Assembled Reduced Graphene Oxide/Tungsten Diselenide/Pd Heterojunction with Matching Energy Bands for Quick Banana Ripeness Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Sensor Fabrication
2.3. Instruments and Measurements
2.4. Theoretical Calculation
2.5. Banana Ripeness Detection Experiments
3. Results
3.1. Theoretical Design of the Ethylene Sensitive Material System
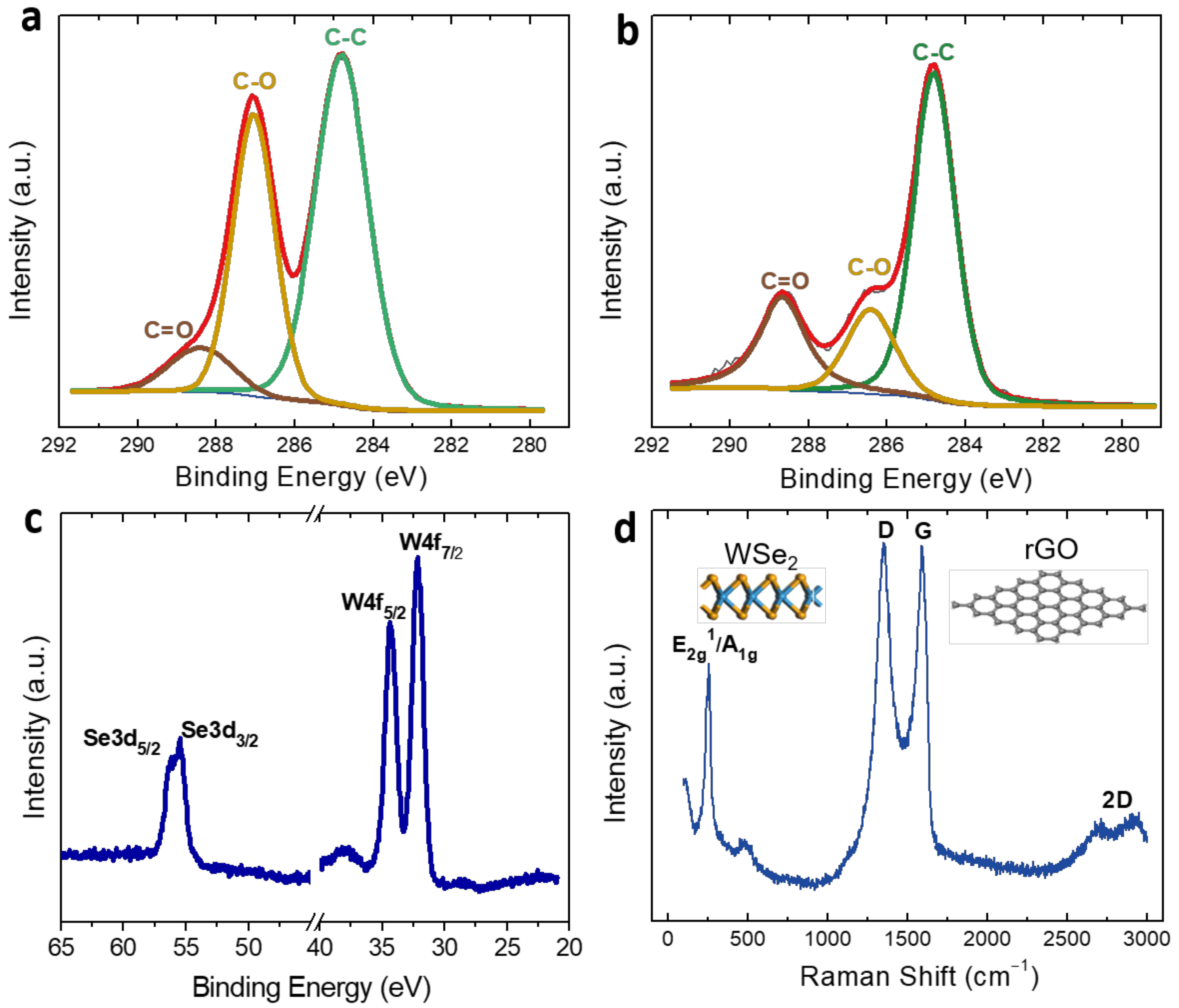
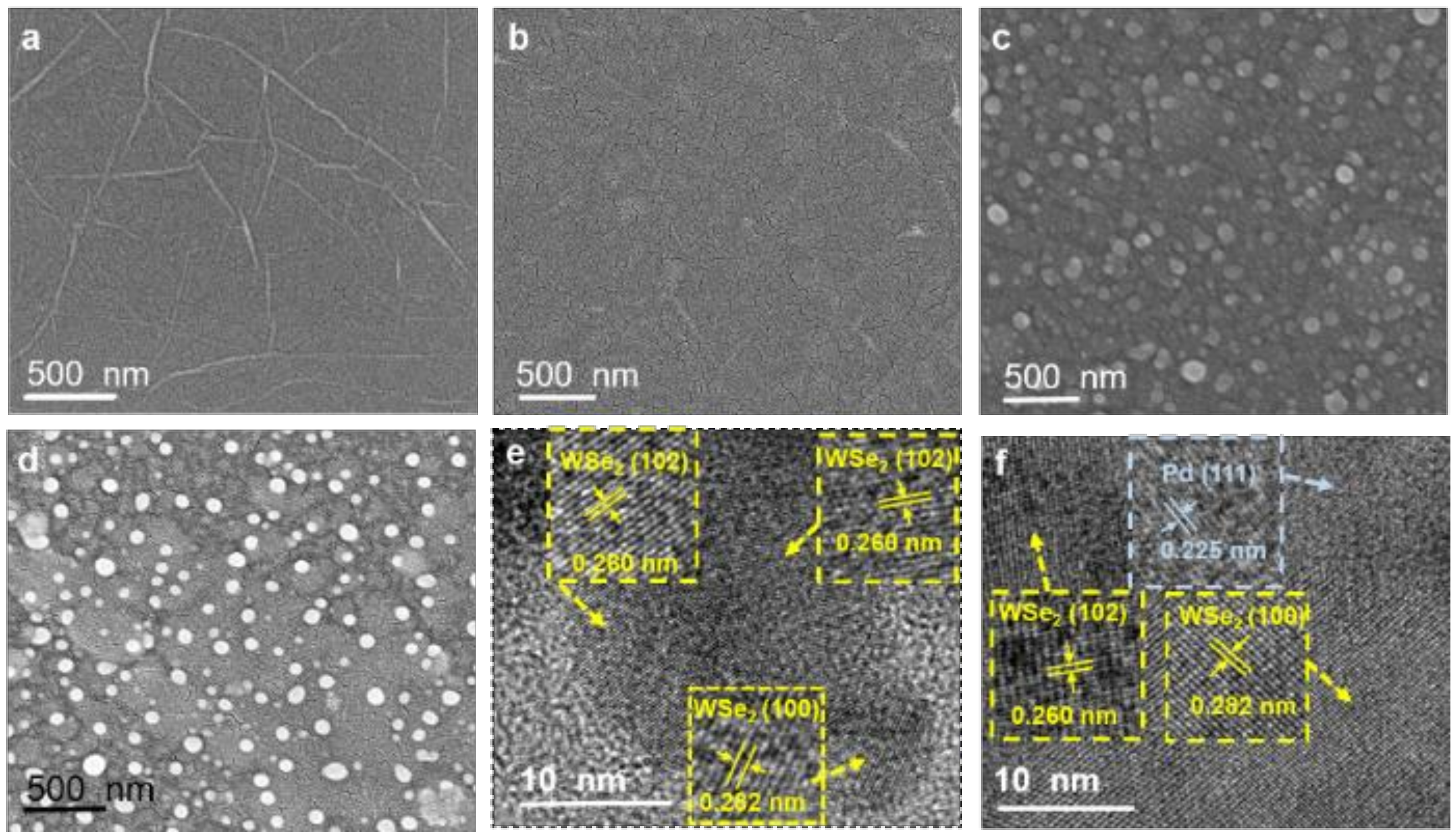
3.2. Fabrication and Characterization of Ethylene Sensitive Films
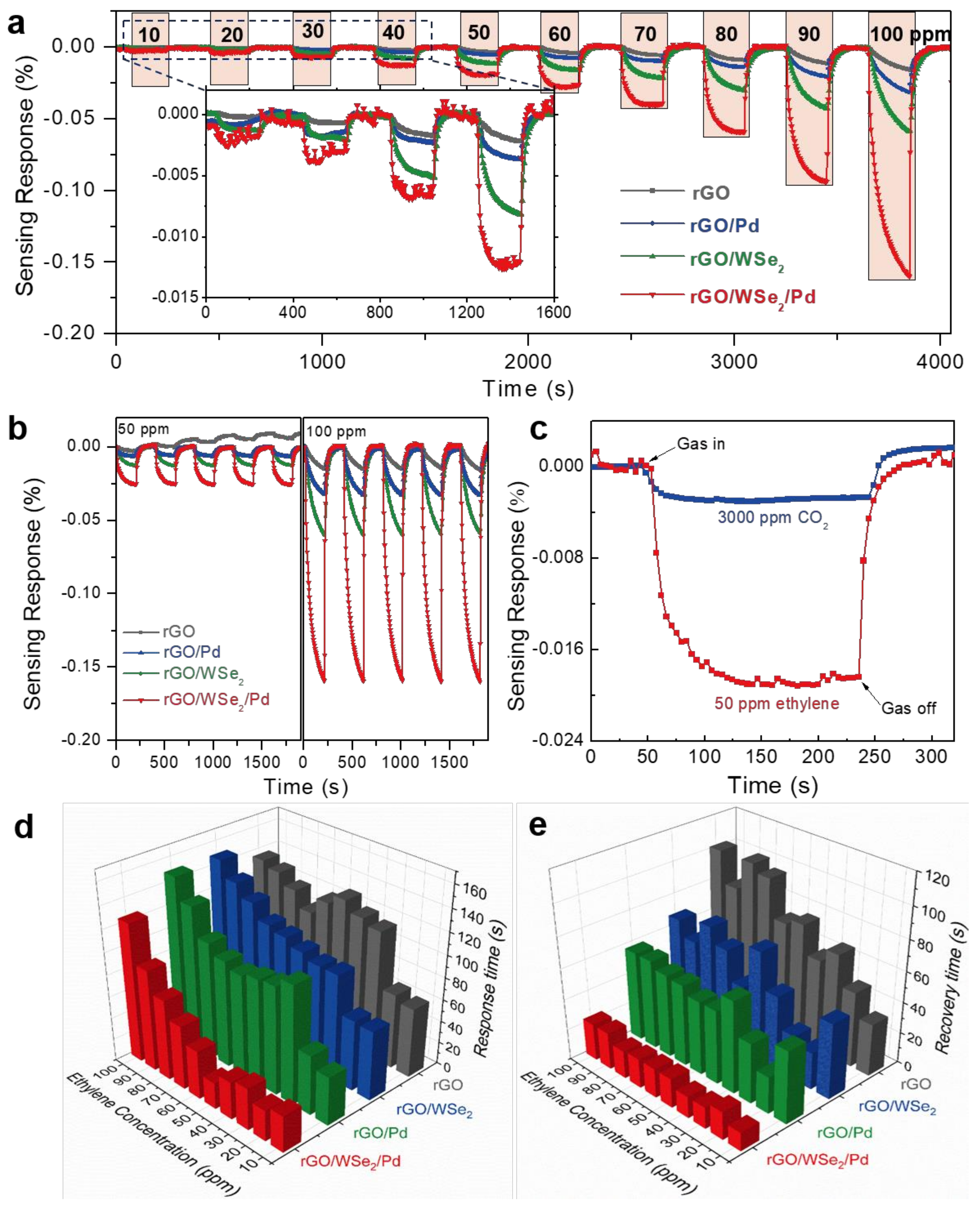
3.3. Ethylene-Sensing Characteristics
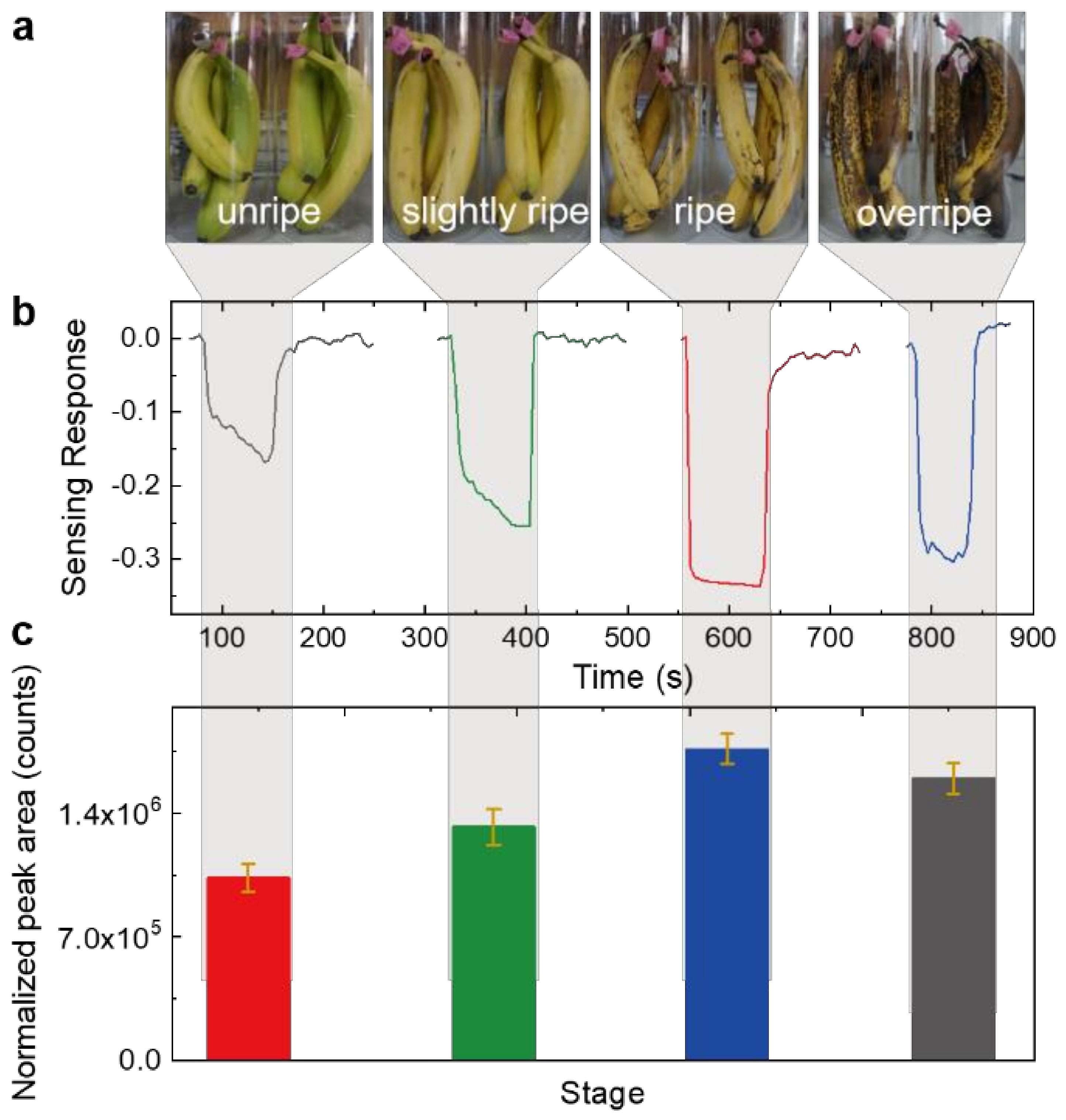
3.4. Banana Ripeness Detection Applications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, S.I.; Star, A. Picking flowers with carbon nanotube sensors. ACS Cent. Sci. 2020, 6, 461–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, W.Y.; Wang, H.J.; Zhou, Z.; Yang, P.; Wu, W.B.; Li, Z.M.; Li, X. Ethylene emission as a potential indicator of Fuji apple flavor quality evaluation under low temperature. Hortic. Plant J. 2020, 6, 231–239. [Google Scholar] [CrossRef]
- Cao, Y.D.; Wang, H.J.; Wu, W.B.; Yang, P.; Zhou, Z.; Wang, Z.; Li, Z.M.; Li, X. Simple and effective characterization of Fuji apple flavor quality by ethylene and sugar content. Food Anal. Methods 2021, 14, 2576–2584. [Google Scholar] [CrossRef]
- East, A.R.; Samarakoon, H.C.; Pranamornkith, T.; Bronlund, J.E. A review of ethylene permeability of films. Packag. Technol. Sci. 2015, 28, 732–740. [Google Scholar] [CrossRef]
- Owino, W.O.; Nakano, R.; Kubo, Y.; Inaba, A. Differential regulation of genes encoding ethylene biosynthesis enzymes and ethylene response sensor ortholog during ripening and in response to wounding in avocados. J. Am. Soc. Hortic. Sci. 2002, 127, 520–527. [Google Scholar] [CrossRef] [Green Version]
- Afreen, H.; Bajwa, I.S. An IoT-based real-time intelligent monitoring and notification system of cold storage. IEEE Access 2021, 9, 38236–38253. [Google Scholar] [CrossRef]
- Nagle, M.; Yasunaga, E.; Mahayothee, B.; Muller, J. Potenial for sensor systems to monitor fruit physiology of mango during long-distance transport. Environ. Control Biol. 2018, 56, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Pujol, M.; Garcia-Mas, J.; Phillips, M.A. Non-invasive quantification of ethylene in attached fruit headspace at 1 p.p.b. by gas chromatography-mass spectrometry. Plant J. 2017, 91, 172–183. [Google Scholar] [CrossRef]
- Eberhardta, A.; Schmitt, K.; Rademacher, S.; Huber, J.; Bauersfeld, M.L.; Wöllenstein, J. Nondispersive infrared photometer based on a rotating interference filter for investigation of climacteric fruit ripening. Procedia Eng. 2016, 168, 1223–1226. [Google Scholar] [CrossRef]
- Tabassum, S.; Kumar, D.P.; Kumar, R. Copper complex-coated nanopatterned fiber-tip guided mode resonance fevice for selective detection of ethylene. IEEE Sens. 2021, 21, 17420–17429. [Google Scholar] [CrossRef]
- Valente, J.; Almeida, R.; Kooistra, L. A comprehensive study of the potential application of flying ethylene-sensitive sensors for ripeness detection in apple orchards. Sensors 2019, 19, 372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, D. Chemical sensors for farm-to-table monitoring of fruit quality. Sensors 2021, 21, 1634. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.; Zhang, Y.; Wang, S.; Wong, M.W.; Ni, R.; Huang, D. Rapid and visual detection and quantitation of ethylene released from ripening fruits: The new use of grubbs catalyst. J. Agric. Food Chem. 2019, 67, 507–513. [Google Scholar] [CrossRef]
- Nimittrakoolchai, O.U.; Supothina, S. High-yield precipitation synthesis of tungsten oxide platelet particle and its ethylene gas-sensing characteristic. Mater. Chem. Phys. 2008, 112, 270–274. [Google Scholar] [CrossRef]
- Ahn, H.; Hoh, J.H.; Kim, S.B.; Overfelt, R.A.; Yoon, Y.S.; Kim, D.J. Effect of annealing and argon-to-oxygen ratio on sputtered SnO2 thin film sensor for ethylene gas detection. Mater. Chem. Phys. 2010, 124, 563–568. [Google Scholar] [CrossRef]
- Sholehah, A.; Faroz, D.F.; Huda, N.; Utari, L.; Septiani, N.L.W.; Yuliarto, B. Synthesis of ZnO flakes on flexible substrate and its application on ethylene sensing at room temperature. Chemosensors 2020, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Pimtong-Ngam, Y.; Jiemsirilers, S.; Supothina, S. Preparation of tungsten oxide-tin oxide nanocomposites and their ethylene sensing characteristics. Sens. Actuators A-Phys. 2007, 139, 7–11. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R.; Thomas, A. Ethylene detection using TiO2-WO3 composite sensor for fruit ripening applications. Sens. Rev. 2017, 37, 147–154. [Google Scholar] [CrossRef]
- Ivanov, P.; Llobet, E.; Vergara, A.; Stankova, M.; Vilanova, X.; Hubalek, J.; Gracia, I.; Cane, C.; Correig, X. Towards a micro-system for monitoring ethylene in warehouses. Sens. Actuators B-Chem. 2005, 111–112, 63–70. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhang, L.Z.; Leng, D.Y.; Ma, F.; Zhang, Z.Y.; Zhang, Y.Y.; Wang, W.; Liang, Q.F.; Gao, J.Z.; Lu, H.B. Nanoscale Pd catalysts decorated WO3-SnO2 heterojunction nanotubes for highly sensitive and selective acetone sensing. Sens. Actuators B-Chem. 2020, 306, 127575. [Google Scholar] [CrossRef]
- Liang, X.Y.; Ding, N.; Ng, S.P.; Wu, C.-M.L. Adsorption of gas molecules on Ga-doped graphene and effect of applied electric field: A DFT study. Appl. Surf. Sci. 2017, 411, 11–17. [Google Scholar] [CrossRef]
- Tabari, L.; Farmanzadeh, D. Yttrium doped graphene oxide as a new adsorbent for H2O, CO, and ethylene molecules: Dispersion-corrected DFT calculations. Appl. Surf. Sci. 2020, 500, 144029. [Google Scholar] [CrossRef]
- Liu, C.H.; Tai, H.L.; Zhang, P.; Ye, Z.B.; Su, Y.J.; Jiang, Y.D. Enhanced ammonia-sensing properties of PANI-TiO2-Au ternary self-assembly nanocomposite thin film at room temperature. Sens. Actuators B-Chem. 2017, 246, 85–95. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Pan, W.J.; Zhou, L.J.; Yu, S.J. Room-temperature benzene sensing with Au-doped ZnO nanorods/exfoliated WSe2 nanosheets and Density Functional Theory simulations. ACS Appl. Mater. Interfaces 2021, 13, 33392–33403. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.L.; Jiang, Y.D.; He, Z.Z.; Liu, B.H.; Xie, H.K.; Li, H.; Li, Z.M.; Wang, Y.; Tai, H.L. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2Tx hybrid sensitive films based gas sensor. Sens. Actuators B-Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of Au-decorated WS2 nanosheets as low power-consumption and selective gas sensors. Sens. Actuators B-Chem. 2019, 296, 126659. [Google Scholar] [CrossRef]
- Neri, G. Thin 2D: The New Dimensionality in Gas Sensing. Chemosensors 2017, 5, 21. [Google Scholar] [CrossRef]
- Phillips, K.M.; McGinty, R.C.; Couture, G.; Pehrsson, P.R.; McKillop, K.; Fukagawa, N.K. Dietary fiber, starch, and sugars in bananas at different stages of ripeness in the retail market. PLoS ONE 2021, 16, e0253366. [Google Scholar] [CrossRef]
- Wang, J.W.; Rathi, S.; Singh, B.; Lee, I.; Maeng, S.; Joh, H.-I.; Kim, G.-H. Dielectrophoretic assembly of Pt nanoparticle-reduced graphene oxide nanohybrid for highly-sensitive multiple gas sensor. Sens. Actuators B-Chem. 2015, 220, 755–761. [Google Scholar] [CrossRef]
- Huang, Q.W.; Zeng, D.W.; Li, H.Y.; Xie, C.S. Room temperature formaldehyde sensors with enhanced performance, fast response and recovery based on zinc oxide quantum dots/graphene nanocomposites. Nanoscale 2012, 4, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Zhao, H.Y.; Li, N.; Zhang, Y.; Zhang, X.Y.; Du, Y.P. Assembled 3D electrocatalysts for efficient hydrogen evolution: WSe2 layers anchored on graphene sheets. Inorg. Chem. Front. 2016, 3, 313. [Google Scholar] [CrossRef]
- Chen, W.Y.Y.; Yerembetova, A.; Washer, B.M.; Jiang, X.F.; Shuvo, S.N.; Peroulis, D.; Wei, A.; Stanciu, L.A. Selective detection of ethylene by MoS2-carbon nanotube networks coated with Cu(I)-Pincer complexes. ACS Sens. 2020, 5, 1699–1706. [Google Scholar] [CrossRef]

| Structure | Energy (eV) |
|---|---|
| Ethylene | −31.75 |
| Graphene | −458.56 |
| Graphene/WSe2 | −817.16 |
| Graphene/Pd | −471.35 |
| Graphene/WSe2/Pd | −805.71 |
| Ethylene adsorbed on graphene surface | −490.45 |
| Ethylene adsorbed on graphene/Pd surface | −503.27 |
| Ethylene adsorbed on graphene/WSe2 surface | −849.10 |
| Ethylene adsorbed on graphene/WSe2/Pd surface | −838.24 |
| Adsorbed System | Adsorption Energy (eV) |
|---|---|
| Ethylene-graphene | −0.14 |
| Ethylene-graphene/Pd | −0.17 |
| Ethylene-graphene/WSe2 | −0.19 |
| Ethylene-graphene/WSe2/Pd | −0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Xu, C.; Du, X.; Wang, Z.; Huang, W.; Sun, J.; Wang, Y.; Li, Z. Assembled Reduced Graphene Oxide/Tungsten Diselenide/Pd Heterojunction with Matching Energy Bands for Quick Banana Ripeness Detection. Foods 2022, 11, 1879. https://doi.org/10.3390/foods11131879
Li X, Xu C, Du X, Wang Z, Huang W, Sun J, Wang Y, Li Z. Assembled Reduced Graphene Oxide/Tungsten Diselenide/Pd Heterojunction with Matching Energy Bands for Quick Banana Ripeness Detection. Foods. 2022; 11(13):1879. https://doi.org/10.3390/foods11131879
Chicago/Turabian StyleLi, Xian, Chengcheng Xu, Xiaosong Du, Zhen Wang, Wenjun Huang, Jie Sun, Yang Wang, and Zhemin Li. 2022. "Assembled Reduced Graphene Oxide/Tungsten Diselenide/Pd Heterojunction with Matching Energy Bands for Quick Banana Ripeness Detection" Foods 11, no. 13: 1879. https://doi.org/10.3390/foods11131879
APA StyleLi, X., Xu, C., Du, X., Wang, Z., Huang, W., Sun, J., Wang, Y., & Li, Z. (2022). Assembled Reduced Graphene Oxide/Tungsten Diselenide/Pd Heterojunction with Matching Energy Bands for Quick Banana Ripeness Detection. Foods, 11(13), 1879. https://doi.org/10.3390/foods11131879







