Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC/LCMS Analysis of Bioactive Compounds from Antrodia cinnamomea
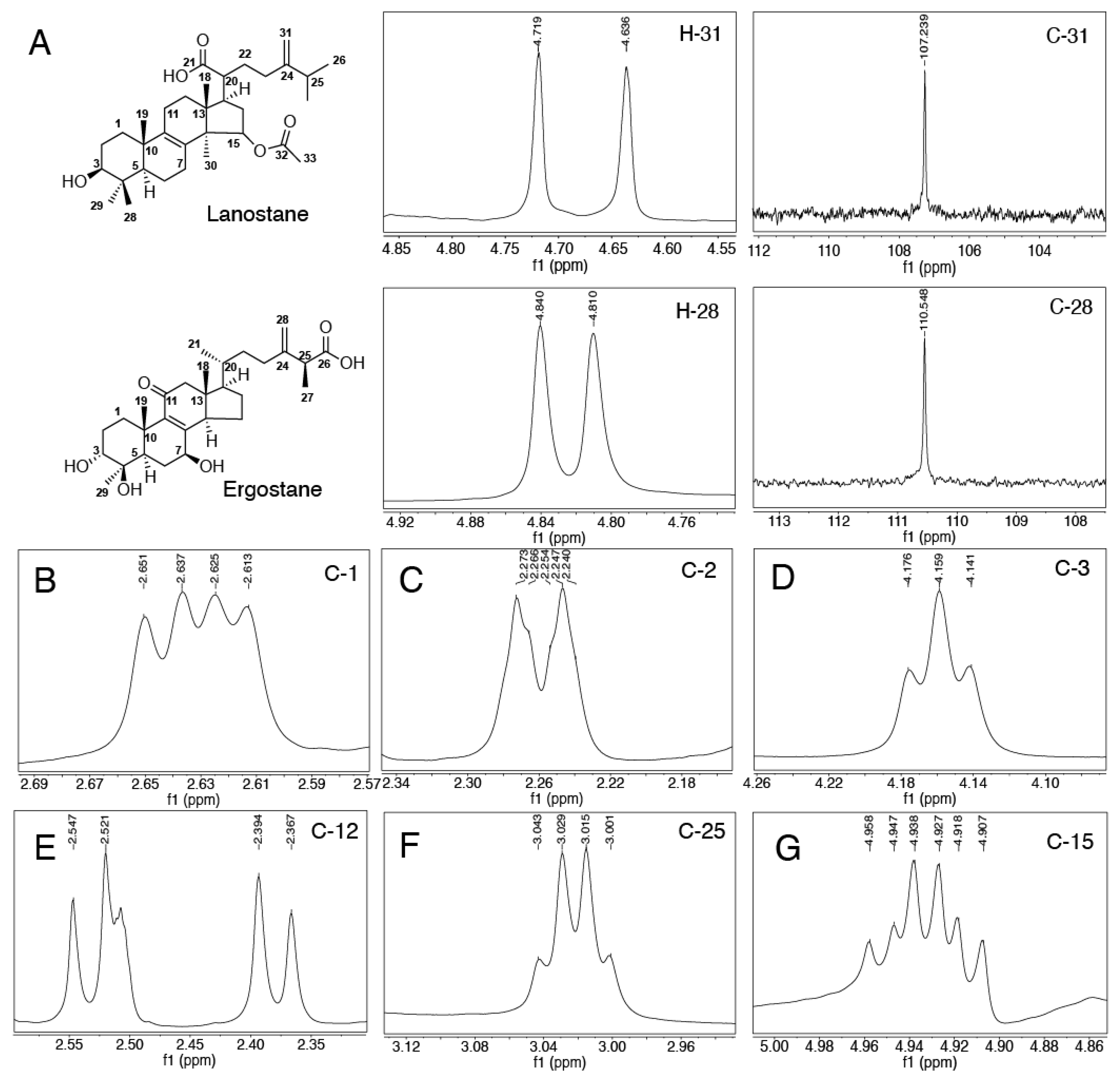
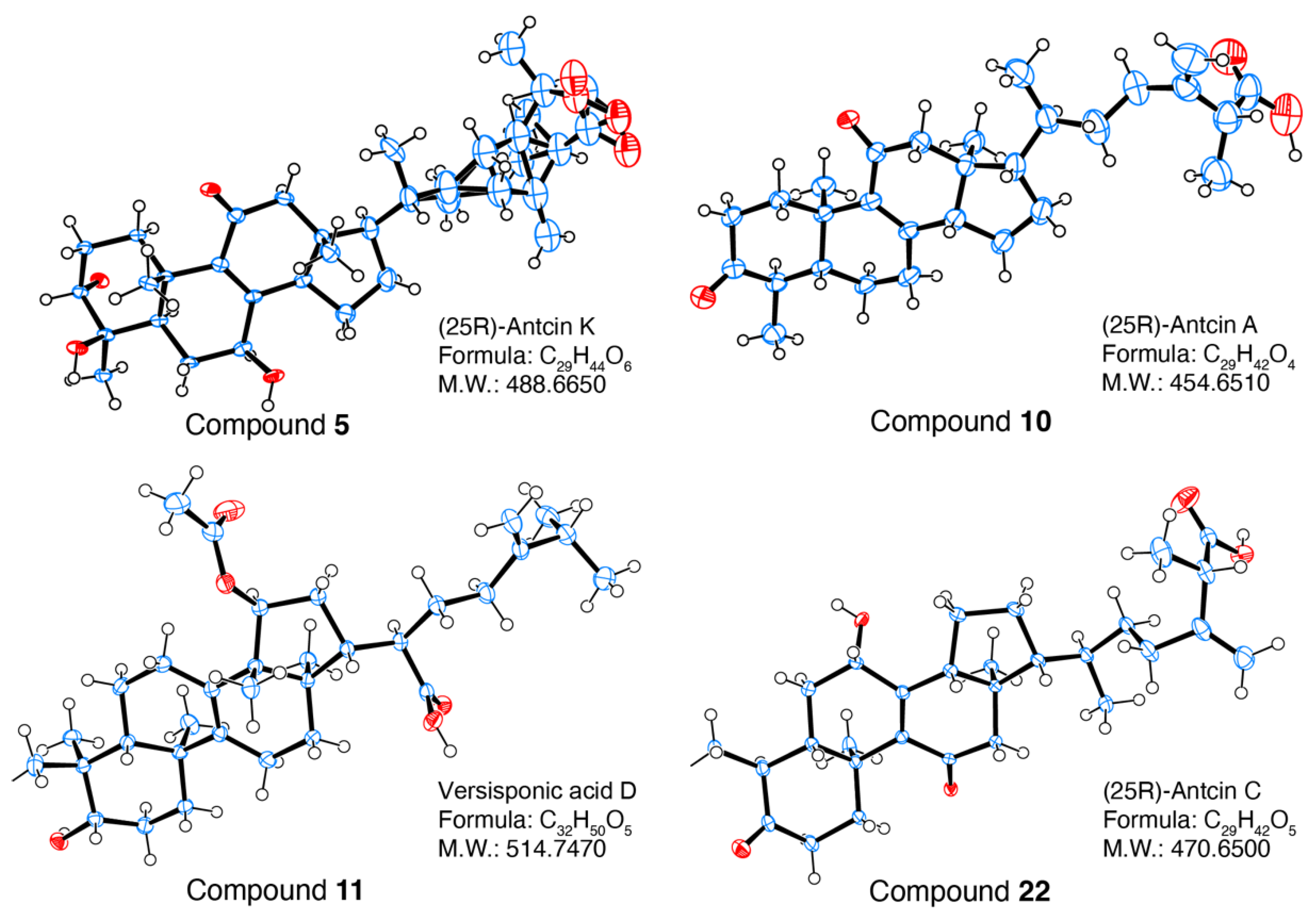
2.2. Structural Identification of Compounds 1-29 Isolated from Antrodia cinnamomea
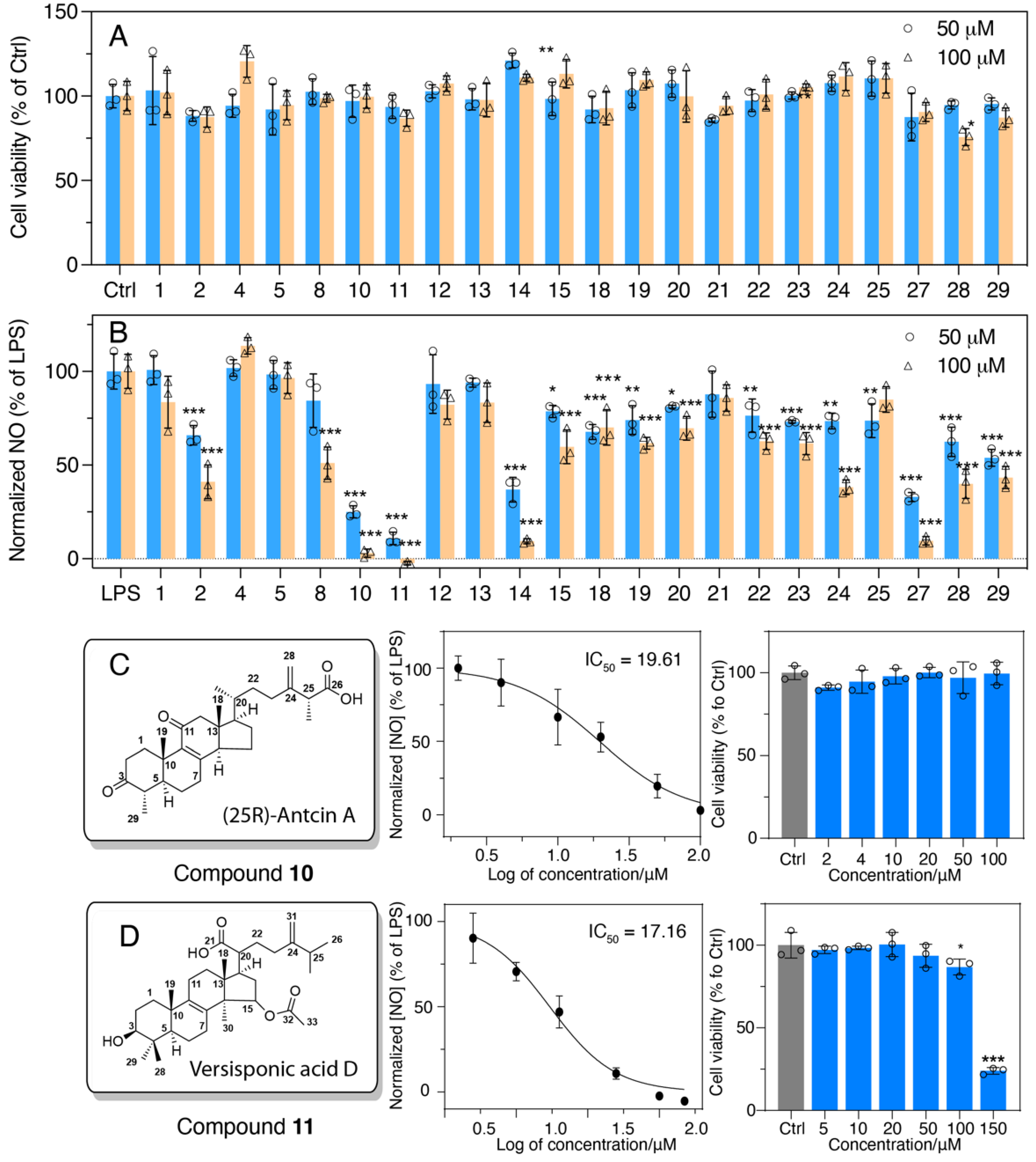
2.3. Evaluation of Anti-Inflammatory Activity of Major Compounds
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Isolated Products from Antrodia cinnamomea
3.5. Cell Culture
3.6. Cytotoxic Activities
3.7. Nitric Oxide (NO) Inhibitory Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.-H.; Ryvarden, L. Antrodia camphorata (“niu-chang-chih”), new combination of a medicinal fungus in Taiwan. Bot. Bull. -Acad. Sin. Taipei 1997, 38, 273–276. [Google Scholar]
- Wang, H.-C.; Chu, F.-H.; Chien, S.-C.; Liao, J.-W.; Hsieh, H.-W.; Li, W.-H.; Lin, C.-C.; Shaw, J.-F.; Kuo, Y.-H.; Wang, S.-Y. Establishment of the metabolite profile for an Antrodia cinnamomea health food product and investigation of its chemoprevention activity. J. Agric. Food Chem. 2013, 61, 8556–8564. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-L.; Chu, Y.-L.; Ho, C.-T.; Chung, J.-G.; Lai, C.-I.; Su, Y.-C.; Kuo, Y.-H.; Sheen, L.-Y. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated Antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J. Agric. Food Chem. 2015, 63, 4561–4569. [Google Scholar] [CrossRef]
- Hu, P.-F.; Huang, J.; Chen, L.; Ding, Z.; Liu, L.; Molnár, I.; Zhang, B.-B. Oxidative stress induction is a rational strategy to enhance the productivity of Antrodia cinnamomea fermentations for the antioxidant secondary metabolite antrodin C. J. Agric. Food Chem. 2020, 68, 3995–4004. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y. Review of pharmacological effects of Antrodia Camphorata and its bioactive compounds. Evid. Based Complementary Altern. 2011, 2011, 110. [Google Scholar]
- Wen, C.-L.; Chang, C.-C.; Huang, S.-S.; Kuo, C.-L.; Hsu, S.-L.; Deng, J.-S.; Huang, G.-J. Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J. Ethnopharmacol. 2011, 137, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.-H.; Xu, Z.-H.; Lu, Z.-M.; Xu, H.-Y.; Zhang, X.-M.; Dou, W.-F. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J. Ethnopharmacol. 2009, 121, 194–212. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Chen, C.-Y.; Chien, S.-C.; Hsiao, W.-W.; Chu, F.-H.; Li, W.-H.; Lin, C.-C.; Shaw, J.-F.; Wang, S.-Y. Metabolite profiles for Antrodia cinnamomea fruiting bodies harvested at different culture ages and from different wood substrates. J. Agric. Food Chem. 2011, 59, 7626–7635. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Hu, P.-F.; Huang, J.; Hu, Y.-D.; Chen, L.; Xu, G.-R. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar] [CrossRef]
- Tazuke, S.; Kurihara, S.; Yamaguchi, H.; Ikeda, T. Photochemically triggered physical amplification of photoresponsiveness. J. Phys. Chem. 1987, 91, 249–251. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.-H.; Tzeng, Y.-M. Evaluation of the anti-inflammatory and anti-proliferation tumoral cells activities of Antrodia camphorata, Cordyceps sinensis, and Cinnamomum osmophloeum bark extracts. J. Ethnopharmacol. 2007, 114, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.-C.; Wu, F.-Y.; Wu, J.-J.; Chen, J.-Y.; Chang, W.-H.; Lu, F.-J.; Lai, Y.-C.; Yang, H.-L. Anti-inflammatory potential of Antrodia camphorata through inhibition of iNOS, COX-2 and cytokines via the NF-κB pathway. Int. Immunopharmacol. 2005, 5, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-H.; Lin, C.-H.; Shih, C.-C. Antidiabetic and antihyperlipidemic properties of a triterpenoid compound, dehydroeburicoic acid, from Antrodia camphorata in vitro and in streptozotocin-induced mice. J. Agric. Food Chem. 2015, 63, 10140–10151. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-S.; Huang, S.-S.; Lin, T.-H.; Lee, M.-M.; Kuo, C.-C.; Sung, P.-J.; Hou, W.-C.; Huang, G.-J.; Kuo, Y.-H. Analgesic and anti-inflammatory bioactivities of eburicoic acid and dehydroeburicoic acid isolated from Antrodia camphorata on the inflammatory mediator expression in mice. J. Agric. Food Chem. 2013, 61, 5064–5071. [Google Scholar] [CrossRef]
- Hsieh, Y.-H.; Chu, F.-H.; Wang, Y.-S.; Chien, S.-C.; Chang, S.-T.; Shaw, J.-F.; Chen, C.-Y.; Hsiao, W.-W.; Kuo, Y.-H.; Wang, S.-Y. Antrocamphin A, an anti-inflammatory principal from the fruiting body of Taiwanofungus camphoratus, and its mechanisms. J. Agric. Food Chem. 2010, 58, 3153–3158. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liu, Y.-W.; Ker, Y.-B.; Wu, Y.-Y.; Lai, E.Y.; Chyau, C.-C.; Hseu, T.-H.; Peng, R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 2007, 55, 5007–5012. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Sheu, M.-T.; Liu, D.-Z.; Liao, C.-K.; Ho, H.-O.; Kao, W.-Y.; Ho, Y.-S.; Lee, W.-S.; Su, C.-H. Pretreatment with an ethanolic extract of Taiwanofungus camphoratus (Antrodia camphorata) enhances the cytotoxic effects of amphotericin B. J. Agric. Food Chem. 2011, 59, 11255–11263. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.-Y.; Lin, K.-H.; Lan, M.-H.; Wu, L.-Y.; Chou, D.-S.; Lin, C.-H.; Su, C.-H.; Sheu, J.-R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef]
- Chen, Y.-c.; Liu, Y.-l.; Li, F.-y.; Chang, C.-I.; Wang, S.-y.; Lee, K.-y.; Li, S.-l.; Chen, Y.-p.; Jinn, T.-r.; Tzen, J.T. Antcin A, a steroid-like compound from Antrodia camphorata, exerts anti-inflammatory effect via mimicking glucocorticoids. Acta Pharmacol. Sin. 2011, 32, 904–911. [Google Scholar] [CrossRef]
- Geethangili, M.; Fang, S.-H.; Lai, C.-H.; Rao, Y.K.; Lien, H.-M.; Tzeng, Y.-M. Inhibitory effect of Antrodia camphorata constituents on the Helicobacter pylori-associated gastric inflammation. Food Chem. 2010, 119, 149–153. [Google Scholar] [CrossRef]
- Shen, C.-C.; Wang, Y.-H.; Chang, T.-T.; Lin, L.-C.; Don, M.-J.; Hou, Y.-C.; Liou, K.-T.; Chang, S.; Wang, W.-Y.; Ko, H.-C. Anti-inflammatory ergostanes from the basidiomata of Antrodia salmonea. Planta Med. 2007, 73, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
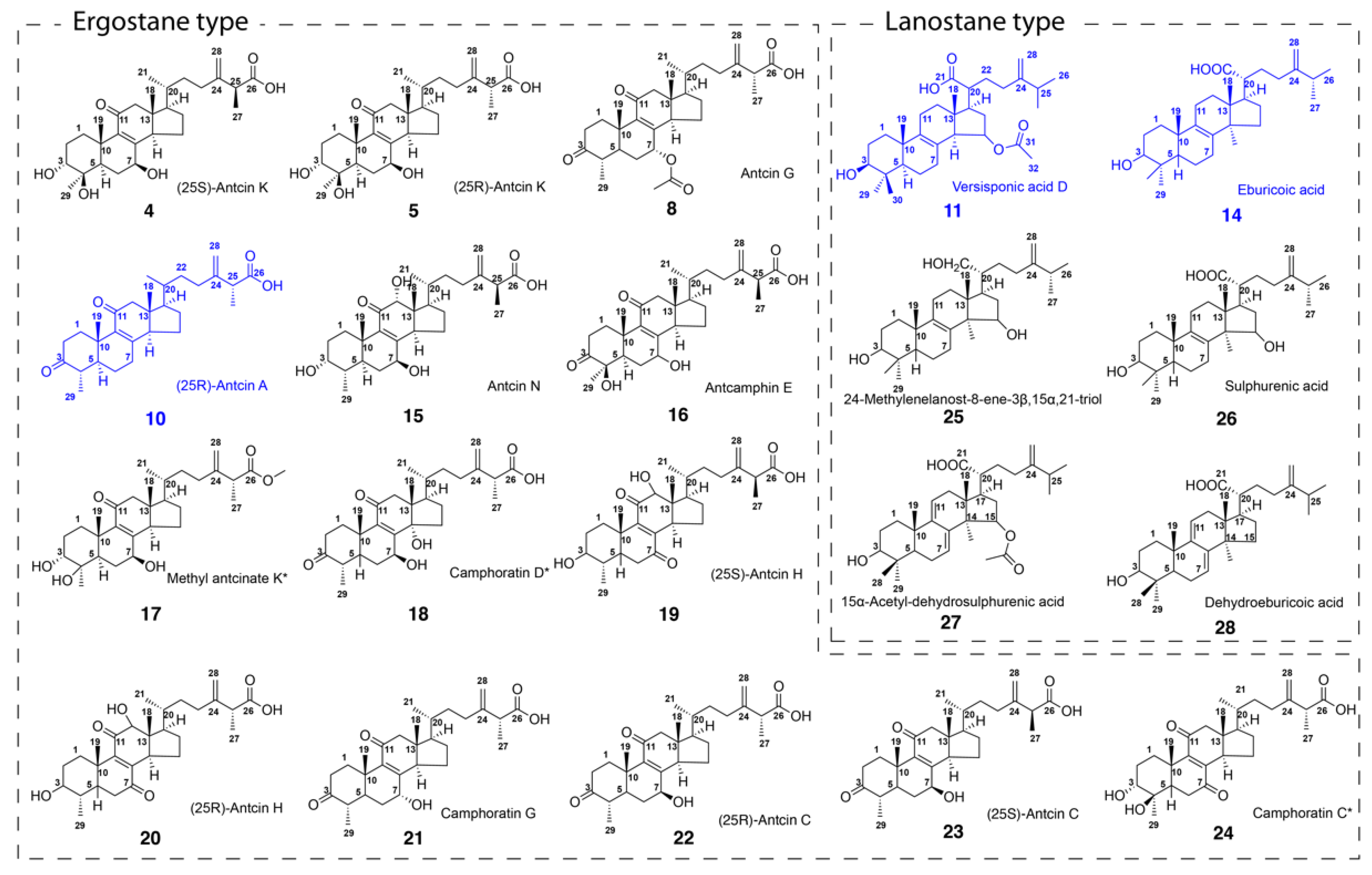
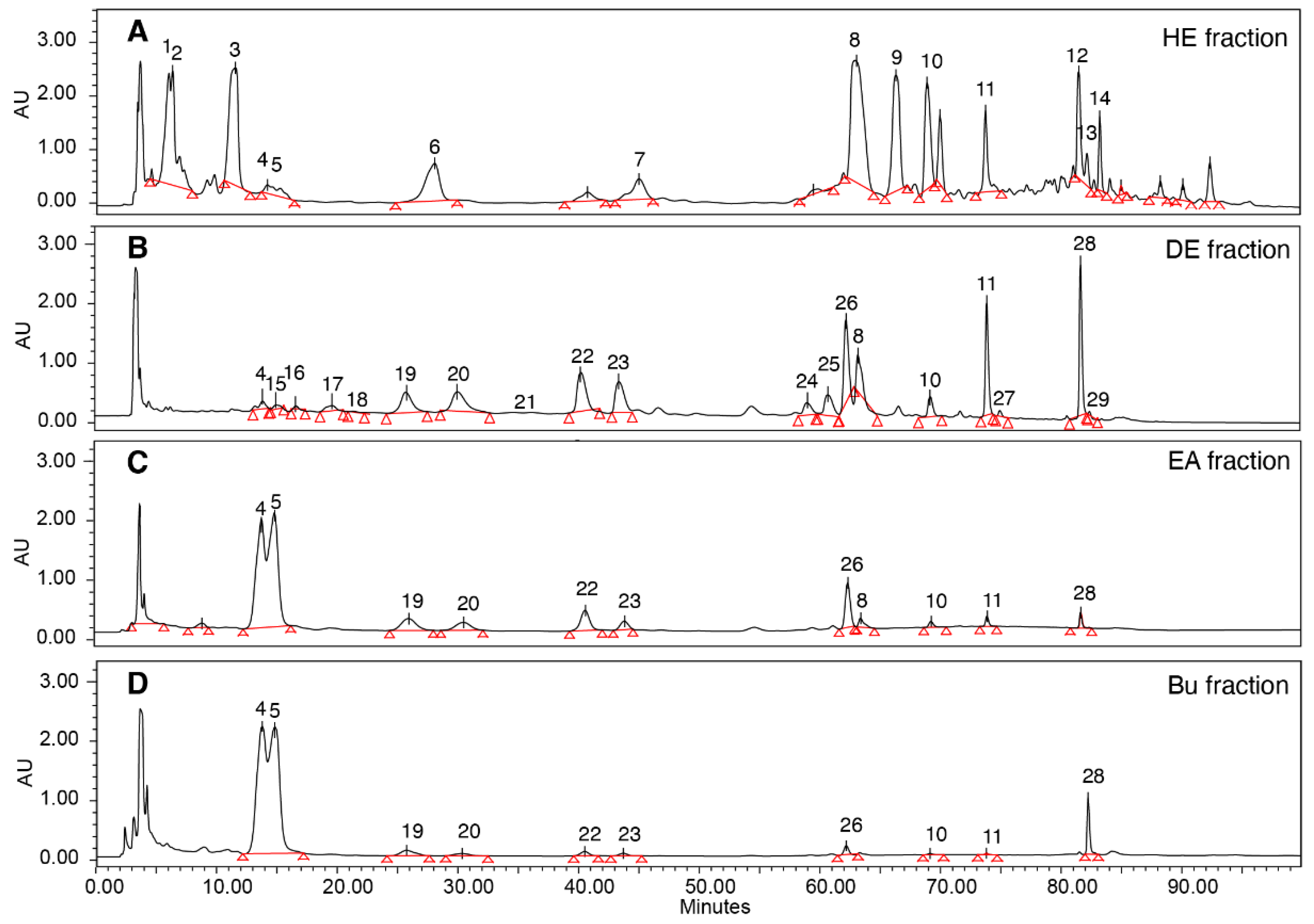
| No | tR | Max | Formula | [M-H]− | MS/MS of [M−H]− | Identification | ||
|---|---|---|---|---|---|---|---|---|
| (min) | (nm) | Pred. | Meas. | Δ(ppm) | ||||
| 1 | 6.355 | 230 | C10H14O4 | 197.0819 | 197.085 | 0.0011 | - | 2,3,4-trimethoxy-6-methylphenol |
| 2 | 6.421 | 230 | C24H19Na2O12- | 545.0677 | 544.9673 | 0.1004 | 118, 168, 396, 480 | Benzenoid (tentative) |
| 3 | 11.564 | 276 | C29H44O6 | 487.3065 | 487.3061 | 0.0004 | 119, 247, 407, 425, 443 | Unknown |
| 4 | 14.23 | 252 | C29H44O6 | 487.3065 | 487.3058 | 0.0007 | 259, 271, 342, 407, 443, 460 | (25S)-Antcin K |
| 5 | 14.988 | 252 | C29H44O6 | 487.3065 | 487.3063 | 0.0002 | 259, 271, 342, 407, 443, 460 | (25R)-Antcin K |
| 6 | 28.088 | 284 | C29H29O9 | 485.1218 | 485.2904 | 0.1686 | 272, 301, 425 | Benzenoid (tentative) |
| 7 | 45.031 | 265 | C25H23O10 | 483.1297 | 483.2762 | −0.1465 | 193, 287, 337, 411, 421, 439 | Benzenoid (tentative) |
| 8 | 63.086 | 250 | C31H44O6 | 511.3065 | 511.3425 | 0.036 | 395, 407, 451, 465 | Antcin G |
| 9 | 68.933 | 251 | C26H23O11 | 452.0822 | 452.0822 | 0 | 126, 143, 262, 316 | Benzenoid (tentative) |
| 10 | 66.352 | 253 | C29H42O4 | 453.301 | 453.3007 | 0.0003 | 123, 257, 337, 393, 411 | (25R)-Antcin A |
| 11 | 70.011 | 249 | C32H50O5 | 527.3742 | 527.3737 | 0.0005 | 397. 467 | Versisponic acid D |
| 12 | 73.777 | 244 | C35H29O9 | 593.1812 | 593.3732 | −0.1920 | 265 | Benzenoid (tentative) |
| 13 | 81.509 | 242 | C25H29O7 | 467.1348 | 467.3529 | −0.1095 | 113, 227, 249, 385, 441 | Benzenoid (tentative) |
| 14 | 83.258 | 245 | C29H41O5 | 469.2959 | 469.3684 | 0.0725 | 113, 227, 281, 299, 385, 441 | Eburicoic acid |
| 15 | 13.935 | 198 | C29H44O6 | 487.3065 | 487.3061 | 0.0004 | 209, 407, 452 | Antcin N |
| 16 | 15.058 | 198 | C29H42O6 | 485.2909 | 485.2903 | 0.0006 | 149, 233, 408, 423, 441, 467 | Antcamphin E |
| 17 | 16.676 | 198 | C30H46O6 | 501.3222 | 501.2854 | 0.0368 | 325, 358, 395, 413, 439, 457, 483 | Methyl antcinate K * |
| 18 | 19.701 | 198 | C29H42O6 | 485.2909 | 485.2906 | 0.0003 | 137, 247, 289, 341, 407, 423, 441 | Camphoratin D * |
| 19 | 25.78 | 256 | C29H42O6 | 485.2909 | 485.2903 | 0.0006 | 149, 207, 233, 399, 423 | (25S)-Anctin H |
| 20 | 30.056 | 256 | C29H42O6 | 485.2909 | 485.2902 | 0.0007 | 193, 233, 397, 441 | (25R)-Anctin H |
| 21 | 34.119 | 252 | C29H42O5 | 469.2959 | 469.2957 | 0.0002 | 247, 301, 341, 407, 425 | Camphoratin G |
| 22 | 40.26 | 255 | C29H42O5 | 469.2959 | 469.3319 | 0.036 | 339, 395, 407, 425 | (25R)-Antcin C |
| 23 | 43.403 | 255 | C29H42O5 | 469.2959 | 469.2953 | 0.0006 | 219, 233, 272, 391, 425 | (25S)-Antcin C |
| 24 | 58.941 | 273 | C29H42O6 | 485.2909 | 485.2906 | 0.0003 | 233, 275, 397, 412, 423, 441 | Antcamphin P/Q |
| 25 | 60.664 | 273 | C31H50O4 | 485.3636 | 485.2903 | 0.0733 | 253, 339, 397, 405, 413, 441 | 24-Methylenelanost-8-ene-3β,15α,21-triol |
| 26 | 62.16 | 242 | C31H50O4 | 485.3636 | 485.2901 | 0.0735 | 160, 369, 397, 423, 441 | Sulphurenic acid |
| 27 | 73.801 | 242 | C33H50O5 | 525.3585 | 525.3577 | 0.0008 | 233, 245, 397, 467 | 15α-Acetyl-dehydrosulphurenic acid |
| 28 | 81.553 | 240 | C31H49O3 | 467.3531 | 467.3526 | 0.0005 | 156, 170, 339, 371 | Dehydroeburicoic acid |
| 29 | 81.965 | 202 | C30H47O4 | 469.3323 | 469.3683 | 0.036 | 339 | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wang, X.; Lin, J.; Lim, S.; Cao, Y.; Chen, S.; Xu, P.; Xu, C.; Zheng, H.; Fu, K.-C.; et al. Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea. Foods 2022, 11, 1831. https://doi.org/10.3390/foods11131831
Yang X, Wang X, Lin J, Lim S, Cao Y, Chen S, Xu P, Xu C, Zheng H, Fu K-C, et al. Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea. Foods. 2022; 11(13):1831. https://doi.org/10.3390/foods11131831
Chicago/Turabian StyleYang, Xin, Xiang Wang, Jiachen Lin, Sophie Lim, Yujia Cao, Siyu Chen, Pingkang Xu, Chunyuhang Xu, Hongling Zheng, Kuo-Chang Fu, and et al. 2022. "Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea" Foods 11, no. 13: 1831. https://doi.org/10.3390/foods11131831
APA StyleYang, X., Wang, X., Lin, J., Lim, S., Cao, Y., Chen, S., Xu, P., Xu, C., Zheng, H., Fu, K.-C., Kuo, C.-L., & Huang, D. (2022). Structure and Anti-Inflammatory Activity Relationship of Ergostanes and Lanostanes in Antrodia cinnamomea. Foods, 11(13), 1831. https://doi.org/10.3390/foods11131831







