Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques
Abstract
:1. Introduction
1.1. Background
1.2. Rationale
2. Materials and Methods
2.1. Culinary Oil Samples
2.2. Thermal Stressing of Culinary Oil Samples
2.3. Continuous Thermo-Oxidation Method
2.4. Discontinuous Thermo-Oxidation Method
2.5. 1H NMR Measurements
2.6. Analysis of 1H NMR Spectra
2.6.1. Analysis of Major Lipid Species and IVs
2.6.2. Analysis of Minor Compounds
2.6.3. Analysis of LOPs
2.7. Statistical Analysis of Experimental Data
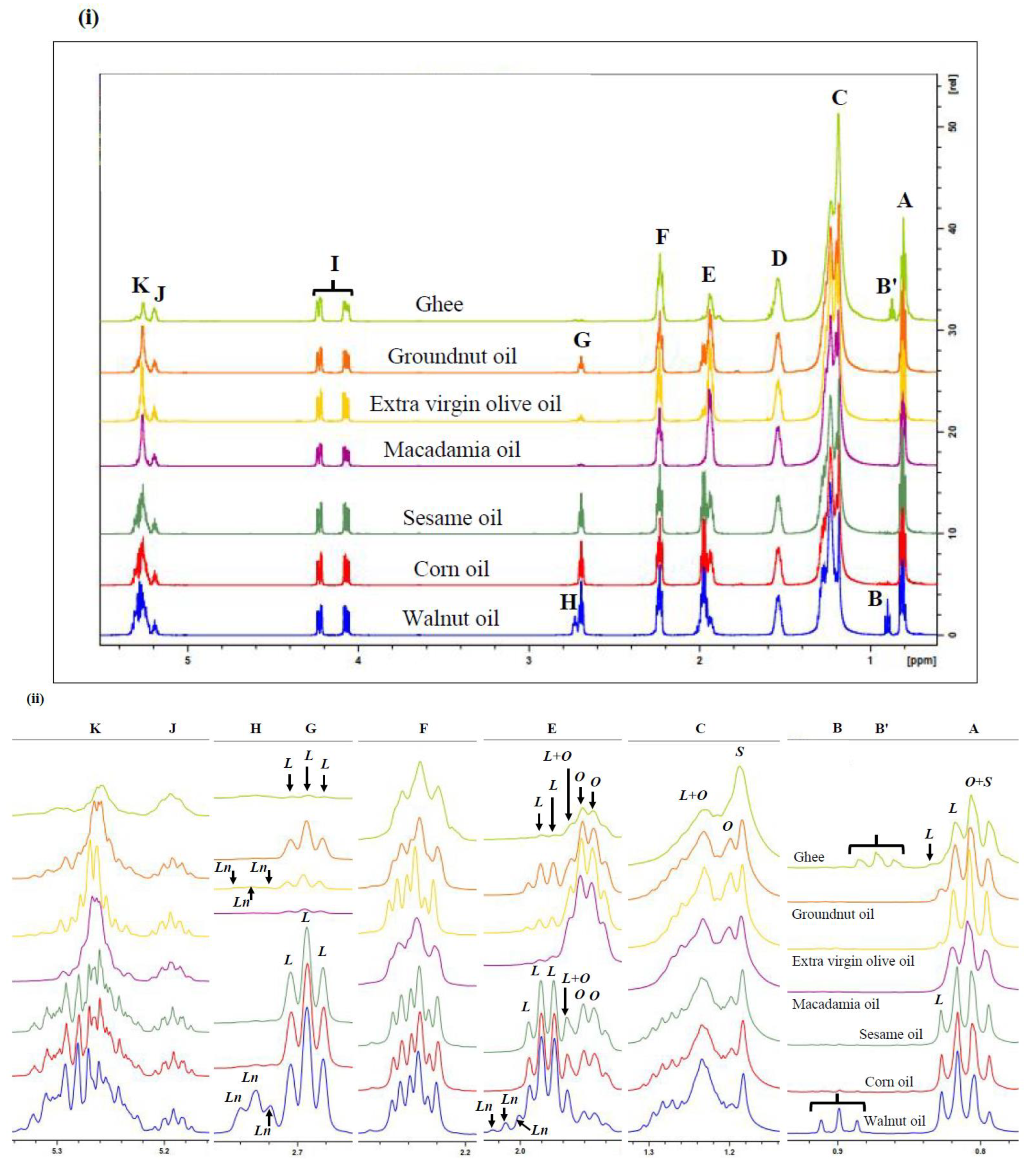
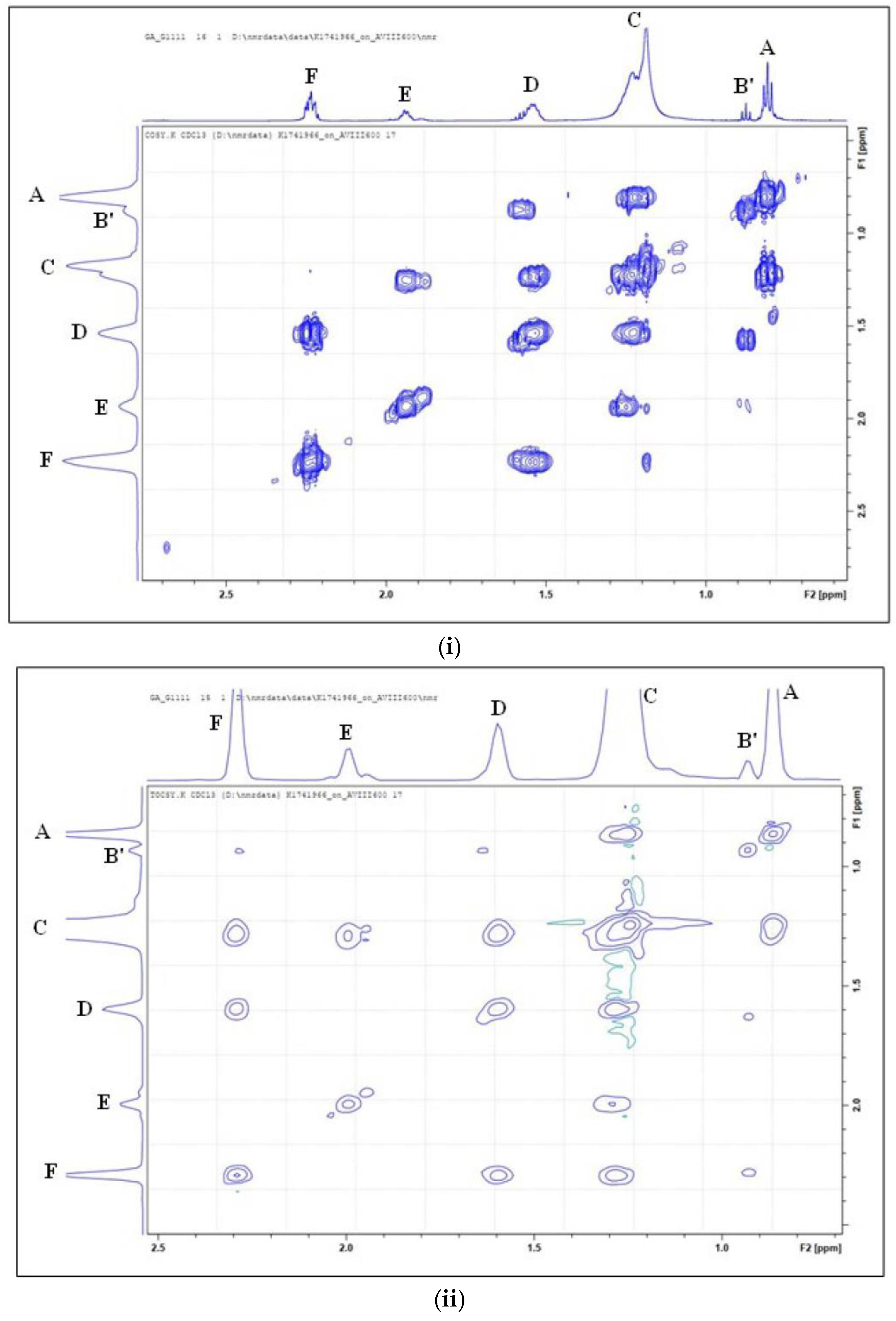
3. Results
3.1. Characterisation of 1H NMR Spectra of Unheated Culinary Oils
Major Compounds Detectable
3.2. Oxidation Products of UFAs
3.2.1. Primary LOPs
3.2.2. Secondary LOPs
Epoxy-FAs and Primary Alcohols
Aldehydes

4. Discussion
4.1. Culinary oil FA Compositions and IVs
4.2. Evolution of LOPs
4.3. Statistical Analysis of Aldehydic LOP Datasets
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grootveld, M.; Rodado, V.R.; Silwood, C.J.L. Detection, monitoring, and deleterious health effects of lipid oxidation products generated in culinary oils during thermal stressing episodes. Inform. Am. Oil Chem. Soc. 2014, 25, 614–624. [Google Scholar]
- Grootveld, M.; Percival, B.C.; Grootveld, K.L. Chronic non-communicable disease risks presented by lipid oxidation products in fried foods. Hepatobiliary Surg. Nutr. 2018, 7, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Percival, B.C.; Wann, A.; Zbasnik, R.; Schlegel, V.; Edgar, M.; Zhang, J.; Ampem, G.; Wilson, P.; Le Gresley, A.; Naughton, D.; et al. Evaluations of the peroxidative susceptibilities of cod liver oils by a 1H NMR analysis strategy: Peroxidative resistivity of a natural collagenous and biogenic amine-rich fermented product. Nutrients 2020, 12, 753. [Google Scholar] [CrossRef] [Green Version]
- US Department of Agriculture (USDA); USDA Foreign Agricultural Service (USDA FAS). Consumption of Vegetable oils Worldwide from 2013/14 to 2020/2021, by Oil Type (in Million Metric Tons). 2021. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 12 September 2021).
- Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Haywood, R.M.; Chander, C.L.; Atherton, M.; Lynch, E.J.; Grootveld, M.C. Generation of lipid peroxidation products in culinary oils and fats during episodes of thermal stressing: A high field 1H NMR study. FEBS Lett. 1994, 355, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Le Gresley, A.; Ampem, G.; Grootveld, M.; Percival, B.C.; Naughton, D.P. Characterisation of peroxidation products arising from culinary oils exposed to continuous and discontinuous thermal degradation processes. Food Funct. 2019, 10, 7952–7966. [Google Scholar] [CrossRef]
- Guillén-Sans, R.; Guzman-Chozas, M. The thiobarbituric acid (TBA) reaction in foods: A review. Crit. Rev. Food Sci. 1998, 38, 315–330. [Google Scholar] [CrossRef]
- Laguerre, M.; Lecomte, J.; Villeneuve, P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Prog. Lipid Res. 2007, 46, 244–282. [Google Scholar] [CrossRef]
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H NMR Spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Le Gresley, A.; Peron, J.R. A semi-automatic approach to the characterisation of dark chocolate by Nuclear Magnetic Resonance and multivariate analysis. Food Chem. 2019, 275, 385–389. [Google Scholar] [CrossRef]
- Poyato, C.; Ansorena, D.; Navarro-Blasco, I.; Astiasarán, I. A novel approach to monitor the oxidation process of different types of heated oils by using chemometric tools. Food Res. Int. 2014, 57, 152–161. [Google Scholar] [CrossRef]
- Goicoechea, E.; Guillén, M.D. Analysis of hydroperoxides, aldehydes and epoxides by 1H Nuclear Magnetic Resonance in sunflower oil oxidized at 70 and 100 °C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef] [PubMed]
- Castejón, D.; Herrera, A.; Heras, Á.; Cambero, I.; Mateos-Aparicio, I. Oil quality control of culinary oils subjected to deep-fat frying based on NMR and EPR Spectroscopy. Food Anal. Methods 2017, 10, 2467–2480. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Siddiqui, N.; Sim, J.; Silwood, C.J.L.; Toms, H.; Iles, R.A.; Grootveld, M. Multicomponent analysis of encapsulated marine oil supplements using high-resolution 1H and 13C NMR techniques. J. Lipid Res. 2003, 44, 2406–2427. [Google Scholar] [CrossRef] [Green Version]
- Le Gresley, A.; Ampem, G.; De Mars, S.; Grootveld, M.; Naughton, D.P. “Real-world” evaluation of lipid oxidation products and trace metals in French fries from two chain fast-food restaurants. Front. Nutr. 2021, 8, 620952. [Google Scholar] [CrossRef]
- Ampem, G.; Le Gresley, A.; Grootveld, M.; Naughton, D.P. The role of polydimethylsiloxane in suppressing the evolution of lipid oxidation products in thermo-oxidised sunflower oil: Influence of Stirring Processes. Front. Nutr. 2021, 8, 721736. [Google Scholar] [CrossRef]
- Ampem, G.; Le Gresley, A.; Grootveld, M.; De Mars, S.; Naughton, D.P. The impact of partial oil substitution and trace metal ions on the evolution of peroxidation products in thermally stressed culinary oils. Food Chem. 2022, 375, 131823. [Google Scholar] [CrossRef]
- Grootveld, M.; Atherton, M.D.; Sheerin, A.N.; Hawkes, J.; Blake, D.R.; Richens, T.E.; Silwood, C.J.L.; Lynch, E.; Claxson, A.W.D. In vivo absorption, metabolism, and urinary excretion of α,β-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998, 101, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Grootveld, M.; Silwood, C.J.L.; Addis, P.; Claxson, A.; Serra, B.B.; Viana, M. Health effects of oxidized heated oils. Food Res. Int. 2001, 13, 41–55. [Google Scholar] [CrossRef]
- Moumtaz, S.; Percival, B.C.; Parmar, D.; Grootveld, K.L.; Jansson, P.; Grootveld, M. Toxic aldehyde generation in and food uptake from culinary oils during frying practices: Peroxidative resistance of a monounsaturate-rich algae oil. Sci. Rep. 2019, 9, 4125. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Uriarte, P.S. Contribution to further understanding of the evolution of sunflower oil submitted to frying temperature in a domestic fryer: Study by 1H nuclear magnetic resonance. J. Agric. Food Chem. 2009, 57, 7790–7799. [Google Scholar] [CrossRef]
- Tociu, M.; Todasca, M.C.; Bratu, A.; Mihalache, M.; Manolache, F. Fast approach for fatty acid profiling of dairy products fats using 1H-NMR spectroscopy. Int. Dairy J. 2018, 83, 52–57. [Google Scholar] [CrossRef]
- Hanganu, A.; Chira, N.A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Ivanova, M.; Hanganu, A.; Dumitriu, R.; Tociu, M.; Ivanov, G.; Stavarache, C.; Popescu, L.; Ghendov-Mosanu, A.; Sturza, R.; Deleanu, C.; et al. Saponification value of fats and oils as determined from 1H-NMR data: The case of dairy fats. Foods 2022, 11, 1466. [Google Scholar] [CrossRef]
- Sacchi, R.; Paduano, A.; Caporaso, N.; Picariello, G.; Romano, R.; Addeo, F. Assessment of milk fat content in fat blends by 13C NMR spectroscopy analysis of butyrate. Food Control 2018, 91, 231–236. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Simultaneous control of the evolution of the percentage in weight of polar compounds, iodine value, acyl groups proportions and aldehydes concentrations in sunflower oil submitted to frying temperature in an industrial fryer. Food Control 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]
- Grootveld, M.; Percival, B.C.; Leenders, J.; Wilson, P.B. Potential adverse public health effects afforded by the ingestion of dietary lipid oxidation product toxins: Significance of fried food sources. Nutrients 2020, 12, 974. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.P.; Hofseth, L.S.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Picklo, M.J.; Azenkeng, A.; Hoffmann, M.R. Trans-4-oxo-2-nonenal potently alters mitochondrial function. Free Radic. Biol. Med. 2011, 50, 400–407. [Google Scholar] [CrossRef]
- Silwood, C.; Grootveld, M. Application of high-resolution, two-dimensional 1H and 13C nuclear magnetic resonance techniques to the characterization of lipid oxidation products in autoxidized linoleoyl/linolenoylglycerols. Lipids 1999, 34, 741–756. [Google Scholar] [CrossRef]
- Percival, B.C.; Wann, A.; Zbasnik, R.; Schlegel, V.; Edgar, M.; Zhang, J.; Grootveld, M. Determinations of the peroxidative susceptibilities of cod liver oils by a newly-developed 1H NMR-based method: Resistance of an antioxidant-fortified product isolated from pre-fermented sources. BMC Res. Notes 2020, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Grootveld, M. Evidence-Based Challenges to the Continued Recommendation and Use of Peroxidatively-Susceptible Polyunsaturated Fatty Acid-Rich Culinary Oils for High-Temperature Frying Practises: Experimental Revelations Focused on Toxic Aldehydic Lipid Oxidation Products. Front. Nutr. 2022, 8, 711640. [Google Scholar] [CrossRef]
- Haywood, R.M.; Claxson, A.W.D.; Hawkes, G.E.; Richardson, D.P.; Naughton, D.P.; Coumbarides, G.; Hawkes, J.; Lynch, E.J.; Grootveld, M.C. Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: Investigations using high resolution proton NMR spectroscopy. Free Radic. Res. 1995, 22, 441–482. [Google Scholar] [CrossRef]
- Percival, B.; Savel, E.; Ampem, G.; Gibson, M.; Edgar, M.; Jafari, F.; Woodason, K.; Frederick, K.; Wilson, P.; Grootveld, M. Molecular composition of and potential health benefits offered by natural East African virgin sunflower oil products: A 400 MHz 1H NMR analysis study. Int. J. Nutr. 2019, 3, 22–43. [Google Scholar] [CrossRef] [Green Version]
- De Stefani, E.; Ronco, A. Squalene: A multi-task link in the crossroads of cancer and aging. Funct. Food Health Dis. 2013, 3, 462–476. [Google Scholar] [CrossRef] [Green Version]
- Sopelana, P.; Arizabaleta, I.; Ibargoitia, M.L.; Guillén, M.D. Characterization of the lipidic components of margarines by 1H nuclear magnetic resonance. Food Chem. 2013, 141, 3357–3364. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. A method based on 1H NMR spectral data useful to evaluate the hydrolysis level in complex lipid mixtures. Food Res. Int. 2014, 66, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.D.; Goicoechea, E. Oxidation of corn oil at room temperature: Primary and secondary oxidation products and determination of their concentration in the oil liquid matrix from 1H nuclear magnetic resonance data. Food Chem. 2009, 116, 183–192. [Google Scholar] [CrossRef]
- Wann, A.I.; Percival, B.C.; Woodason, K.; Gibson, M.; Vincent, S.; Grootveld, M. Comparative 1H NMR-based chemometric evaluations of the time-dependent generation of aldehydic lipid oxidation products in culinary oils exposed to laboratory-simulated shallow frying episodes: Differential patterns observed for omega-3 fatty acid-containing soybean oils. Foods 2021, 10, 2481. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Crowley, J.R.; Alejandro, M.A.; Shoulars, K.; Casajuna, N.; Mani, S.; Reyna, A.; Sharp, J. Leukotoxins diols from ground corncob bedding disrupt estrus cyclicity in rats and stimulate MCF-7 breast cancer cell proliferation. Environ. Health Perspect. 2005, 113, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.A.; Hammock, B.D. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 2007, 32, 279–291. [Google Scholar] [CrossRef] [PubMed]
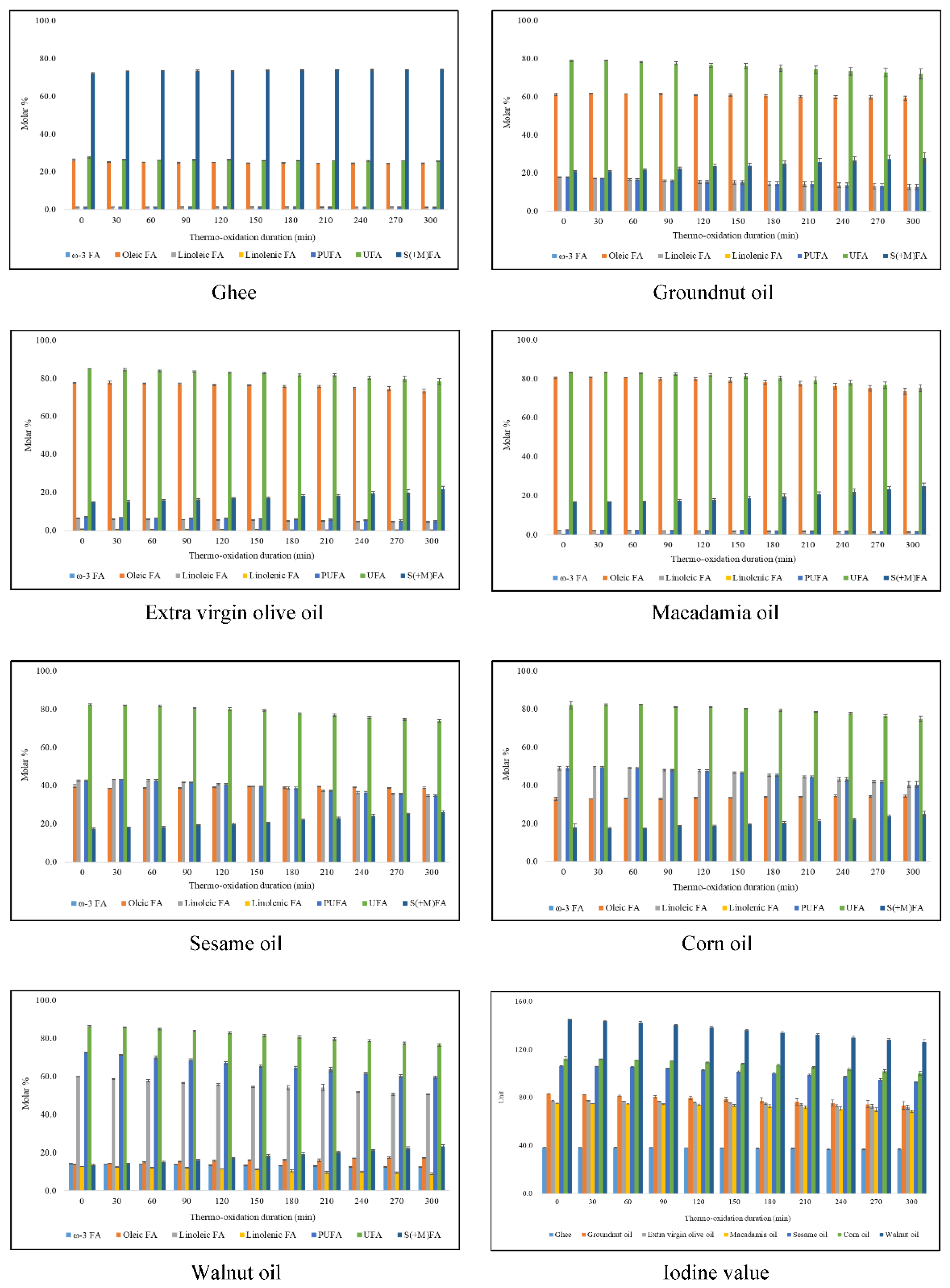
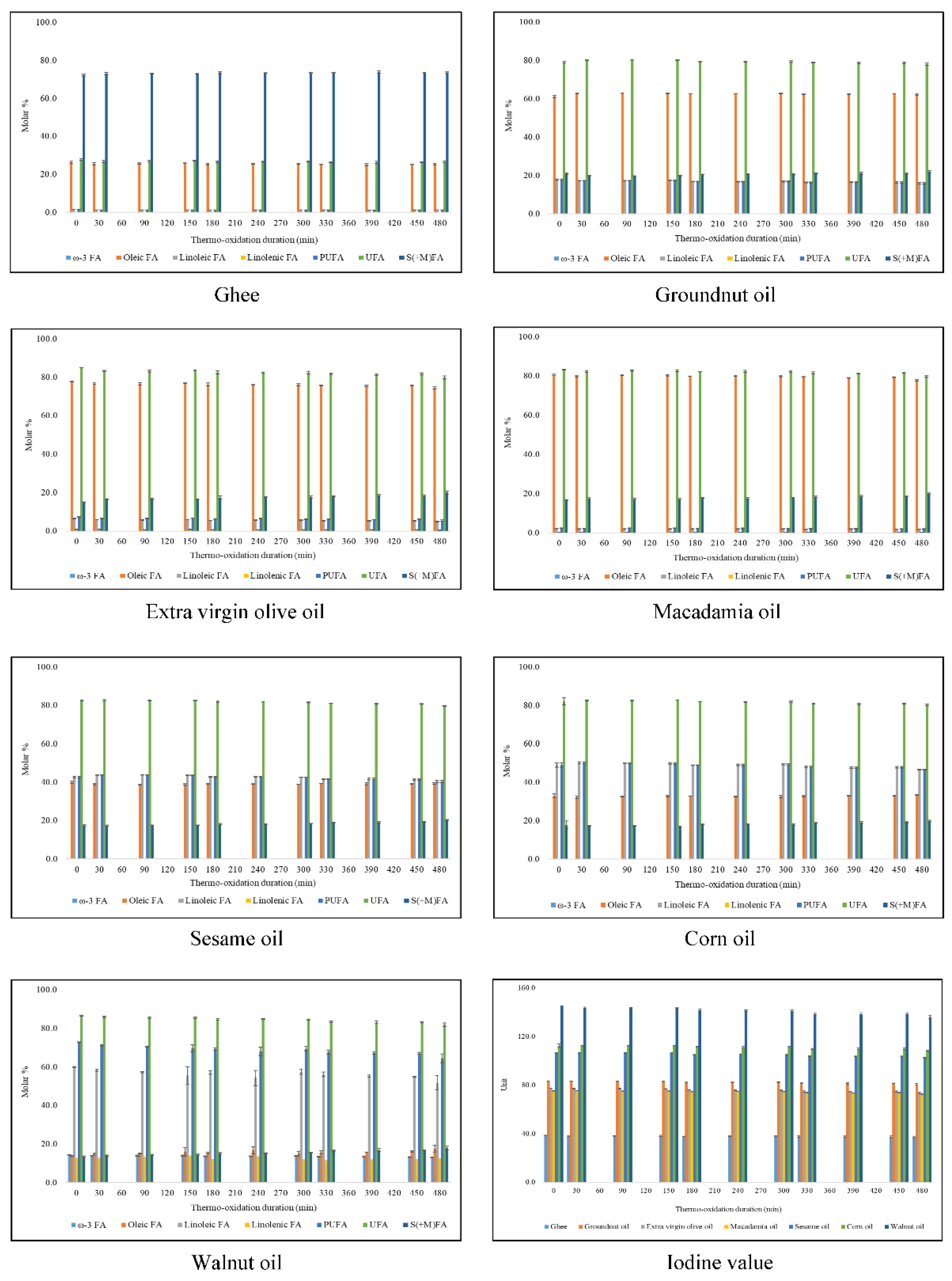


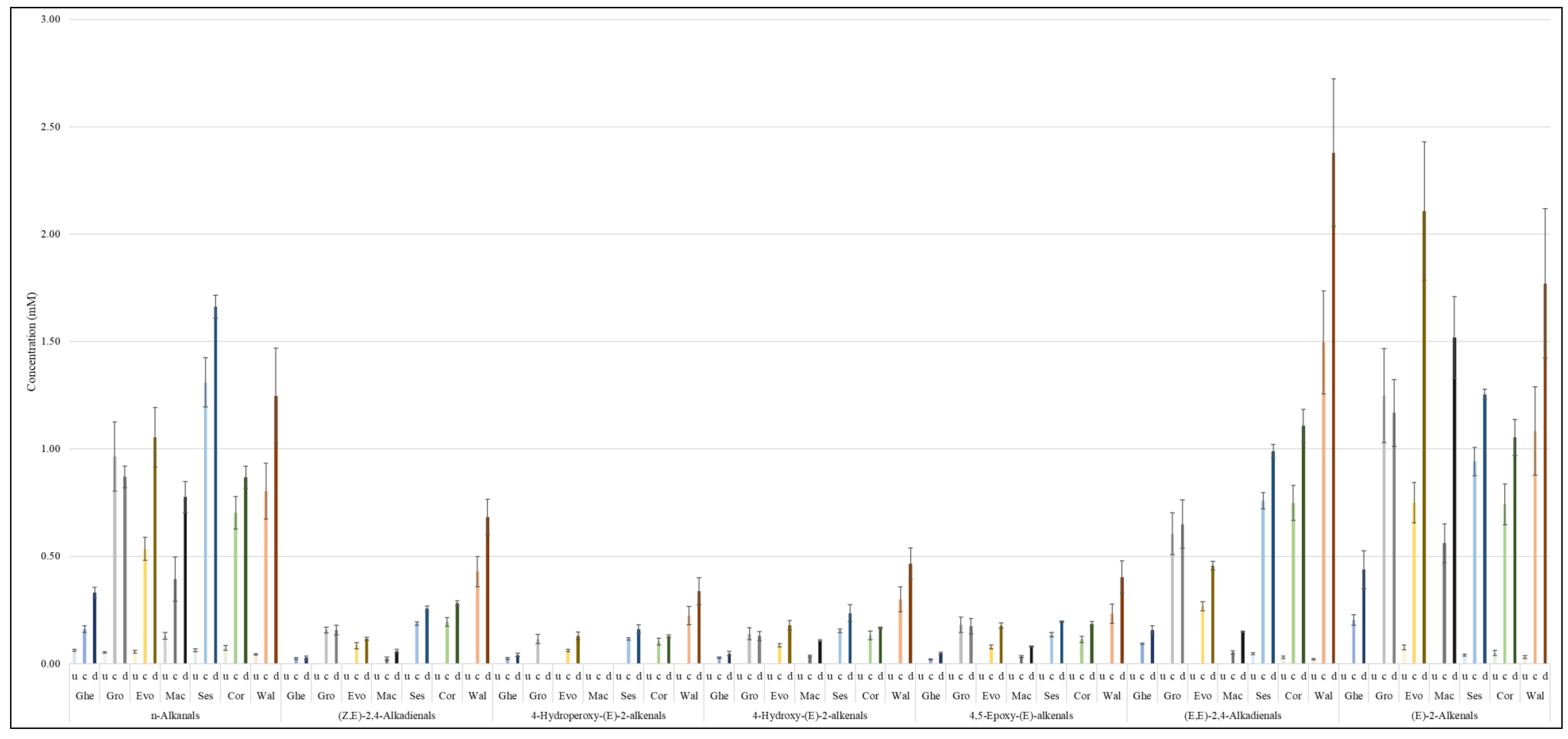
| Culinary Oil | Product Label (% (w/w)) | 1H NMR-Derived (Molar %) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA | MUFA | PUFA | UFA | IVu | SFA | MUFA | PUFA | UFA | IVu | |||||||
| ω-3 | O | L | Ln | L+Ln | O+L+Ln | ω-3 | O | L | Ln | L+Ln | O+L+Ln | |||||
| Ghee | 67.13 | - | - | - | - | 0.47 | - | - | 72.28 ± 0.51 | - | 26.31 ± 0.52 | 1.41 ± 0.03 | - | 1.41 ± 0.03 | 27.72 ± 0.51 | 38.48 ± 0.12 |
| Groundnut oil | 17.14 | - | 59.61 | - | - | 18.45 | 78.06 | - | 20.99 ± 0.34 | - | 60.87 ± 0.64 | 17.81 ± 0.31 | 0.34 ± 0.03 | 18.15 ± 0.33 | 79.01 ± 0.34 | 83.23 ± 0.12 |
| Extra virgin olive oil | 16.98 | - | 71.30 | - | - | 11.72 | 83.02 | - | 15.02 ± 0.09 | 2.01 ± 0.05 | 77.66 ± 0.10 | 6.44 ± 0.12 | 0.88 ± 0.07 | 7.32 ± 0.18 | 84.98 ± 0.09 | 77.43 ± 0.13 |
| Macadamia oil | 12.09 | - | 75.82 | - | - | 7.95 | 83.77 | - | 16.84 ± 0.24 | - | 80.57 ± 0.26 | 2.34 ± 0.05 | 0.25 ± 0.02 | 2.58 ± 0.07 | 83.16 ± 0.24 | 75.29 ± 0.20 |
| Sesame oil | 14.60 | - | - | - | - | - | - | - | 17.48 ± 0.34 | - | 39.84 ± 0.68 | 42.68 ± 0.39 | - | 42.68 ± 0.39 * | 82.52 ± 0.34 | 106.31 ± 0.22 |
| Corn oil | 13.46 | - | 29.53 | - | - | 57.00 | 86.54 | - | 17.91 ± 2.00 | 2.68 ± 0.16 | 31.80 ± 0.93 | 49.03 ± 1.09 | 1.26 ± 0.10 | 50.30 ± 1.06 | 82.09 ± 2.00 | 112.47 ± 1.37 |
| Walnut oil | 9.10 | - | - | - | - | - | - | - | 13.50 ± 0.30 | 14.25 ± 0.12 | 13.74 ± 0.26 | 59.97 ± 0.16 | 12.79 ± 0.05 | 72.76 ± 0.20 | 86.50 ± 0.30 | 145.20 ± 0.09 |
| Functional Group | ||||
|---|---|---|---|---|
| Signal | Chemical Shift (ppm) | Multiplicity | Condensed Function | Classification |
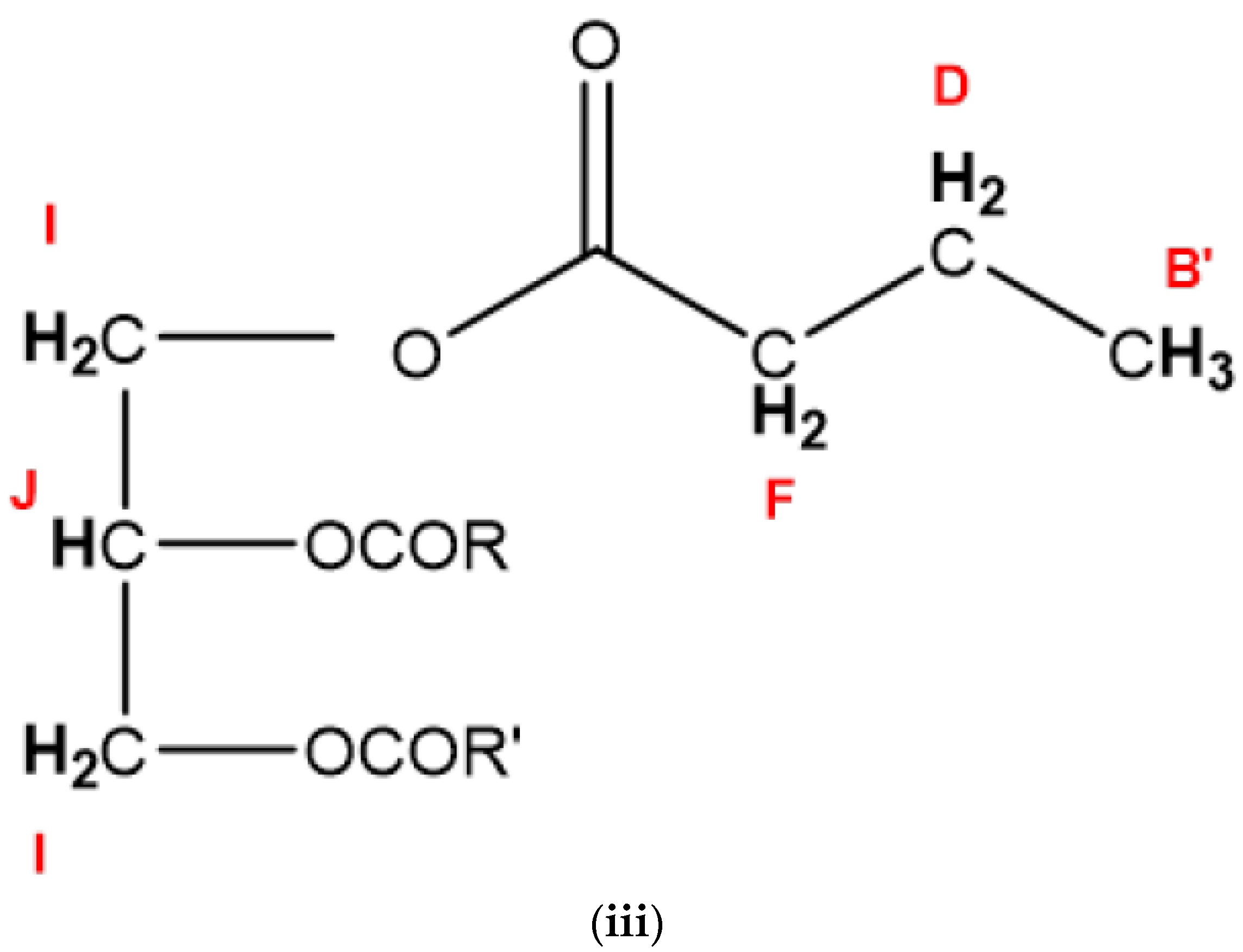
| A | 0.780–0.839 | t | –CH3 | Saturated, oleic, and linoleic acyl groups |
| B’ | 0.856–0.893 | t | –CH3 | n-Butyric acid/ester acyl groups |
| B | 0.883–0.918 | t | –CH3 | Unsaturated ω-3 acyl groups |
| C | 1.119–1.344 | t | –(CH2)n– | Bulk chain acyl groups |
| D | 1.494–1.596 | m | –OCO–CH2–CH2– | All acyl groups |
| E | 1.894–2.028 | m | –CH2–CH=CH– | All acyl groups |
| F | 2.200–2.286 | dt | –OCO–CH2– | All acyl groups |
| G | 2.669–2.715 | dd | =HC–CH2–CH= | Diunsaturated fatty acid ω-6 acyl groups |
| H | 2.715–2.755 | dd | =HC–CH2–CH= | Triunsaturated ω-3 acyl groups |
| I | 4.032–4.266 | ABX (AB) | –CH2OCOR | Glyceryl backbone functions |
| J | 5.164–5.221 | ABX (X) | >CHOCOR | Glyceryl backbone functions |
| K | 5.221–5.347 | m | –CH=CH– | Acyl chain olefinic functions |
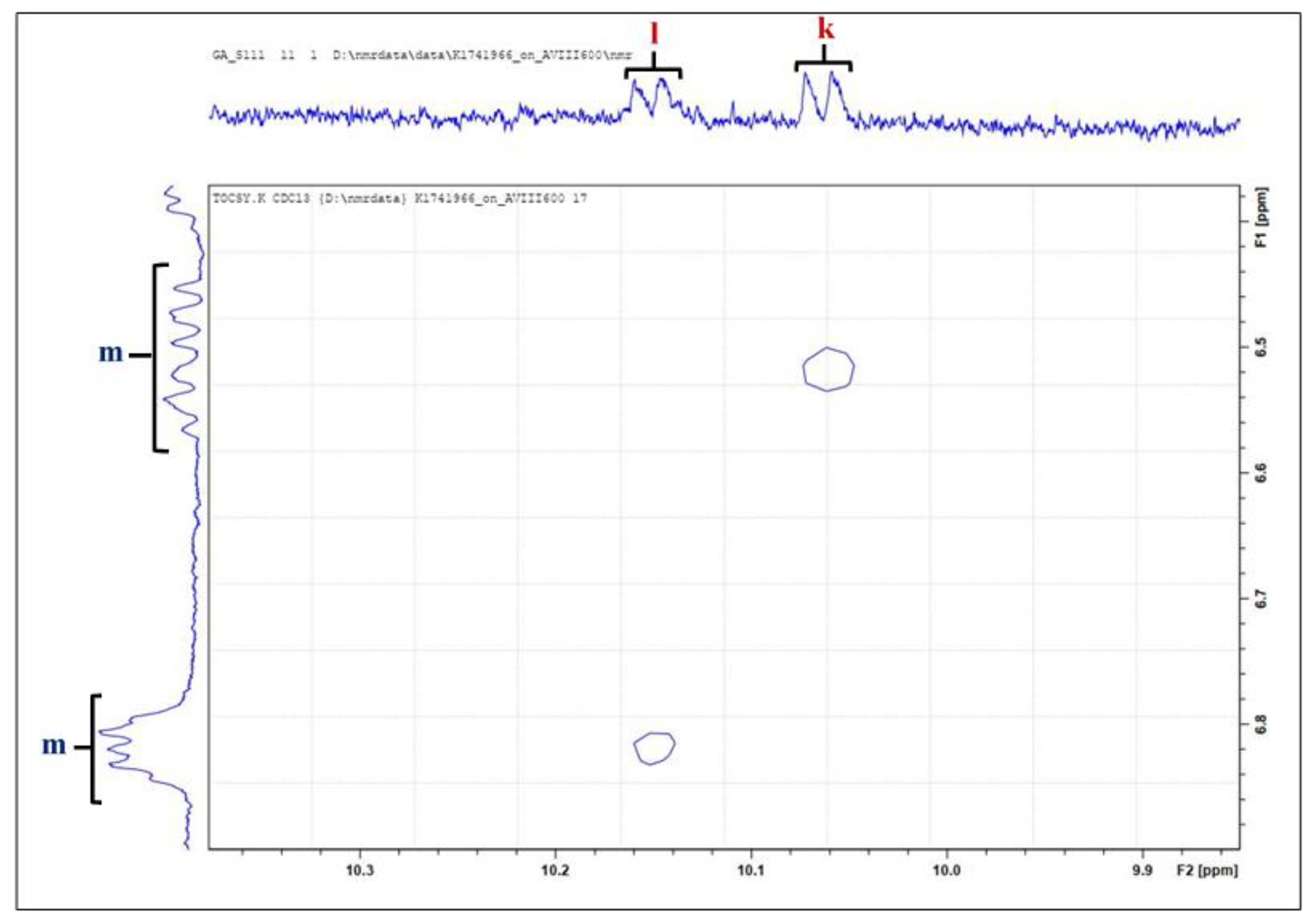
| Functional Group | ||||
|---|---|---|---|---|
| Signal | Chemical Shift (Ppm) | Multiplicity | Condensed Function | Classification |
| a | 9.412–9.443 | d | –CHO | (E)-2-Alkenals |
| b | 9.443–9.470 | d | –CHO | (E,E)-2,4-Alkadienals |
| c | 9.470–9.494 | d | –CHO | 4,5-Epoxy-(E)-alkenals |
| d | 9.499–9.520 | d | –CHO | 4-Hydroxy-(E)-2-alkenals |
| e | 9.507–9.523 | d | –CHO | 4-Hydroperoxy-(E)-2-alkenals |
| f | 9.523–9.547 | d | –CHO | (Z,E)-2,4-Alkadienals |
| g | 9.661–9.697 | t | –CHO | n-Alkanals |
| h | 9.712–9.727 | t | –CHO | 4-Oxo-n-alkanals |
| i | 9.733–9.748 | t | –CHO | Low-molecular-mass n-alkanals (ethanal, propanal and n-butanal) |
| j | 9.985–10.011 | d | –CHO | (Z)-2-Alkenals |
| k | 10.074–10.098 | d | –CHO | Unidentified unsaturated aldehyde |
| Factor | Oleic FA | Linoleic FA | Linolenic FA | Total ω-3 FAs | Total PUFAs | Total UFAs | Total S(+M) FAs | IV |
|---|---|---|---|---|---|---|---|---|
| C/D | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Time (min) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Time (min) | <0.0001 | <0.0001 | 0.001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Oil × Time (min) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Factor | Alkenal Species (Signal k) | (Z)-2-Alkenals | n-Alkanals (Low mwt) | 4-Oxo-n-Alkanals | n-Alkanals | (Z,E)-2,4-Alkadienals | 4-Hydroperoxy-(E)-2-Alkenals | 4-Hydroxy-(E)-2-Alkenals | 4,5-Epoxy-(E)-Alkenals | (E,E)-2,4-Alkadienals | (E)-2-Alkenals |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C/D | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Time (min) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Time (min) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Oil × Time (min) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Factor | Oleic FA | Linoleic FA | Linolenic FA | Total ω-3 FAs | Total PUFAs | Total UFAs | Total S(+M) FAs | IV |
|---|---|---|---|---|---|---|---|---|
| C/D | ns | <0.0001 | 0.086 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ns |
| Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Oil | <0.0001 | <0.0001 | 0.032 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Factor | Alkenal Species (Signal k) | (Z)-2-Alkenals | n-Alkanals (Low mwt) | 4-Oxo-n-alkanals | n-Alkanals | (Z,E)-2,4-Alkadienals | 4-Hydroperoxy-(E)-2-Alkenals | 4-Hydroxy-(E)-2-Alkenals | 4,5-Epoxy-(E)-Alkenals | (E,E)-2,4-Alkadienals | (E)-2-Alkenals |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C/D | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| C/D × Oil | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ampem, G.; Le Gresley, A.; Grootveld, M.; Naughton, D.P. Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques. Foods 2022, 11, 1864. https://doi.org/10.3390/foods11131864
Ampem G, Le Gresley A, Grootveld M, Naughton DP. Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques. Foods. 2022; 11(13):1864. https://doi.org/10.3390/foods11131864
Chicago/Turabian StyleAmpem, Gilbert, Adam Le Gresley, Martin Grootveld, and Declan P. Naughton. 2022. "Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques" Foods 11, no. 13: 1864. https://doi.org/10.3390/foods11131864
APA StyleAmpem, G., Le Gresley, A., Grootveld, M., & Naughton, D. P. (2022). Nuclear Magnetic Resonance Spectroscopic Analysis of the Evolution of Peroxidation Products Arising from Culinary Oils Exposed to Thermal Oxidation: An Investigation Employing 1H and 1H-1H COSY and TOCSY Techniques. Foods, 11(13), 1864. https://doi.org/10.3390/foods11131864







