. Agric. Food Chem. 2006, 54, 3391–3401. [Google Scholar] [CrossRef]
- Stołyhwo, A.; Kołodziejska, I.; Sikorski, Z.E. Long chain polyunsaturated fatty acids in smoked Atlantic mackerel and Baltic sprats. Food Chem. 2006, 94, 589–595. [Google Scholar] [CrossRef]
- Bai, J.; Baker, S.M.; Goodrich-Schneider, R.M.; Montazeri, N.; Sarnoski, P.J. Aroma Profile Characterization of Mahi-Mahi and Tuna for Determining Spoilage Using Purge and Trap Gas Chromatography-Mass Spectrometry. J. Food Sci. 2019, 84, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Ben Hamed, S.; Ranzani-Paiva, M.J.T.; Tachibana, L.; Dias, D.D.; Ishikawa, C.M.; Esteban, M.A. Fish pathogen bacteria: Adhesion, parameters influencing virulence and interaction with host cells. Fish Shellfish Immunol. 2018, 80, 550–562. [Google Scholar] [CrossRef]
- Sternisa, M.; Klancnik, A.; Mozina, S.S. Spoilage Pseudomonas biofilm with Escherichia coli protection in fish meat at 5 degrees C. J. Sci. Food Agric. 2019, 99, 4635–4641. [Google Scholar] [CrossRef]
- Feng, L.; Bi, W.; Chen, S.; Zhu, J.; Liu, X. Regulatory function of sigma factors RpoS/RpoN in adaptation and spoilage potential of Shewanella baltica. Food Microbiol. 2021, 97, 103755. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Y.; Jin, X.; Lv, X.; Liu, Z.; Ni, L. Spoilage of tilapia by Pseudomonas putida with different adhesion abilities. Curr. Res. Food Sci. 2022, 5, 710–717. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Identification of compounds characterizing the aroma of fresh whitefish (Coregonus clupeaformis). J. Agric. Food Chem. 1983, 31, 326–330. [Google Scholar] [CrossRef]
- Oerlemans, M.M.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2020, 76, 104289. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.-P.; Shen, Y.-Q. A systematic review of advances in intestinal microflora of fish. Fish Physiol. Biochem. 2021, 47, 2041–2053. [Google Scholar] [CrossRef]
- Gaulke, C.A.; Martins, M.L.; Watral, V.G.; Humphreys, I.R.; Spagnoli, S.T.; Kent, M.L.; Sharpton, T.J. A longitudinal assessment of host-microbe-parasite interactions resolves the zebrafish gut microbiome’s link to Pseudocapillaria tomentosa infection and pathology. Microbiome 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Yan, Q.; Yu, Y.; Zhang, T. Factors influencing the grass carp gut microbiome and its effect on metabolism. FEMS Microbiol. Ecol. 2013, 87, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Pérez, T.; Balcázar, J.; Ruiz-Zarzuela, I.; Halaihel, N.; Múzquiz, J. Host-microbiota interactions within the fish intestinal ecosystem. Mucosal Immunol. 2010, 3, 355–360. [Google Scholar] [CrossRef] [PubMed]


| Strain | GenBank Accession Number |
|---|---|
| S. putrefaciens JR-6 | ON505449 |
| S. putrefaciens HR-15 | ON505450 |
| S. putrefaciens JR-18 | ON505451 |
| S. putrefaciens JR-20 | ON505452 |
| S. putrefaciens JC-48 | ON505453 |
| S. putrefaciens HC-71 | ON505454 |
| Strain | Primer | Sequence (5′→3′) |
|---|---|---|
| Total bacteria | 27-F | AGAGTTTGATCCTGGCTCAG |
| 1429-R | GGTTACCTTGTTACGACTT | |
| Shewanella spp. | She3-F | GGAAACCCTGATGCAGCCAT |
| She3-R | AAGCTGCTACCTTTCCTCCCT |
| S. putrefaciens | The Total Number of Colonies/(CFU/g) | TVB-N/(mg/100 g) | YTVB-N/CFU/(mgTVB-N/CFU) | ||
|---|---|---|---|---|---|
| N0 | Ns | TVB-N0 | TVB-Ns | ||
| Control | 1.61 × 104 | 2.45 × 107 | 14.37 | 32.15 | - |
| HR-15 | 4.42 × 104 | 3.21 × 1010 | 16.10 | 39.81 | 1.85 × 10−10 |
| JR-18 | 5.56 × 104 | 3.89 × 1011 | 18.25 | 43.68 | 1.97 × 10−11 |
| HC-71 | 7.50 × 104 | 5.52 × 1012 | 20.35 | 47.74 | 1.74 × 10−12 |
| S. putrefaciens | The Total Number of Colonies/(CFU/g) | TVB-N/(mg/100 g) | YTVB-N/CFU/(mgTVB-N/CFU) | ||
|---|---|---|---|---|---|
| N0 | Ns | TVB-N0 | TVB-Ns | ||
| Control | 3.54 × 109 | 3.70 × 1011 | 8.96 | 25.20 | - |
| HR-15 | 4.63 × 109 | 4.29 × 1011 | 11.25 | 31.27 | 6.53 × 10−11 |
| JR-18 | 5.38 × 109 | 4.87 × 1011 | 12.93 | 37.80 | 7.49 × 10−11 |
| HC-71 | 6.21 × 109 | 5.22 × 1011 | 14.65 | 42.93 | 8.06 × 10−11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yu, Y.; He, H.; Lv, X.; Liu, Z.; Ni, L. The Adhesion and Spoilage of Shewanella putrefaciens in Tilapia. Foods 2022, 11, 1913. https://doi.org/10.3390/foods11131913
Zhang W, Yu Y, He H, Lv X, Liu Z, Ni L. The Adhesion and Spoilage of Shewanella putrefaciens in Tilapia. Foods. 2022; 11(13):1913. https://doi.org/10.3390/foods11131913
Chicago/Turabian StyleZhang, Wen, Ying Yu, Huihui He, Xucong Lv, Zhibin Liu, and Li Ni. 2022. "The Adhesion and Spoilage of Shewanella putrefaciens in Tilapia" Foods 11, no. 13: 1913. https://doi.org/10.3390/foods11131913
APA StyleZhang, W., Yu, Y., He, H., Lv, X., Liu, Z., & Ni, L. (2022). The Adhesion and Spoilage of Shewanella putrefaciens in Tilapia. Foods, 11(13), 1913. https://doi.org/10.3390/foods11131913








