The Effect of Polyphenol Extract from Rosa Roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Polyphenols’ Extraction from Rosa Roxbunghii Fruit
2.2. Nontargeted Metabolic Profiling Analysis of Rosa Roxburghii Fruit Polyphenols’ Extract
2.3. Animal Models and Experimental Design
2.4. Biochemical Parameters
2.5. Plasma Non-Targeted Metabolomics Analysis
2.6. Analysis of Gut Microbiota
2.7. Statistical Analysis
3. Results
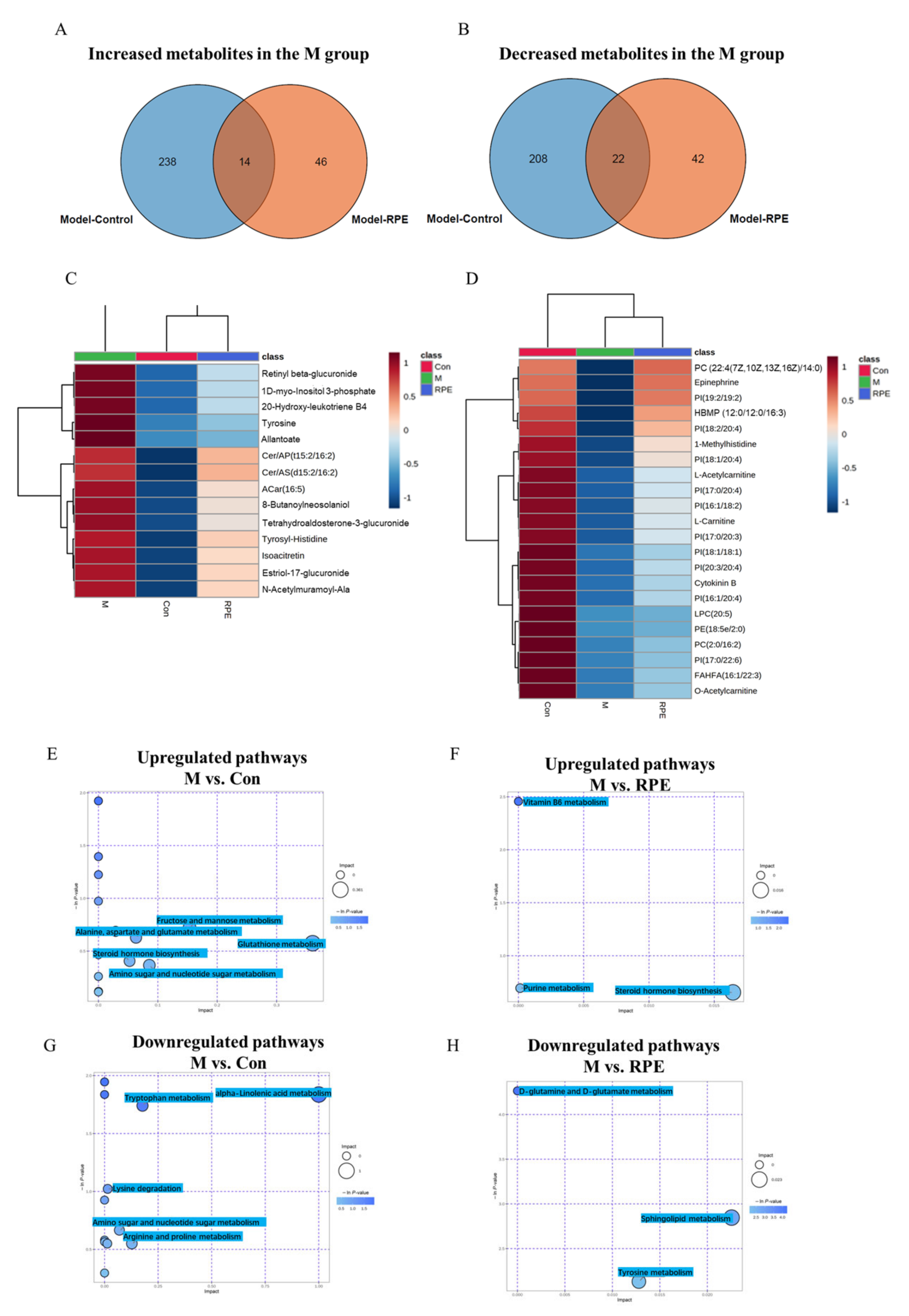
3.1. Compound Composition Analysis of RPE Based on Nontargeted Metabolic Profiling
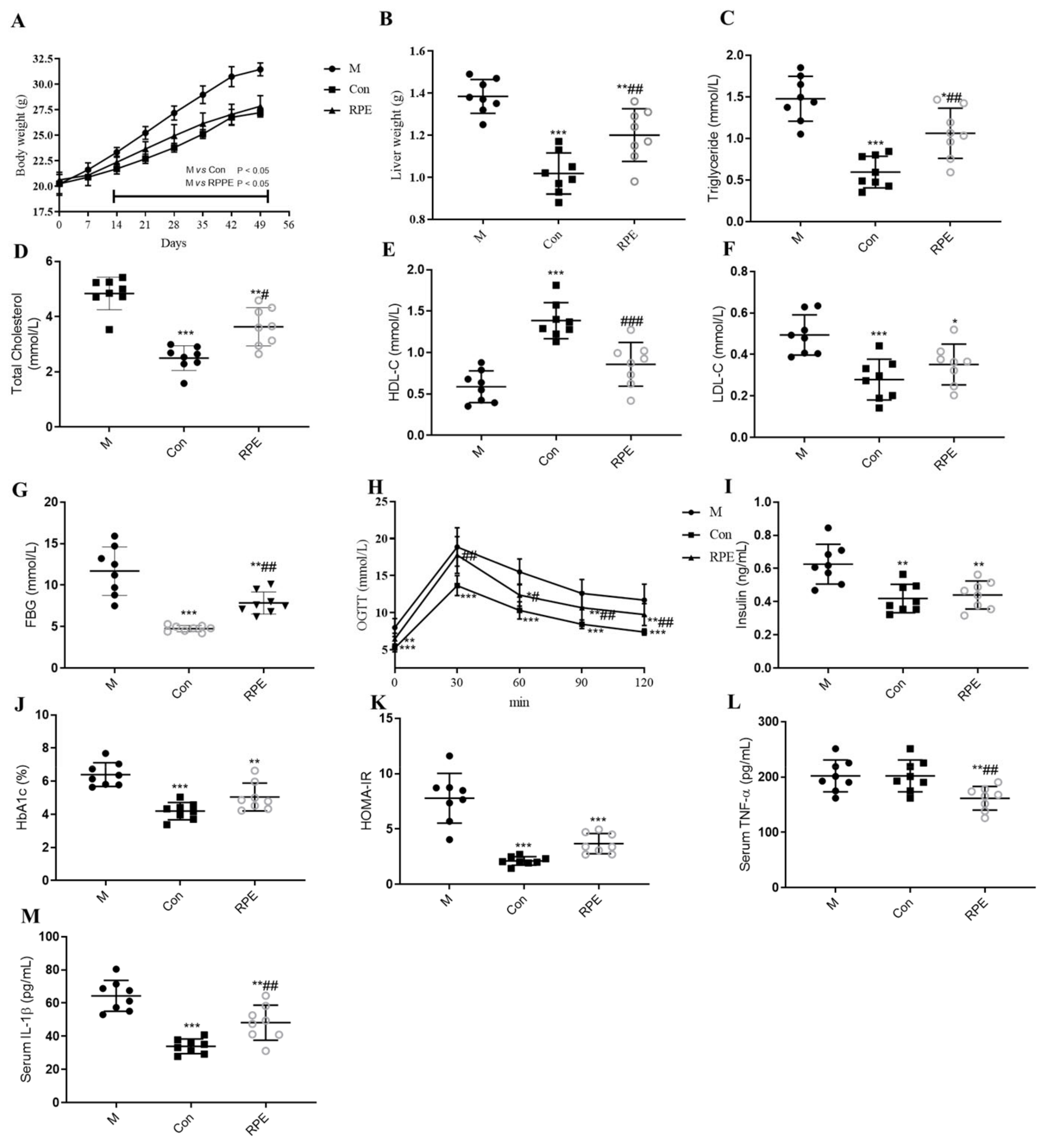
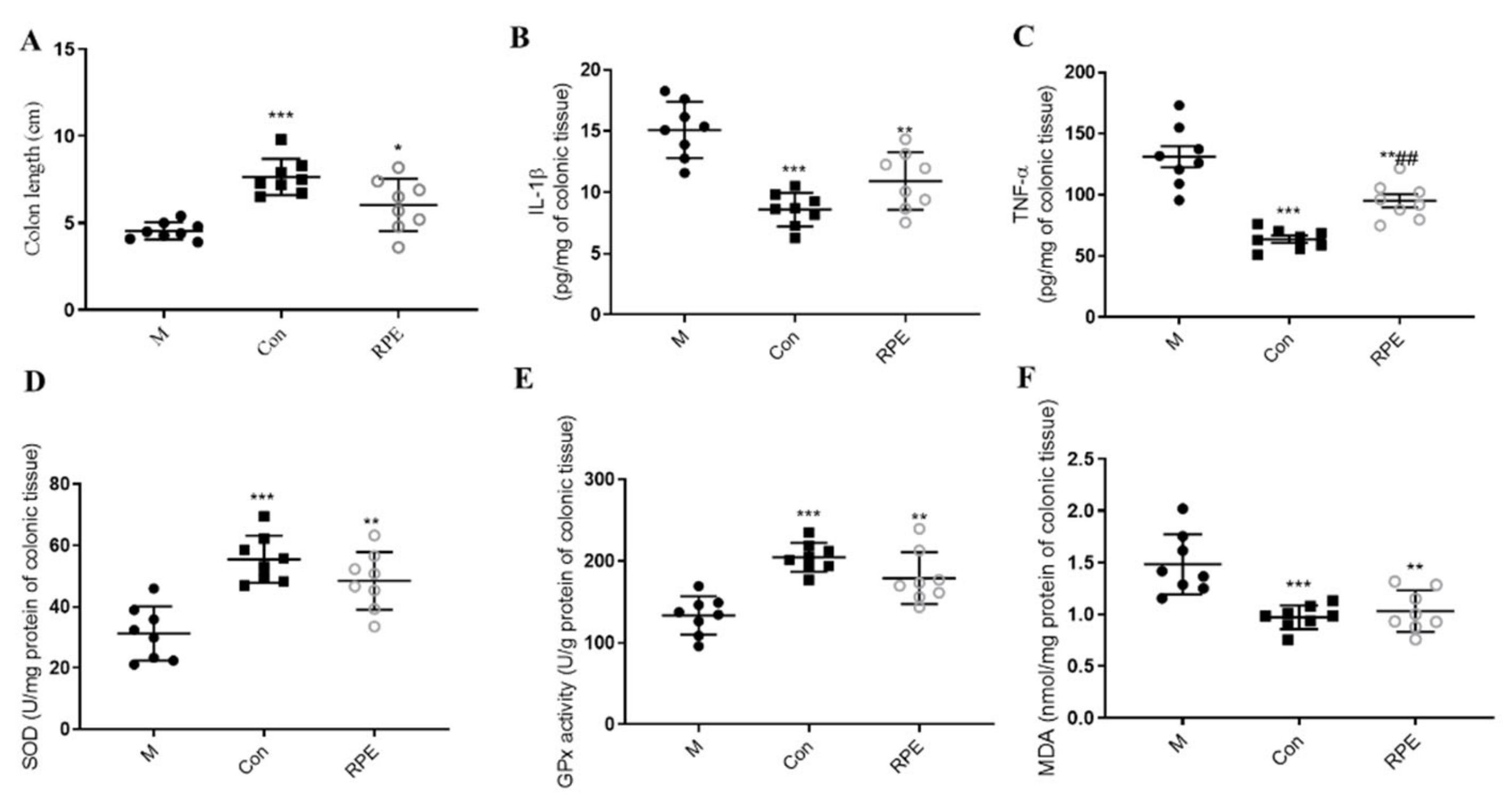
3.2. Effects of RPE on Lipid and Glucose Metabolism Parameters and Oxidative Stress Markers
3.3. Effects of RPE on the Plasma Metabolites
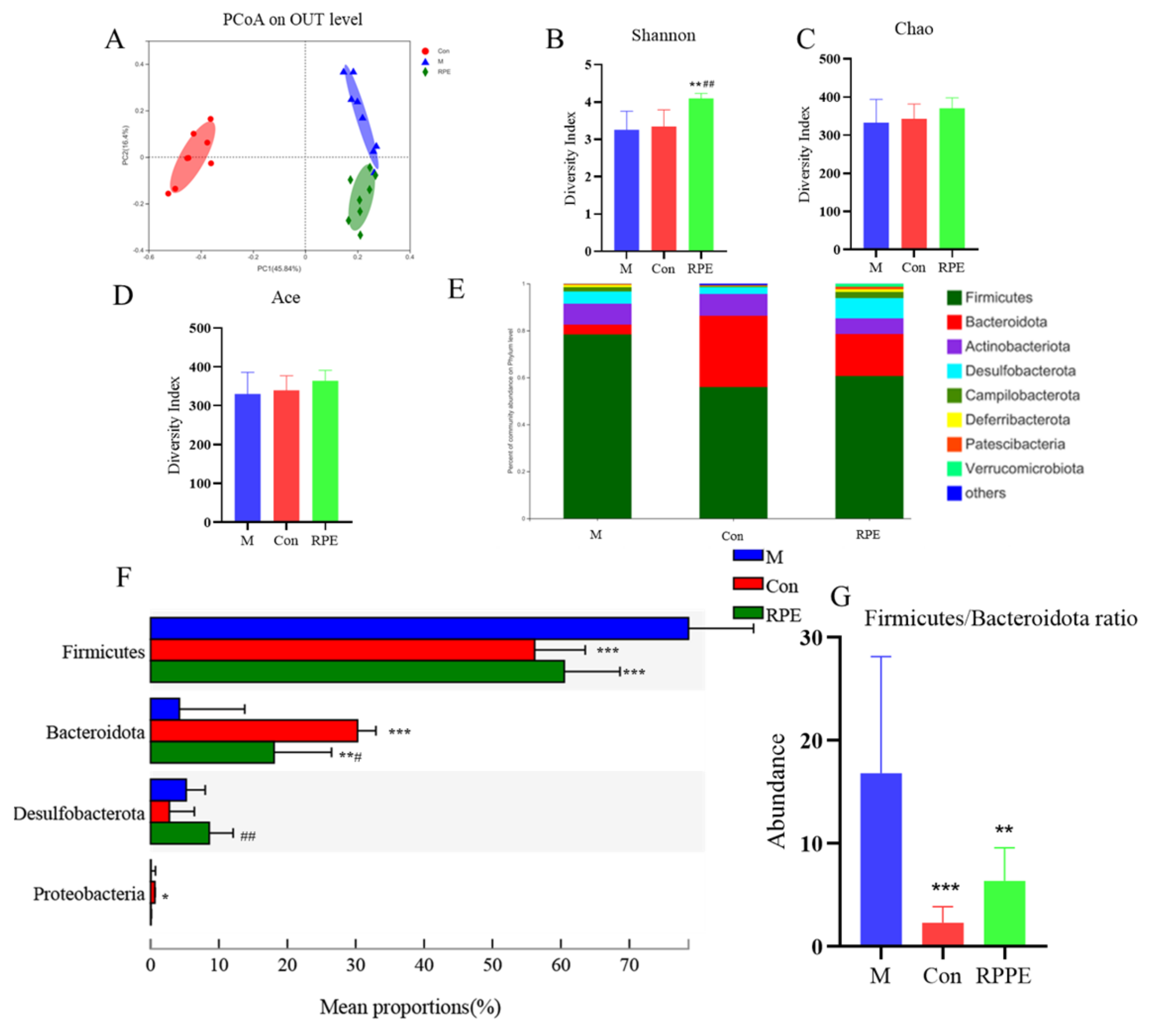
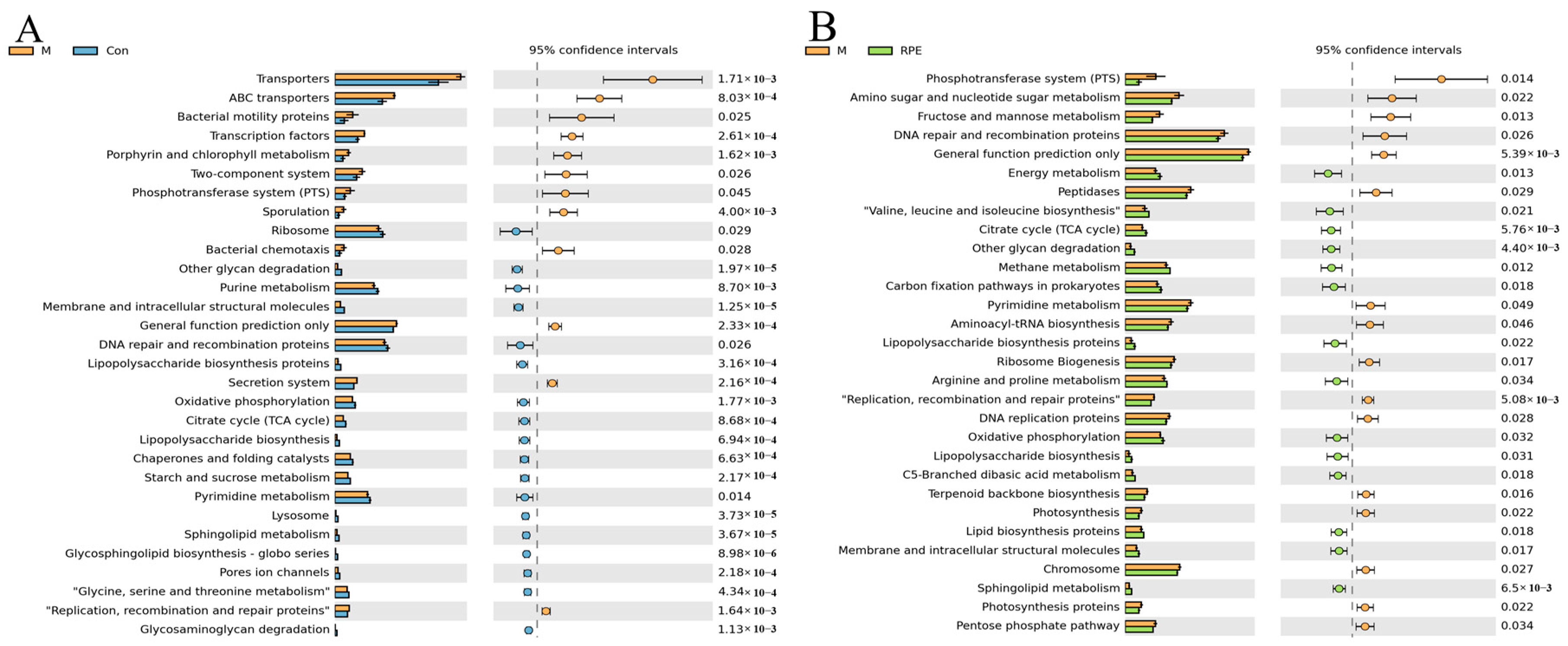
3.4. Effects of RPE on the Gut Microbiota Composition and Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burgos-Morón, E.; Abad-Jiménez, Z.; de Marañón, A.M.; Iannantuoni, F.; López, E.; López-Domènech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between Oxidative Stress, ER Stress, and Inflammation in Type 2 Diabetes: The Battle Continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, S.; Samocha-Bonet, D.; Heilbronn, L.; Campbell, L.V. Inflammatory and Oxidative Stress Responses to High-Carbohydrate and High-Fat Meals in Healthy Humans. J. Nutr. Metab. 2012, 2012, 238056. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Jia, Z.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Bahadoran, Z. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef]
- Wang, L.T.; Lv, M.J.; An, J.Y.; Fan, X.H.; Dong, M.Z.; Zhang, S.D.; Wang, J.D.; Wang, Y.Q.; Cai, Z.H.; Fu, Y.J. Botanical Characteristics, Phytochemistry and Related Biological Activities of: Rosa Roxburghii Tratt Fruit, and Its Potential Use in Functional Foods: A Review. Food Funct. 2021, 12, 1432–1451. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Selma, M.V.; Espiín, J.C.; Tomaás-Barberaán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Huang, D.; Li, C.; Chen, Q.; Xie, X.; Fu, X.; Chen, C.; Huang, Q.; Huang, Z.; Dong, H. Identification of polyphenols from Rosa roxburghii Tratt pomace and evaluation of in vitro and in vivo antioxidant activity. Food Chem. 2022, 377, 131922. [Google Scholar] [CrossRef]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 3, 2101019. [Google Scholar] [CrossRef]
- Huang, X.-L.; He, Y.; Ji, L.-L.; Wang, K.-Y.; Wang, Y.-L.; Chen, D.-F.; Geng, Y.; Ouyang, P.; Lai, W.-M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017, 8, 101545–101559. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Huang, Q.; Fu, X. Polysaccharide from Rosa roxburghii Tratt Fruit Attenuates Hyperglycemia and Hyperlipidemia and Regulates Colon Microbiota in Diabetic db/db Mice. J. Agric. Food Chem. 2019, 68, 147–159. [Google Scholar] [CrossRef]
- Fan, W.; Li, B.; Tian, H.; Li, X.; Ren, H.; Zhou, Q. Metabolome and transcriptome analysis predicts metabolism of violet-red color change in Lilium bulbs. J. Sci. Food Agric. 2021, 102, 2903–2915. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Liu, T.; Yang, N.; Li, Y.; He, J.; Qian, M.; Kuang, Z.; Zhang, W.; Ni, C.; et al. Prebiotics Regulation of Intestinal Microbiota Attenuates Cognitive Dysfunction Induced by Surgery Stimulation in APP/PS1 Mice. Aging Dis. 2020, 11, 1029–1045. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Liu, J.; Xu, D.; Chen, S.; Yuan, F.; Mao, L.; Gao, Y. Superfruits in China: Bioactive phytochemicals and their potential health benefits—A Review. Food Sci. Nutr. 2021, 9, 6892–6902. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Pariyani, R.; Kortesniemi, M.; Zhang, Y.; Yang, B. 1H NMR Metabolomics and Full-Length RNA-Seq Reveal Effects of Acylated and Nonacylated Anthocyanins on Hepatic Metabolites and Gene Expression in Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2021, 69, 4423–4437. [Google Scholar] [CrossRef]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.-F.; Zheng, G.-H.; Wang, A.-M.; Sun, C.-H.; Qin, S.-P.; Zhuang, J.; Lu, J.; Ma, D.-F.; Zheng, Y.-L. The Inhibitory Effects of Purple Sweet Potato Color on Hepatic Inflammation Is Associated with Restoration of NAD+ Levels and Attenuation of NLRP3 Inflammasome Activation in High-Fat-Diet-Treated Mice. Molecules 2017, 22, 1315. [Google Scholar] [CrossRef]
- Luo, Y.; Fang, J.-L.; Yuan, K.; Jin, S.-H.; Guo, Y. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods 2019, 59, 223–233. [Google Scholar] [CrossRef]
- Eltahawy, N.; El-Hady, A.A.; Badawi, M.; Hammad, A. Gamma Amino Butyric Acid Ameliorates Jejunal Oxidative Damage in Diabetic Rats. Indian J. Pharm. Educ. Res. 2017, 51, 588–596. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Faas, M. Specific Inulin-Type Fructan Fibers Protect against Autoimmune Diabetes by Modulating Gut Immunity, Barrier Function, and Microbiota Homeostasis. Mol. Nutr. Food Res. 2017, 357, 201–207. [Google Scholar] [CrossRef]
- Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and Antioxidative Potential of the Blue Congo Variety of Purple Potato Extract in Streptozotocin-Induced Diabetic Rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Guo, J.; Wu, G.; Dong, C.; Pang, Y.; Gao, S.; Wang, Y. Identification of biomarkers and mechanisms of diabetic cardiomyopathy using microarray data. Cardiol. J. 2020, 27, 807–816. [Google Scholar] [CrossRef]
- Karolczak, K.; Kubalczyk, P.; Głowacki, R.; Pietruszynski, R.; Watala, C. An inverse relationship between plasma glutathione concentration and fasting glycemia in patients with coronary artery disease and concomitant type 2 diabetes: A pilot study. Adv. Clin. Exp. Med. 2017, 26, 1359–1366. [Google Scholar] [CrossRef]
- Sumińska, M.; Podgórski, R.; Fichna, P.; Fichna, M. Steroid Metabolism in Children and Adolescents with Obesity and Insulin Resistance: Altered SRD5A and 20α/20βHSD Activity. Front. Endocrinol. 2021, 12, 1399. [Google Scholar] [CrossRef]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front. Endocrinol. 2020, 11, 569250. [Google Scholar] [CrossRef]
- Gorden, D.; Myers, D.S.; Ivanova, P.T.; Fahy, E.; Maurya, M.R.; Gupta, S.; Min, J.; Spann, N.J.; McDonald, J.G.; Kelly, S.L.; et al. Biomarkers of NAFLD progression: A lipidomics approach to an epidemic. J. Lipid Res. 2015, 56, 722–736. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, M.; Gordillo, R.; Koliaki, C.; Gancheva, S.; Jelenik, T.; De Filippo, E.; Herder, C.; Markgraf, D.; Jankowiak, F.; Esposito, I.; et al. Specific Hepatic Sphingolipids Relate to Insulin Resistance, Oxidative Stress, and Inflammation in Nonalcoholic Steatohepatitis. Diabetes Care 2018, 41, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, C.; Kirchberg, F.F.; Lass, N.; Harder, U.; Peissner, W.; Koletzko, B.; Reinehr, T. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J. Diabetes Res. 2016, 2016, 2108909. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Korner, A. Effect of glucagon, insulin, and thyroxine on tyrosine transaminase and tryptophan pyrrolase of rat liver. Arch. Biochem. Biophys. 1969, 129, 75–78. [Google Scholar] [CrossRef]
- Yu, Z.; Ning, Y.; Yu, H.; Tang, N. A HPLC-Q-TOF-MS-based urinary metabolomic approach to identification of potential biomarkers of metabolic syndrome. J. Huazhong Univ. Sci. Technol. 2014, 34, 276–283. [Google Scholar] [CrossRef]
- Catania, V.A.; Carrillo, M.C. Intestinal phase II detoxification systems: Effect of low-protein diet in weanling rats. Toxicol. Lett. 1990, 54, 263–270. [Google Scholar] [CrossRef]
- Mingrone, G.; Greco, A.V.; Capristo, E.; Benedetti, G.; Giancaterini, A.; De Gaetano, A.; Gasbarrini, G. L-Carnitine Improves Glucose Disposal in Type 2 Diabetic Patients. J. Am. Coll. Nutr. 1999, 18, 77–82. [Google Scholar] [CrossRef]
- Morino, K.; Petersen, K.F.; Shulman, G.I. Molecular Mechanisms of Insulin Resistance in Humans and Their Potential Links with Mitochondrial Dysfunction. Diabetes 2006, 55, S9–S15. [Google Scholar] [CrossRef]
- Shamsuddin, A.K.M.; Yang, G.Y. Inositol & Its Phosphates: Basic Science to Practical Applications; Bentham Science Publishers: Sharjah, United Arab Emirates, 2015. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Dash, N.R.; Al Bataineh, M.T.; Tahseen, M.; Bataineh, A. Metagenomic Analysis of the Gut Microbiome Reveals Enrichment of Menaquinones (Vitamin K2) Pathway in Diabetes Mellitus. Diabetes Metab. J. 2020, 45, 77–85. [Google Scholar] [CrossRef]
- Gotoh, A.; Nara, M.; Sugiyama, Y.; Sakanaka, M.; Yachi, H.; Kitakata, A.; Nakagawa, A.; Minami, H.; Okuda, S.; Katoh, T.; et al. Use of Gifu Anaerobic Medium for culturing 32 dominant species of human gut microbes and its evaluation based on short-chain fatty acids fermentation profiles. Biosci. Biotechnol. Biochem. 2017, 81, 2009–2017. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Kortesniemi, M.; Pariyani, R.; Zhang, Y.; Yang, B. Effects of acylated and nonacylated anthocyanins extracts on gut metabolites and microbiota in diabetic Zucker rats: A metabolomic and metagenomic study. Food Res. Int. 2022, 153, 110978. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.I.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Zhang, H.-H.; Liu, J.; Lv, Y.-J.; Jiang, Y.-L.; Pan, J.-X.; Zhu, Y.-J.; Huang, M.-G.; Zhang, S.-K. Changes in Intestinal Microbiota of Type 2 Diabetes in Mice in Response to Dietary Supplementation with Instant Tea or Matcha. Can. J. Diabetes 2019, 44, 44–52. [Google Scholar] [CrossRef]
- Remely, M.; Aumueller, E.; Jahn, D.; Hippe, B.; Brath, H.; Haslberger, A. Microbiota and epigenetic regulation of inflammatory mediators in type 2 diabetes and obesity. Benef. Microbes 2014, 5, 33–43. [Google Scholar] [CrossRef]
- Moya-Pérez, A.; Neef, A.; Sanz, Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PLoS ONE 2015, 10, e0126976. [Google Scholar] [CrossRef]
- Hou, Y.-P.; He, Q.-Q.; Ouyang, H.-M.; Peng, H.-S.; Wang, Q.; Li, J.; Lv, X.-F.; Zheng, Y.-N.; Li, S.-C.; Liu, H.-L.; et al. Human Gut Microbiota Associated with Obesity in Chinese Children and Adolescents. BioMed Res. Int. 2017, 2017, 7585958. [Google Scholar] [CrossRef]
- Mrozinska, S.; Kapusta, P.; Gosiewski, T.; Sroka-Oleksiak, A.; Ludwig-Słomczyńska, A.H.; Matejko, B.; Kiec-Wilk, B.; Bulanda, M.; Malecki, M.T.; Wolkow, P.P.; et al. The Gut Microbiota Profile According to Glycemic Control in Type 1 Diabetes Patients Treated with Personal Insulin Pumps. Microorganisms 2021, 9, 155. [Google Scholar] [CrossRef]
- Deutscher, J.; Francke, C.; Postma, P.W. How Phosphotransferase System-Related Protein Phosphorylation Regulates Carbohydrate Metabolism in Bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Chen, Z.; Wang, M.; Long, M.; Ren, T.; Chen, C.; Dai, X.; Yang, S.; Tan, S. The Effect of Polyphenol Extract from Rosa Roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice. Foods 2022, 11, 1747. https://doi.org/10.3390/foods11121747
Wang H, Chen Z, Wang M, Long M, Ren T, Chen C, Dai X, Yang S, Tan S. The Effect of Polyphenol Extract from Rosa Roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice. Foods. 2022; 11(12):1747. https://doi.org/10.3390/foods11121747
Chicago/Turabian StyleWang, Hui, Zhaojun Chen, Mei Wang, Mingxiu Long, Tingyuan Ren, Chao Chen, Xiaotong Dai, Sheng Yang, and Shuming Tan. 2022. "The Effect of Polyphenol Extract from Rosa Roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice" Foods 11, no. 12: 1747. https://doi.org/10.3390/foods11121747
APA StyleWang, H., Chen, Z., Wang, M., Long, M., Ren, T., Chen, C., Dai, X., Yang, S., & Tan, S. (2022). The Effect of Polyphenol Extract from Rosa Roxburghii Fruit on Plasma Metabolome and Gut Microbiota in Type 2 Diabetic Mice. Foods, 11(12), 1747. https://doi.org/10.3390/foods11121747






