Impacts of pH and Base Substitution during Deaerator Treatments of Herring Milt Hydrolysate on the Odorous Content and the Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Herring Milt Hydrolysate (HMH)
2.2. Methods
2.2.1. Protocol
2.2.2. Analyses
3. Results and Discussion
3.1. Volatile Compound Analyses
3.1.1. DMA, TMA, and TMAO
3.1.2. Most Potent Odor-Active Compounds
3.1.3. General Discussion on Volatile Compounds
3.2. Sensory Analysis
3.3. Antioxidant Activity
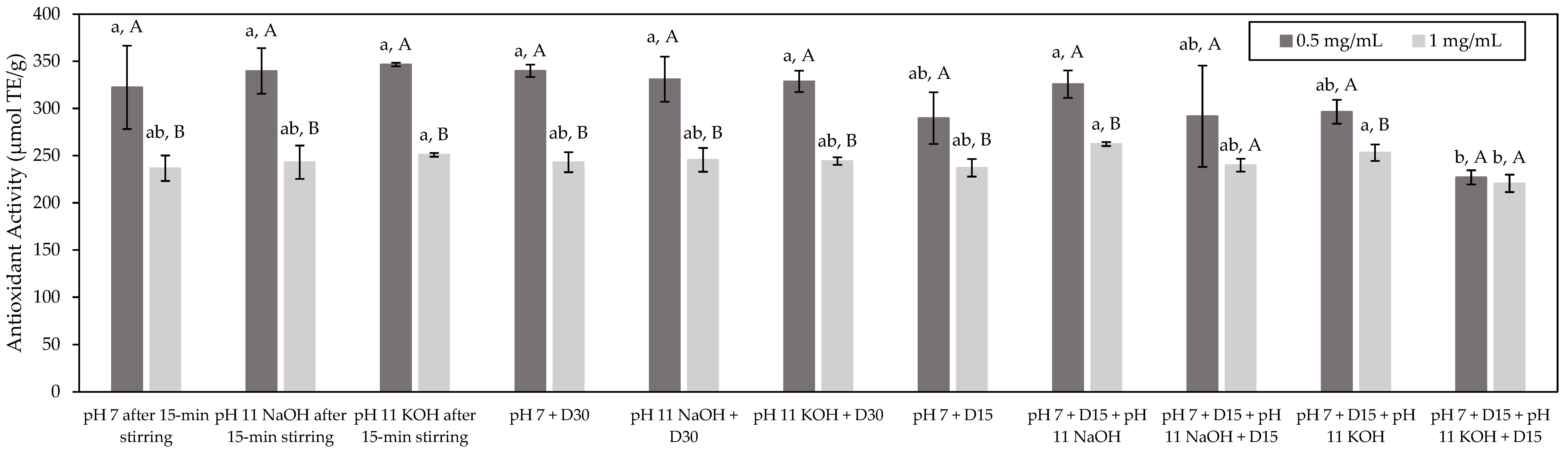
3.3.1. ORAC
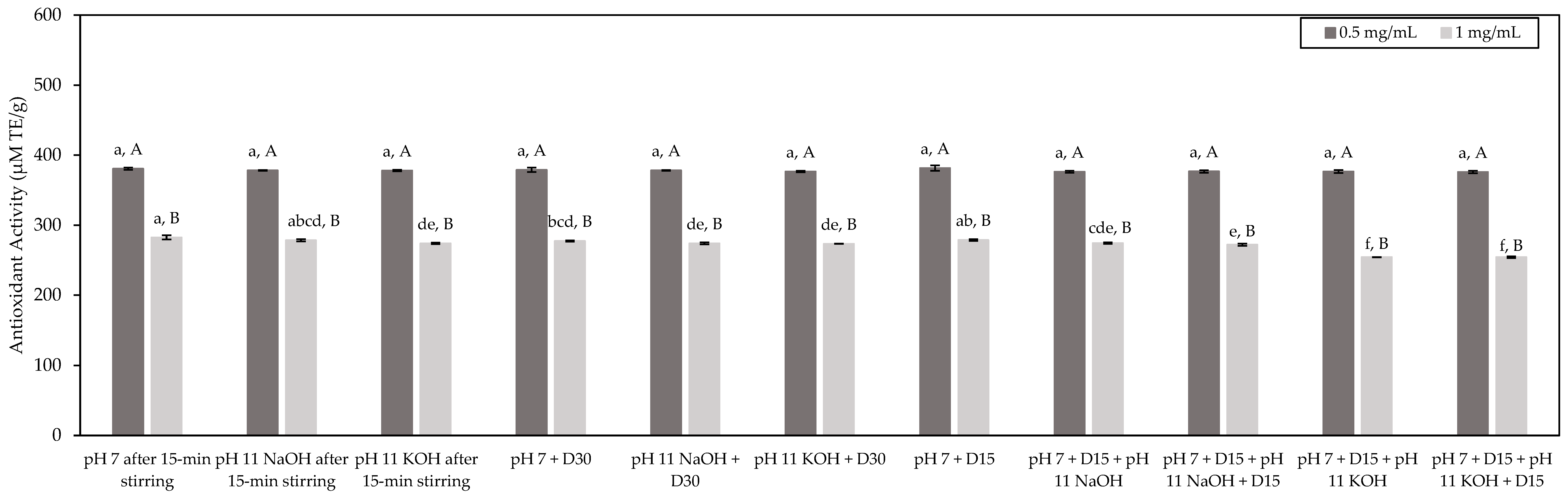
3.3.2. DPPH Radical-Scavenging Capacity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villamil, O.; Váquiro, H.; Solanilla, J.F. Fish Viscera Protein Hydrolysates: Production, Potential Applications and Functional and Bioactive Properties. Food Chem. 2017, 224, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.; Fraboulet, E.; Marette, A.; Bazinet, L. Simultaneous Double Cationic and Anionic Molecule Separation from Herring Milt Hydrolysate and Impact on Resulting Fraction Bioactivities. Sep. Purif. Technol. 2019, 210, 431–441. [Google Scholar] [CrossRef]
- Durand, R.; Pellerin, G.; Thibodeau, J.; Fraboulet, E.; Marette, A.; Bazinet, L. Screening for Metabolic Syndrome Application of a Herring By-Product Hydrolysate after Its Separation by Electrodialysis with Ultrafiltration Membrane and Identification of Novel Anti-Inflammatory Peptides. Sep. Purif. Technol. 2020, 235, 116205. [Google Scholar] [CrossRef]
- Wang, Y.; Gagnon, J.; Nair, S.; Sha, S. Herring Milt Protein Hydrolysate Improves Insulin Resistance in High-Fat-Diet-Induced Obese Male C57BL/6J Mice. Mar. Drugs 2019, 17, 456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Nair, S.; Gagnon, J. Herring Milt and Herring Milt Protein Hydrolysate Reduce Weight Gain and Improve Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese Mice. Curr. Dev. Nutr. 2020, 4, 1699. [Google Scholar] [CrossRef]
- Durand, R.; Ouellette, A.; Houde, V.P.; Guénard, F.; Varin, T.V.; Marcotte, B.; Pilon, G.; Fraboulet, E.; Vohl, M.-C.; Marette, A.; et al. Animal and Cellular Studies Demonstrate Some of the Beneficial Impacts of Herring Milt Hydrolysates on Obesity-Induced Glucose Intolerance and Inflammation. Nutrients 2020, 12, 3235. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, X.; Chen, H.; Cai, B.; Wan, P.; Zhu, X.; Sun, H.; Sun, H.; Pan, J. Identification of Odor Volatile Compounds and Deodorization of Paphia Undulata Enzymatic Hydrolysate. J. Ocean Univ. China 2016, 15, 1101–1110. [Google Scholar] [CrossRef]
- Todeschini, S.; Perreault, V.; Goulet, C.; Bouchard, M.; Dubé, P.; Boutin, Y.; Bazinet, L. Assessment of the Performance of Electrodialysis in the Removal of the Most Potent Odor-Active Compounds of Herring Milt Hydrolysate: Focus on Ion-Exchange Membrane Fouling and Water Dissociation as Limiting Process Conditions. Membranes 2020, 10, 127. [Google Scholar] [CrossRef]
- Baliño-Zuazo, L.; Barranco, A. A Novel Liquid Chromatography–Mass Spectrometric Method for the Simultaneous Determination of Trimethylamine, Dimethylamine and Methylamine in Fishery Products. Food Chem. 2016, 196, 1207–1214. [Google Scholar] [CrossRef]
- Park, S.-K.; Jo, D.-M.; Yu, D.; Khan, F.; Lee, Y.B.; Kim, Y.-M. Reduction of Trimethylamine Off-Odor by Lactic Acid Bacteria Isolated from Korean Traditional Fermented Food and Their In Situ Application. J. Microbiol. Biotechnol. 2020, 30, 1510–1515. [Google Scholar] [CrossRef]
- Jo, D.-M.; Park, S.-K.; Khan, F.; Kang, M.-G.; Lee, J.-H.; Kim, Y.-M. An Approach to Extend the Shelf Life of Ribbonfish Fillet Using Lactic Acid Bacteria Cell-Free Culture Supernatant. Food Control 2021, 123, 107731. [Google Scholar] [CrossRef]
- Song, G.; Zhang, M.; Peng, X.; Yu, X.; Dai, Z.; Shen, Q. Effect of Deodorization Method on the Chemical and Nutritional Properties of Fish Oil during Refining. LWT 2018, 96, 560–567. [Google Scholar] [CrossRef]
- Boraphech, P.; Thiravetyan, P. Trimethylamine (Fishy Odor) Adsorption by Biomaterials: Effect of Fatty Acids, Alkanes, and Aromatic Compounds in Waxes. J. Hazard. Mater. 2015, 284, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, S.; Perreault, V.; Goulet, C.; Bouchard, M.; Dubé, P.; Boutin, Y.; Bazinet, L. Development of a New Deodorization Method of Herring Milt Hydrolysate: Impacts of PH, Stirring with Nitrogen and Deaerator Treatment on the Odorous Content. Foods 2021, 10, 884. [Google Scholar] [CrossRef]
- Tremblay, A.; Corcuff, R.; Goulet, C.; Godefroy, S.B.; Doyen, A.; Beaulieu, L. Valorization of Snow Crab (Chionoecetes Opilio) Cooking Effluents for Food Applications. J. Sci. Food Agric. 2020, 100, 384–393. [Google Scholar] [CrossRef]
- Chan, S.T.; Yao, M.W.Y.; Wong, Y.C.; Wong, T.; Mok, C.S.; Sin, D.W.M. Evaluation of Chemical Indicators for Monitoring Freshness of Food and Determination of Volatile Amines in Fish by Headspace Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry. Eur. Food Res. Technol. 2006, 224, 67. [Google Scholar] [CrossRef]
- Chang, G.W.; Chang, W.L.; Lew, K.B.K. Trimethylamine-Specific Electrode for Fish Quality Control. J. Food Sci. 1976, 41, 723–724. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Effect of Pork Meat Proteins on the Binding of Volatile Compounds. Food Chem. 2008, 108, 1226–1233. [Google Scholar] [CrossRef]
- Wang, K.; Arntfield, S. Effect of Salts and PH on Selected Ketone Flavours Binding to Salt-Extracted Pea Proteins: The Role of Non-Covalent Forces. Food Res. Int. 2015, 77, 17. [Google Scholar] [CrossRef]
- Tappi, S.; De Aguiar Saldanha Pinheiro, A.C.; Mercatante, D.; Picone, G.; Soglia, F.; Rodriguez-Estrada, M.T.; Petracci, M.; Capozzi, F.; Rocculi, P. Quality Changes during Frozen Storage of Mechanical-Separated Flesh Obtained from an Underutilized Crustacean. Foods 2020, 9, 1485. [Google Scholar] [CrossRef]
- Pérez-Juan, M.; Flores, M.; Toldrá, F. Effect of Ionic Strength of Different Salts on the Binding of Volatile Compounds to Porcine Soluble Protein Extracts in Model Systems. Food Res. Int. 2007, 40, 687–693. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, W.; Cao, Y.; Gong, X.; Li, J.; Lu, X.; Dai, Y. Analysis of Volatile Components of Tilapia Enzymolysis Solution after Different Deodorization Treatments. IOP Conf. Ser. Earth Environ. Sci. 2020, 571, 012121. [Google Scholar] [CrossRef]
- Güner, M.; Yılmaz, E.; Yüceer, Y. Off-Odor Removal from Fish Oil by Adsorbent Treatment with Selected Metal-Organic Frameworks. Flavour Fragr. J. 2019, 34, 163–174. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric Characterization of Aroma Active Compounds in Fermented Fish Paste in Comparison with Fish Sauce, Fermented Soy Paste and Sauce Products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Marsili, R.T.; Laskonis, C.R. Odorant Synergy Effects as the Cause of Fishy Malodors in Algal Marine Oils. J. Agric. Food Chem. 2014, 62, 9676–9682. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Let, M.B.; Meyer, A.S.; Jacobsen, C. Modeling the Sensory Impact of Defined Combinations of Volatile Lipid Oxidation Products on Fishy and Metallic Off-Flavors. J. Agric. Food Chem. 2004, 52, 1635–1641. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Valette, J.; Guichard, É.; Gunata, Z. Impact of Fruit Texture on the Release and Perception of Aroma Compounds during in Vivo Consumption Using Fresh and Processed Mango Fruits. Food Chem. 2018, 239, 806–815. [Google Scholar] [CrossRef]
- Friel, E.N.; Taylor, A.J. Effect of Salivary Components on Volatile Partitioning from Solutions. J. Agric. Food Chem. 2001, 49, 3898–3905. [Google Scholar] [CrossRef]
- Sabeena Farvin, K.H.; Andersen, L.L.; Nielsen, H.H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Jessen, F. Antioxidant Activity of Cod (Gadus Morhua) Protein Hydrolysates: In Vitro Assays and Evaluation in 5% Fish Oil-in-Water Emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef]
- Nazlić, M.; Kremer, D.; Grubešić, R.J.; Soldo, B.; Vuko, E.; Stabentheiner, E.; Ballian, D.; Bogunić, F.; Dunkić, V. Endemic Veronica Saturejoides Vis. Ssp. Saturejoides–Chemical Composition and Antioxidant Activity of Free Volatile Compounds. Plants 2020, 9, 1646. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Fons, F.; Froissard, D.; Morel, S.; Bessière, J.-M.; Buatois, B.; Sol, V.; Fruchier, A.; Rapior, S. Pteridaceae Fragrant Resource and Bioactive Potential: A Mini-Review of Aroma Compounds. Nat. Prod. Commun. 2018, 13, 1934578X1801300531. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish Protein Hydrolysates: Proximate Composition, Amino Acid Composition, Antioxidant Activities and Applications: A Review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and Identification of Antioxidant Peptides from Grass Carp Muscle Hydrolysates by Consecutive Chromatography and Electrospray Ionization-Mass Spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef]
- Zhong, S.; Ma, C.; Lin, Y.C.; Luo, Y. Antioxidant Properties of Peptide Fractions from Silver Carp (Hypophthalmichthys Molitrix) Processing by-Product Protein Hydrolysates Evaluated by Electron Spin Resonance Spectrometry. Food Chem. 2011, 126, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC Assays Comparison to Measure the Antioxidant Capacity of Food Products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Autolysis-Assisted Production of Fish Protein Hydrolysates with Antioxidant Properties from Pacific Hake (Merluccius Productus). Food Chem. 2008, 107, 768–776. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Kitts, D.D.; Li-Chan, E.C.Y. Antioxidative and Angiotensin-I-Converting Enzyme Inhibitory Potential of a Pacific Hake (Merluccius Productus) Fish Protein Hydrolysate Subjected to Simulated Gastrointestinal Digestion and Caco-2 Cell Permeation. J. Agric. Food Chem. 2010, 58, 1535–1542. [Google Scholar] [CrossRef]
- Halldorsdottir, S.M.; Sveinsdottir, H.; Gudmundsdottir, A.; Thorkelsson, G.; Kristinsson, H.G. High Quality Fish Protein Hydrolysates Prepared from By-Product Material with Fucus Vesiculosus Extract. J. Funct. Foods 2014, 9, 10–17. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein Hydrolysate from Salmon Frames: Production, Characteristics and Antioxidative Activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef]
- Paśko, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S.; Fołta, M.; Zachwieja, Z. Anthocyanins, Total Polyphenols and Antioxidant Activity in Amaranth and Quinoa Seeds and Sprouts during Their Growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Theodore, A.E.; Raghavan, S.; Kristinsson, H.G. Antioxidative Activity of Protein Hydrolysates Prepared from Alkaline-Aided Channel Catfish Protein Isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar] [CrossRef] [PubMed]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in Vitro Antioxidant Properties of a Cod (Gadus Morhua) Protein Hydrolysate and Peptide Fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef] [PubMed]
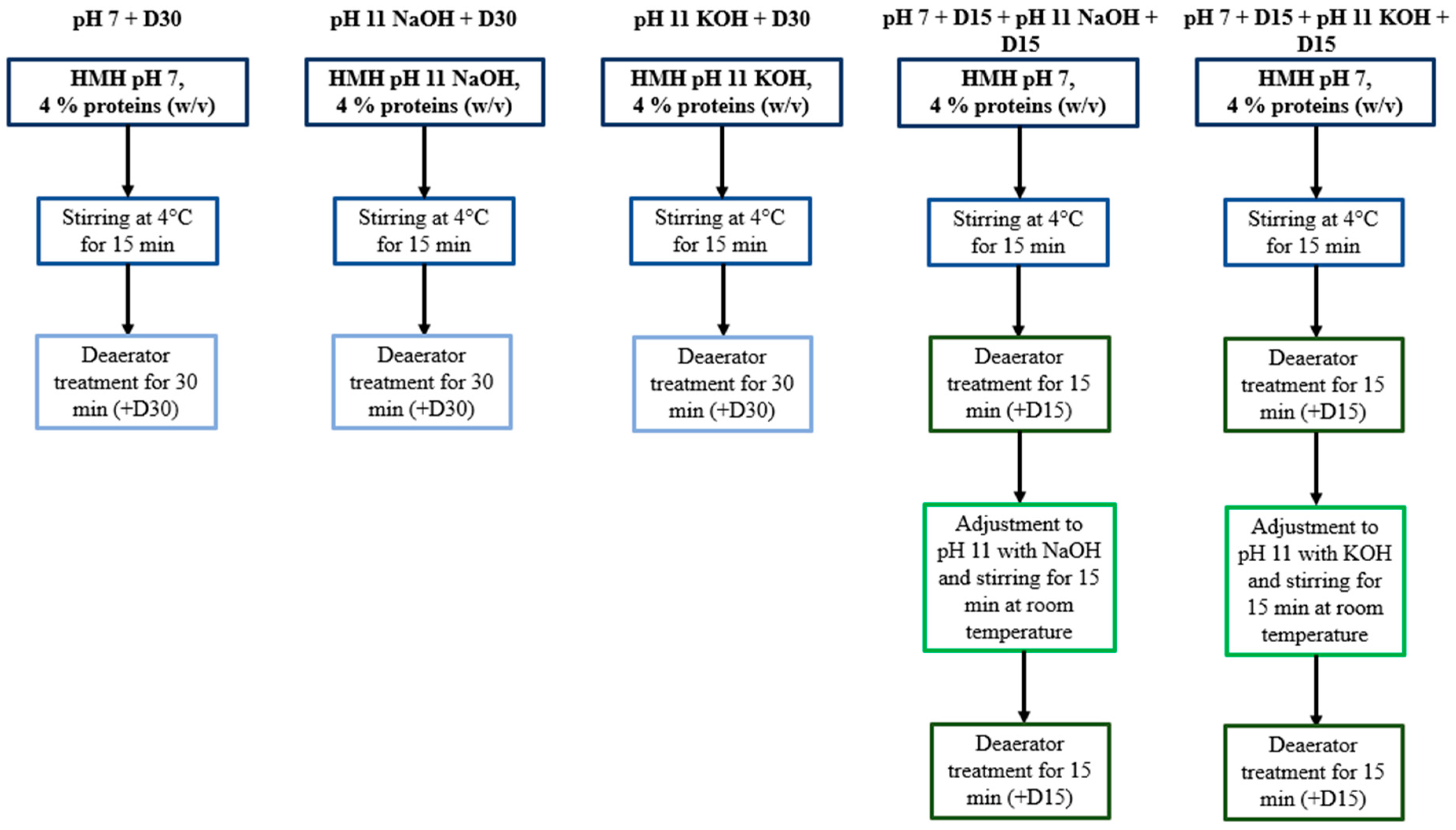

| 15 min Stirring | Single Deaerator Treatment | Combined Deaerator Treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 7 | pH 11 NaOH | pH 11 KOH | pH 7 + D30 | pH 11 NaOH + D30 | pH 11 KOH + D30 | pH 7 + D15 | pH 7 + D15 + pH 11 NaOH | pH 7 + D15 + pH 11 NaOH + D15 | pH 7 + D15 + pH 11 KOH | pH 7 + D15 + pH11 KOH + D15 | |
| DMA | 21.22 ± 1.95 ab | 16.69 ± 1.08 bc | 14.29 ± 0.66 cde | 23.32 ± 1.86 a | 11.41 ± 1.36 def | 9.18 ± 0.76 f | 23.44 ± 2.08 a | 15.13 ± 0.94 cd | 9.65 ± 0.34 ef | 12.28 ± 2.78 cdef | 7.80 ± 2.11 f |
| TMA | 6.54 ± 0.16 a | 7.50 ± 0.60 a | 7.38 ± 0.38 a | 6.61 ± 0.12 a | 3.74 ± 1.60 b | 2.38 ± 0.18 bc | 6.61 ± 0.40 a | 6.99 ± 0.25 a | 2.24 ± 0.51 bc | 6.19 ± 0.81 a | 1.86 ± 0.49 c |
| TMAO | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a | <2.50 a |
| 15 min Stirring | Single Deaerator Treatment | Combined Deaerator Treatments | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 7 | pH 11 NaOH | pH 11 KOH | pH 7 + D30 | pH 11 NaOH + D30 | pH 11 NaOH + D30 + pH 7 | pH 11 KOH + D30 | pH 11 KOH + D30 + pH 7 | pH 7 + D15 | pH 7 + D15 + pH 11 NaOH | pH 7 + D15 + pH 11 NaOH + D15 | pH 7 + D15 + pH 11 KOH | pH 7 + D15 + pH 11 KOH + D15 | |
| 3-Methylbutanal | 7.72 ± 0.53 a | 5.50 ± 0.08 bcd | 3.99 ± 0.61 de | 4.47 ± 0.30 cde | 4.88 ± 0.54 bcde | 5.71 ± 0.52 bc | 3.76 ± 0.22 e | 3.61 ± 0.42 e | 6.32 ± 0.65 ab | 3.56 ± 0.40 e | 3.50 ± 0.70 e | 4.62 ± 0.09 cde | 3.88 ± 0.88 e |
| 2-Methylbutanal | 4.07 ± 0.24 a | 3.33 ± 0.09 ab | 2.24 ± 0.44 bcde | 1.35 ± 0.19 de | 2.64 ± 0.45 bcd | 2.96 ± 0.74 abc | 1.93 ± 0.43 cde | 1.41 ± 0.32 de | 2.47 ± 0.89 bcde | 1.17 ± 0.37 e | 1.37 ± 0.33 de | 2.22 ± 0.35 bcde | 1.56 ± 0.37 de |
| 1-Methyl-1H-tetrazole | 4.66 ± 1.06 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 3.14 ± 2.73 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| 2,3-Pentanedione | 2.70 ± 0.31 a | 0.18 ± 0.07 ef | 0.13 ± 0.09 f | 1.18 ± 0.29 c | 0.071 ± 0.032 f | 1.00 ± 0.07 cd | 0.069 ± 0.017 f | 0.59 ± 0.03 de | 2.21 ± 0.33 b | 0.066 ± 0.047 f | 0.078 ± 0.025 f | 0.12 ± 0.02 f | 0.077 ± 0.013 f |
| Pentanal | 4.33 ± 0.47 a | 2.06 ± 0.15 cde | 1.33 ± 0.28 de | 2.31 ± 0.52 bcd | 1.40 ± 0.20 de | 2.71 ± 0.39 bc | 1.10 ± 0.07 e | 1.77 ± 0.43 cde | 3.28 ± 0.69 ab | 1.00 ± 0.27 e | 1.04 ± 0.27 e | 1.82 ± 0.09 cde | 1.30 ± 0.42 de |
| Hexanal | 5.66 ± 0.85 a | 5.24 ± 0.65 a | 2.79 ± 0.40 bcd | 5.32 ± 0.64 a | 4.07 ± 1.53 abc | 4.76 ± 0.33 ab | 3.50 ± 0.42 abcd | 3.63 ± 0.39 abcd | 4.70 ± 0.67 ab | 1.77 ± 0.29 d | 2.15 ± 0.92 cd | 5.30 ± 0.25 a | 4.67 ± 1.20 ab |
| (Z)-4-Heptenal | 8.80 ± 1.41 a | 1.87 ± 0.22 de | 1.99 ± 0.41 de | 2.57 ± 0.21 de | 1.91 ± 0.53 de | 5.29 ± 0.22 bc | 1.77 ± 0.08 de | 3.93 ± 0.83 cd | 6.60 ± 1.73 b | 1.69 ± 0.33 e | 1.57 ± 0.50 e | 1.83 ± 0.34 de | 1.55 ± 0.45 e |
| Heptanal | 0.83 ± 0.36 abc | 0.46 ± 0.05 bc | 0.41 ± 0.11 bc | 1.04 ± 0.20 ab | 0.32 ± 0.14 bc | 1.02 ± 0.15 ab | 0.34 ± 0.04 bc | 0.80 ± 0.25 bc | 1.62 ± 0.78 a | 0.18 ± 0.03 c | 0.18 ± 0.05 c | 0.41 ± 0.06 bc | 0.45 ± 0.13 bc |
| Methional | 0.053 ± 0.018 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.041 ± 0.005 b | 0.00 ± 0.00 c | 0.083 ± 0.017 a | 0.00 ± 0.00 c | 0.050 ± 0.009 b | 0.041 ± 0.007 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| Benzaldehyde | 8.03 ± 0.61 a | 2.27 ± 0.13 c | 2.15 ± 0.28 c | 2.14 ± 0.22 c | 2.24 ± 0.73 c | 7.71 ± 0.20 ab | 2.67 ± 0.21 c | 5.91 ± 0.56 b | 6.29 ± 1.93 ab | 1.73 ± 0.23 c | 1.65 ± 0.40 c | 2.32 ± 0.24 c | 1.91 ± 0.28 c |
| (Z)-6-Octen-2-one | 1.83 ± 0.62 a | 1.54 ± 0.19 ab | 1.28 ± 0.17 abc | 0.133 ± 0.008 d | 0.93 ± 0.46 abcd | 0.63 ± 0.40 bcd | 0.61 ± 0.08 bcd | 0.14 ± 0.06 d | 1.04 ± 0.56 abcd | 1.05 ± 0.14 abcd | 0.73 ± 0.19 bcd | 1.24 ± 0.30 abc | 0.58 ± 0.12 cd |
| (E,E)-2,4-Heptadienal | 0.68 ± 0.17 a | 0.016 ± 0.005 b | 0.044 ± 0.035 b | 0.56 ± 0.11 a | 0.041 ± 0.006 b | 0.46 ± 0.11 ab | 0.029 ± 0.017 b | 0.67 ± 0.57 a | 0.77 ± 0.03 a | 0.019 ± 0.004 b | 0.039 ± 0.002 b | 0.037 ± 0.028 b | 0.032 ± 0.003 b |
| Octanal | 1.80 ± 0.19 a | 0.60 ± 0.03 cd | 0.49 ± 0.11 d | 1.27 ± 0.27 b | 0.46 ± 0.22 d | 1.21 ± 0.08 b | 0.53 ± 0.04 cd | 0.94 ± 0.07 bc | 1.91 ± 0.22 a | 0.39 ± 0.05 d | 0.47 ± 0.15 d | 0.68 ± 0.03 cd | 0.51 ± 0.15 cd |
| 2-Nonanone | 1.27 ± 0.08 a | 1.05 ± 0.12 ab | 0.89 ± 0.17 abc | 0.12 ± 0.02 e | 0.54 ± 0.37 bcde | 0.53 ± 0.32 bcde | 0.255 ± 0.008 de | 0.13 ± 0.03 e | 0.83 ± 0.43 abcd | 0.40 ± 0.04 cde | 0.21 ± 0.07 e | 0.68 ± 0.18 bcde | 0.21 ± 0.05 e |
| (E,Z)-2,6-Nonadienal | 0.85 ± 0.09 a | 0.049 ± 0.006 d | 0.050 ± 0.012 d | 0.61 ± 0.09 b | 0.061 ± 0.037 d | 0.77 ± 0.10 ab | 0.054 ± 0.003 d | 0.42 ± 0.05 c | 0.91 ± 0.08 a | 0.055 ± 0.018 d | 0.053 ± 0.014 d | 0.061 ± 0.011 d | 0.065 ± 0.020 d |
| pH 7 after 15-min Stirring | pH 7 + D30 | pH 11 KOH after 15-min Stirring | pH 11 KOH + D30 | pH 7 + D15 + pH 11 KOH + D15 | |
|---|---|---|---|---|---|
| S scores | 88.0 a | 75.0 a | 51.0 b | 43.0 b | 43.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todeschini, S.; Perreault, V.; Goulet, C.; Bouchard, M.; Dubé, P.; Boutin, Y.; Bazinet, L. Impacts of pH and Base Substitution during Deaerator Treatments of Herring Milt Hydrolysate on the Odorous Content and the Antioxidant Activity. Foods 2022, 11, 1829. https://doi.org/10.3390/foods11131829
Todeschini S, Perreault V, Goulet C, Bouchard M, Dubé P, Boutin Y, Bazinet L. Impacts of pH and Base Substitution during Deaerator Treatments of Herring Milt Hydrolysate on the Odorous Content and the Antioxidant Activity. Foods. 2022; 11(13):1829. https://doi.org/10.3390/foods11131829
Chicago/Turabian StyleTodeschini, Sarah, Véronique Perreault, Charles Goulet, Mélanie Bouchard, Pascal Dubé, Yvan Boutin, and Laurent Bazinet. 2022. "Impacts of pH and Base Substitution during Deaerator Treatments of Herring Milt Hydrolysate on the Odorous Content and the Antioxidant Activity" Foods 11, no. 13: 1829. https://doi.org/10.3390/foods11131829
APA StyleTodeschini, S., Perreault, V., Goulet, C., Bouchard, M., Dubé, P., Boutin, Y., & Bazinet, L. (2022). Impacts of pH and Base Substitution during Deaerator Treatments of Herring Milt Hydrolysate on the Odorous Content and the Antioxidant Activity. Foods, 11(13), 1829. https://doi.org/10.3390/foods11131829







