Abstract
Lactobacillus acidophilus NCFM is widely used in the fermentation industry; using it as a freeze-dried powder can greatly reduce the costs associated with packaging and transportation, and even prolong the storage period. Previously published research has reported that the expression of galU (EC: 2.7.7.9) is significantly increased as a result of freezing and drying. Herein, we aimed to explore how galU plays an important role in improving the resistance of Lactobacillus acidophilus NCFM to freeze-drying. For this study, galU was first knocked out and then re-expressed in L. acidophilus NCFM to functionally characterize its role in the pertinent metabolic pathways. The knockout strain ΔgalU showed lactose/galactose deficiency and displayed irregular cell morphology, shortened cell length, thin and rough capsules, and abnormal cell division, and the progeny could not be separated. In the re-expression strain pgalU, these inhibited pathways were restored; moreover, the pgalU cells showed a strengthened cell wall and capsule, which enhanced their resistance to adverse environments. The pgalU cells showed GalU activity that was 229% higher than that shown by the wild-type strain, and the freeze-drying survival rate was 84%, this being 4.7 times higher than that of the wild-type strain. To summarize, expression of the galU gene can significantly enhance gene expression in galactose metabolic pathway and make the strain form a stronger cell wall and cell capsule and enhance the resistance of the bacteria to an adverse external environment, to improve the freeze-drying survival rate of L. acidophilus NCFM.
1. Introduction
Lactobacillus acidophilus NCFM is one of the most widely used probiotic species in the fermentation industry [1,2]. It has been reported to improve intestinal flora composition [3], regulate metabolism levels [4], enhance immune function, and also prevent cancer [5]. Using the freeze-drying method to obtain L. acidophilus powder can greatly reduce the costs associated with packaging and transportation, and even prolong the storage period [6]. However, upon exposure to stress in the form of freezing and drying, the survival rate of L. acidophilus is adversely impacted, which is not conducive to the industrial production of L. acidophilus powder [7]. To improve the survival rate of freeze-dried strains, methods such as improving culture conditions [8], adding sugars to protective solutions [9], and optimizing the freeze-drying parameters [10] have been employed, but the survival rate of strains continues to remain low in mass-production environments.
Upon freezing and drying L. acidophilus NCMF, the mRNA transcription of UTP-glucose-1-phosphate uridylyltransferase (GalU, EC: 2.7.7.9, encoded by galU) has been reported to significantly increase [11]. Furthermore, it has been speculated that the survival rate of freeze-dried L. acidophilus NCFM can be improved via galU (903 nt, Gene ID: 3253049). Therefore, in this study, we tried to knock out the galU gene and then re-express it to establish whether its lactose metabolism was affected. The effect of the galU gene on L. acidophilus NCFM in freeze-drying was evaluated by observing the morphological changes, growth curves, and freeze-dried survival rates after knockout and re-expression.
The galU gene plays a key role in glycogen synthesis in animals [12] and regulates the conversion process between starch and polysaccharides in plants [13]. In Streptococcus pneumoniae, galU directly affects growth, adhesion, in vitro phagocytosis, and in vivo pathogenicity [14]. Moreover, in uropathogenic Escherichia coli, the mutation of galU has been observed to result in the loss of the O-polysaccharide sidechain of lipopolysaccharides, consequently affecting the post-translational modification of proteins [15]. However, to date, only a few studies have explored how galU improves the resistance of L. acidophilus NCFM to freeze-drying. Therefore, in this study, transcriptomes are used to further analyze the differences among L. acidophilus NCFM and its knockout and re-expression offspring.
2. Materials and Methods
2.1. Strains and Growth Conditions
The bacterial strains and plasmids are listed in Table 1. The LA strain was statically cultured in Man–Rogosa–Sharpe (MRS) medium at 37 °C, with 2% inoculation [16]. For knockout plasmid preparation, E. coli strain DH10BT1 carrying pK18mobsacB was cultured in 50 mL Luria–Bertani (LB) medium containing 50 µg/mL kanamycin, followed by incubation at 37 °C in a rotary shaker (150 rpm) for 18 h [17]. MRS medium, containing 5 µg/mL ampicillin, was used for screening positive clones harboring low-copy recombinant knockout vectors. SAMRS (MRS medium with 10% sucrose) medium was used for the negative screening of galU-deleted strains [18]. M17 medium, with lactose as the sole source of carbon, was used for identifying and screening the lactose-deficient strains [19]. GM17 medium (M17 medium with 5% glucose) was used to extract pNZ8149 and culture the lactose-deficient strains [20]. Then, 0.04% bromocresol violet was added to the M17 medium (BM17 medium), which served as an indicator (colonies appeared yellow) when lactose was fermented by Lactobacillus to produce acid [21].

Table 1.
The bacterial strains and plasmids used in this study.
2.2. Knockout of galU
The upstream and downstream homologous arms of galU and the gene responsible for ampicillin resistance (amp) in the pUC57 plasmid were linked using a CV19 One-Step Seamless Cloning kit (Aidlab Biotechnologies Co., Ltd., Beijing, China) to construct Knock, a target segment for galU knockout. The upstream and downstream homologous arms of galU were amplified using galU-1-F/R and galU-2-F/R primers, and amp was amplified using amp-F/R primers, with pUC57 serving as the template. The primer sequences are listed in Table 2.

Table 2.
The primers used in this study.
After double-digestion with the restriction endonucleases BamHI and PstI, the linear Knock fragment and the pK18mobsacB vector were ligated (2:1 ratio) using the T4 DNA ligase, followed by incubation at 37 °C overnight [22]. The product was transferred into E. coli Trans-T1 cells and positive clones were verified by performing PCR with galU-1-F and galU-2-R primers. The DNA sequence of positive clones with a 99.9% matching rate was named Knock-pK18mobsacB (i.e., the recombinant knockout vector).
Subsequently, Knock-pK18mobsacB was electro-transformed into competent L. acidophilus cells (1.2 kV, 25 μF, 200 Ω, 5.1 ms pulses) using a gene pulser transfection apparatus (Xinyi-2E, Ningbo Xinyi Co., Ltd., Ningbo, China). After recovery for 3 h in MRS broth, the bacterial solution was evenly spread onto an MRS medium plate containing 5 mg/mL ampicillin [23]. After incubation for 3 days, colonies were selected for expanded culture, and PCR was performed with galU-1-F and galU-2-R primers for validation. Positive bacterial cells harboring the target segment were spread onto a SAMRS-medium plate and allowed to grow for 3 days; colonies were then selected for validation via PCR. Positive strains with a matching rate of > 99.9% by sequencing were named ΔgalU (i.e., the galU knockout strain). Using the wild-type strain, LA, three pairs of primers for galU (galU-4/5/6-F/R) were designated to confirm that galU was knocked out.
In order to verify whether the lactose metabolic pathway of ΔgalU was knocked out, the LA and ΔgalU strains were adjusted to OD600 1.0 and then diluted 106 times with sterile physiological saline; we then drew an S-shaped curve on a lactose plate to observe whether growth could be seen after culturing at 37 °C for 36 h.
2.3. Expression of galU in ΔgalU
The galU (903nt) gene was amplified using galU-7-F/R primers and LA-strain DNA as the template, and pNZ8149 from L. lactis was digested by incubation with NcoI and XbaI at 37 °C overnight [24]. The DNA was denatured at 94 °C for 2 min, annealed at 60 °C, and then extended at 72 °C for 1 min in 30 cycles for Polymerase Chain Reaction (PCR) amplification. The purified products were linked using a CV19-One Step Seamless Cloning kit (Aidlab Biotechnologies Co.,Ltd., Beijing of Chian) to obtain the recombinant expression plasmid pNZ8149-galU, which was transfected into competent ∆galU cells via electroporation [25]. After incubation in MRS broth for 3 h, positive clones were screened on BM17 medium plates and identified via PCR with galU-8-F/R primers. The ΔgalU strain harboring pNZ8149-galU with a 99% matching rate by sequencing was named pgalU (i.e., the galU re-expression strain). LA, ΔgalU, and pgalU strains were placed on the S line of a BM17 medium plate, and colony morphology was observed after incubation at 37 °C for 36 h. The three strains, pgalU, LA, and ΔgalU, were expanded in MRS broth for 18 h and then collected; the sediment was then resuspended with 2 mL of sterile saline, 100 µL of lactose (purple) and galactose (green) was added to the fermentation tube, and incubation at 37 °C for more than 18 h was used to observe the color change. If the strain could ferment lactose or galactose to produce acid, the solution turned yellow.
2.4. Determination of GalU Activity
Growth curves were constructed for the LA, ΔgalU, and pgalU strains grown in an MRS medium with 1% of inoculation; we measured the OD600 value every 2 h and plotted the measured OD600 value and corresponding culture time, then collected the stable stage of the strain according to the growth curve, which was followed by centrifugation of 50 mL bacterial cell suspension at 5000× g. The cells were then washed with 0.1 M phosphate-buffered saline, resuspended, and lysed using an ultrasonic cell disrupter (Scientz-IID, Scientz Biotechnology Co., Ltd., Ningbo, China). Cell lysis was performed at 300 W, with 100 s pulses and 3 s pauses, on an ice bath to prevent protein denaturation [26]. Subsequently, a 2 mL sample of lysed cells was centrifuged at 12,000× g for 10 min at 4 °C. The supernatant was transferred to a new centrifuge tube, and the remaining precipitate was dissolved in 2 mL denaturant buffer (8 M urea, 100 mM NaH2PO4, 10 mM Tris-HCl, pH 8.0). The total protein content was determined with a bicinchoninic acid kit for protein determination (Sigma-Aldrich, Shanghai, China). According to the determined results, the total protein concentration of each copy was adjusted to 0.1 mg/mL [27], and the enzyme activity of GalU (34.46 kDa) was detected with an ELISA Kit (Shanghai Keshun Biotechnology Co., Ltd., Shanghai, China) according to the instructions: we added 0.05 mL of sample to reaction wells that had been coated with GalU antibodies, incubated them at 37 °C for 1 h and then washed them, establishing the blank and standard curves at the same time. Each well was washed after adding 0.05 mL of microplate antibody and then incubated at 37 °C for 1 h. We then added 0.1 mL of TMB substrate solution and incubated the wells at 37 °C for 30 min; finally, we added 0 05 mL of 2 M sulfuric acid to terminate the reaction. Immediately afterward, we determined the absorbance value at 450 nm with a microplate reader and calculated the GalU activity, according to the standard curve.
2.5. Effect of Freeze-Drying on Bacterial Survival Rate
Growth curves were used to determine the effects of freeze-drying on bacterial survival rate. The strains were cultured to the end of the stationary phase (OD600 of around 1.2), followed by centrifugation of 50 mL bacterial cell suspension at 5000× g for 10 min, the precipitates were collected and frozen overnight at −80 °C, and then dried in an Alpha 1-4 LD Plus freeze-dryer (Christ Goema, Germany) for 24 h at −49 °C and 9 Pa. After the cells were freeze-dried for 24 h, they were rehydrated immediately after being taken out of the freeze dryer (at room temperature) without storage. At the same time, pre-frozen and freeze-dried samples were placed in 50 mL sterile tubes. We then took 1 mL of bacterial solution before and after lyophilization (adding 50 mL sterile saline for re-dissolution), diluted it by 103, 104, 105, 106, 107, and 108 times, and coated the plates, with three parallels in each group. Plate colony-counting was performed after 3 days of incubation, and the freeze-drying survival rate was calculated as the number of live bacteria after lyophilization, divided by the number of live bacteria before lyophilization × 100% [28].
2.6. Transmission Electron Microscopy (TEM) to Assess the Cell Structure
To obtain the bacterial cells, the LA, ΔgalU, and pgalU strains were centrifuged at 3000× g for 10 min at 4 °C. After washing twice with 0.1 M phosphate-buffered saline, the cells were fixed in 2.5% glutaraldehyde solution for > 12 h at 4 °C. The samples were immersed in 0.1 M phosphate-buffered saline thrice for 15 min each time, and then fixed in 1% osmium acid, followed by incubation for 1–2 h in a dark room [29]. After three times washes with 30%, 50%, 70%, and 90% alcohol for 15 min respectively, the samples were treated three times with 90% acetone and anhydrous acetone for 15 min each time. After overnight incubation with an embedding agent, fresh embedding agent was added, and polymerization was allowed to proceed at 37 °C for 12 h, then the samples were dried at 60 °C for 36 h. Subsequently, the samples were sliced into 50–60 nm slices using an LKB-1 ultrathin slicer. The cells were observed using an H-800 transmission electron microscope (Hitachi, Tokyo, Japan) after double-staining with 3% uranium acetate.
2.7. Transcriptome Sequencing
LA, ΔgalU, and pgalU strains were cultured in an MRS medium to an OD600 of around 1.2, and total RNA was then extracted using a kit (Qubit 4.0). After rRNA removal, oligo-(dT) magnetic beads were added for mRNA enrichment, and short mRNA fragments were obtained. After synthesizing, modifying, purifying, and segmenting the fragments, they were sequenced on an Illumina HiSeq 2000. The NGS QC software was used to filter and count the reads, in order to identify the different genes and analyze the metabolic pathways [30].
3. Results
3.1. Acquisition of the galU Knockout Strain ΔgalU
The 2180 bp Knock target segment was synthesized from the 662 bp upstream and 592 bp downstream homologous arms of galU and the 974 bp amp gene, followed by ligation in pK18mobsacB to obtain Knock-pK18mobsacB (Figure 1A); BamHI–PstI double-digestion was then performed for validation (Figure 1B). After introducing Knock-pk18mobsacb into the LA strain, the knockout strain ΔgalU was obtained, as verified through sequencing. In the case of the wild-type strain LA, PCR using three pairs of galU primers (galU-4/5/6-F/R) generated the corresponding bands, but no amplicons were observed in the case of ΔgalU (Figure 1C), indicating that the galU in ΔgalU had been successfully knocked out. As shown in Figure 1D, the LA strain could grow on an M17 agar plate, but ΔgalU could not grow on an M17 agar plate, signifying that ΔgalU was unable to decompose lactose into glucose so as to maintain growth.
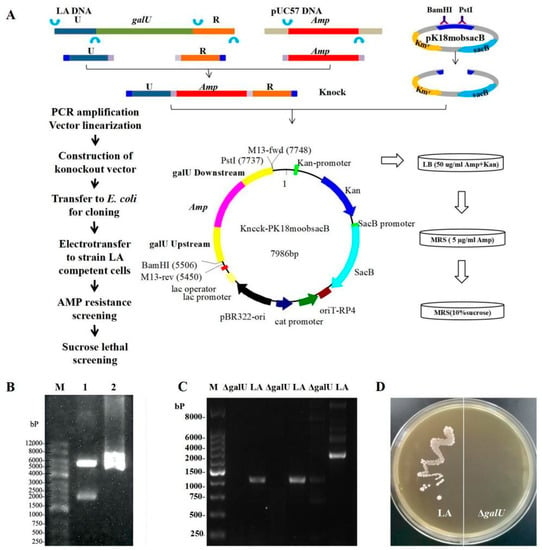
Figure 1.
Schematics and results for the knockout of the galU gene. (A) Schematics for the knockout of the galU gene. (B) BamHI-PstI double-digestion map. Lane M, Marker 12,000 bp; Lane 1, fragments of the digested plasmid, Lane2, recombinant plasmid Knock-pK18mobsacB. (C) PCR amplification of the knockout strain ΔgalU and original LA strain. Lane M, Marker 8000 bp; ΔgalU, PCR amplification of the knockout strain ΔgalU; LA, PCR amplification of the original LA strain. (D) The growth of the original strain LA and knockout strain ΔgalU on an M17 plate, with lactose as the sole glycogen. The left side of the plate is the original strain LA, which can form normal colonies. The right side of the plate is the knockout strain, ΔgalU, without colony formation.
3.2. Acquisition of the galU Re-Expression Strain pgalU
Considering the fact that the knockout strain ΔgalU showed lactose deficiency, we concluded that the food-grade expression vector pNZ8149 could be used for galU expression. Next, pNZ8149 and galU (obtained by PCR amplification of DNA obtained from the LA strain, Figure 2B) were recombined to obtain the food-grade expression plasmid, pNZ8149-galU (verified by NcoI–XbaI double digestion, Figure 2C), which was then introduced into ΔgalU, and the positive clones were screened on BM17 agar. As is evident from Figure 2A, ΔgalU showed growth on BM17 agar only upon the successful integration of pNZ8149-galU. There was no colony of ΔgalU found on the BM17 plate, while the LA and pgalU strains were similar (Figure 2D, left). The lactose fermentation tubes (purple) of the pgalU, LA, and ΔgalU strains have not changed color, indicating that none of the three strains can directly use lactose fermentation to produce acid. The galactose fermentation tubes of the pgalU and LA strains have become yellow, while the ΔgalU strain has not (Figure 2D, right), indicating that the galactose fermentation pathway of the ΔgalU strain has been blocked and has been fixed in the pgalU strains.
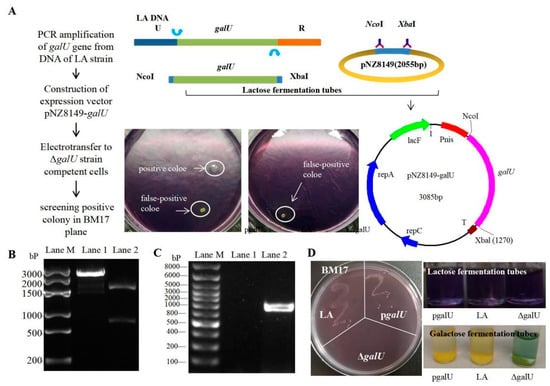
Figure 2.
Schematics and results of preparation of the pgalU complementation strain. (A) Schematics for preparation of the overexpression strain, pgalU. (B)Electrophoresis of recombinant expression plasmid pNZ8149-galU. Lane M, Marker 3000 bp; Lane 1, fragments of the digested plasmid; Lane 2, plasmid pNZ8149-galU digested by NcoI-XbaI restriction endonucleases. (C) PCR validation of a positive pgalU strain, screened on a BM17 plate. Lane M, Marker 8000 bp; Lane 1, a pseudo-positive clone; Lane 2, positive pgalU strain. (D) BM17 plate colony (left) and the lactose/galactose fermentation (right) of the LA, ΔgalU, and pgalU strains.
3.3. GalU Activity of the LA, ΔgalU, and pgalU Strains
The three strains entered the logarithmic phase of growth from around 4 h onward and the stable phase at 8 h; the maximum OD600 value stabilized at 1.45–1.50, then gradually declined after 20 h (Figure 3A). According to the standard curve of GalU activity determination, the GalU content in the LA, ΔgalU, and pgalU strains was evaluated (Figure 3B). The knockout strain ΔgalU showed almost no GalU activity, while the re-expression strain pgalU showed GalU activity that was 229% higher than that of the wild-type strain, with an increased amount of precipitate.
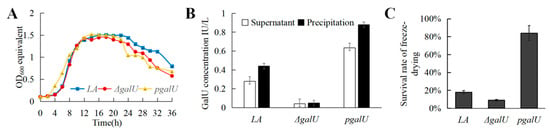
Figure 3.
Growth curves, GalU enzyme activity and freeze-drying survival rate for strains LA, ΔgalU and pgalU. (A) Growth curves of strains LA, ΔgalU and pgalU. (B) GalU enzyme activity in strains LA, ΔgalU, and pgalU. (C) The freeze-drying survival rate for strains LA, ΔgalU, and pgalU.
3.4. Effect of galU on Freeze-Drying Survival Rate
In the freeze-drying experiment, the survival rate of ΔgalU was only 9%, while that of pgalU was 84%, which was 4.7 times that of LA (17.9%; Figure 3C). These results indicated that galU expression substantially contributes to increasing the survival rate of freeze-dried strains; this may be related to the strengthening of the cell wall and capsule.
3.5. TEM of LA, ΔgalU, and pgalU Strains
TEM revealed that the wild-type LA cells (Figure 4A–C) were short, rod-shaped, and 1 μm long. A dense capsule was present around them, conferring higher resistance to adverse environments. In contrast, ΔgalU cells showed obvious changes in their cell structure (Figure 4D–F); the cells were irregular and the capsule was thin and rough. Although ΔgalU cells could continue to replicate and divide, the progeny could not be separated and only shared the original cell shell. In the TEM experiment, the first-generation knockout strain ΔgalU that had just been selected was used. The growth of the first-generation ΔgalU monoclonal strain was very slow and the survival rate was very low. According to the uniform treatment of the strains in the pre-TEM stage, the monoclonal strains were picked out and incubated for the same time, then centrifuged. After the cells were collected, they were fixed with formaldehyde. It can be observed that the precipitation of the knockout bacteria was significantly less than that of the wild-type strains. After many iterations, the growth status of ΔgalU gradually became consistent with that of the wild type. Furthermore, the re-expression strain pgalU showed normal morphology and cell division; the pgalU cells showed significant growth and the capsule appeared thick (Figure 4G–I).
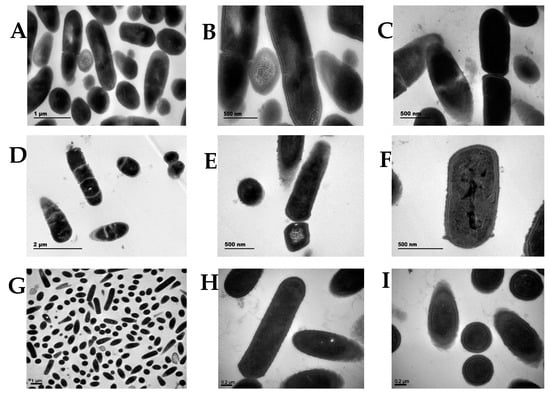
Figure 4.
Colony morphology and microscopic morphology of the LA, ΔgalU, and pgalU strains. (A–C) Transmission electron microscopy of strain LA. (D–F) The galU gene knockout strain, ΔgalU. (G–I) The galU gene expression strain, pgalU.
3.6. Regulation of Metabolic Pathways by galU
We found that 410 genes were upregulated and 1196 genes were downregulated in the metabolic pathways of L. acidophilus after galU knocked out. In the amino sugar metabolism pathway, we found that the part of the galU genes we edited were mainly involved in the regulation of galactose metabolism (here we only compared the start strain LA with the re-expression strain pgalU, because the knockout strain ΔgalU could not be cultured in lactose) (Figure 5). Through gene enrichment analysis, eight genes with the highest expression difference were identified: galactokinase (galK), UDPglucose--hexose-1-phosphate uridylyltransferase (galT), UDP-glucose 4-epimerase (galE), UDP-galactopyranose mutase (glf), glutamine---fructose-6-phosphate transaminase (glmS), UDP-N-acetylglucosamine 2-epimerase (wecB), UDP-N-acetylmuramate dehydrogenase (murB), hexosaminidase (HEXA_B). Their Q value values were <0.01, and the degree of enrichment was very significant. These genes are related to the transformation of galactose into UDP-ManNAc. This result could be attributed to the recovery of galactose metabolism and improvement of freeze-drying resistance in pgalU.
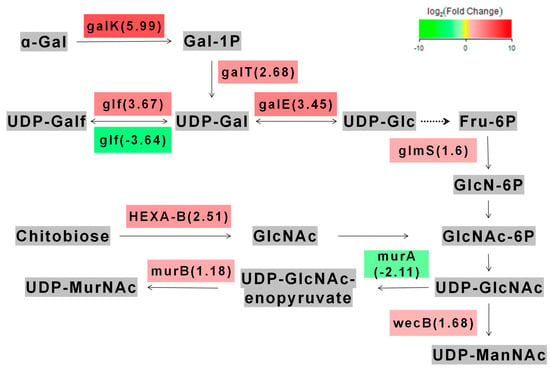
Figure 5.
The main metabolic pathways affected by galU gene.
4. Discussion
We herein investigated the mechanisms responsible for improving freeze-drying resistance by first knocking out and then re-expressing galU in L. acidophilus NCFM. The knockout strain ΔgalU showed lactose deficiency, irregular cell morphology, abnormal cell division, and thin and rough capsule; moreover, lactose metabolism ability was lost. After galU was re-expressed, galactose metabolism ability was restored and genes are related to the transformation of galactose into UDP-ManNAc showed higher expression levels, and the cell wall and capsule became thicker. Our previous work found that mannose as antifreeze factors can improve the survival rate of L. acidophilus after freeze-drying, and the enzyme activities detection also showed the activity of glycosyltransferases such as GalU had significant difference in adding mannose as antifreeze factors [31]. Therefore, we supposed and verified that the galU gene as an important regulatory site for L. acidophilus for resisting freeze-drying and UDP-ManNAc-related amino sugars can be used as antifreeze factors for L. acidophilus.
During sample preparation for transcriptome sequencing, MRS medium (contains glucose, not lactose, as the main source of carbon) was used to culture all three strains (LA, ΔgalU and pgalU), that may underestimate the espressions of genes in galactose metabolism pathway. In L. acidophilus NCFM, lactose can be hydrolyzed to glucose and galactose under the action of β-galactosidase after being transported into the cell [32,33]. After β-galactosidase binds to lactose, glucose is first released [34,35]. Therefore, lactose metabolism in ΔgalU must be inhibited before glucose is released in the reaction of galactosidase and lactose. We speculate that galU knockout may resulted in ΔgalU losing its ability to hydrolyze lactose.
In this study, one of the reasons for using pNZ8149 was that it is a food-grade expression vector [36], making it ideal for food research and development [37]. The other reason was that NZ3900, the standard host strain of pNZ8149, is also a lactose-deficient strain [38,39]. The NZ3900 strain requires the presence of lacF/repA/C in pNZ8149 to ensure a functional lactose metabolism pathway [40]. Theoretically, both pNZ8149 and galU can ensure the growth of the knockout strain ΔgalU on the M17 plate, but the experimental results showed that pNZ8149 makes the colony yellow, while galU makes the colony white. In this manner, we could distinguish whether the recovery of lactose metabolism in the different strains was affected by pNZ8149 or galU. The reason why the colonies appear to have different colors needs further exploration. What is more, it is hard to understand why gene expression often needs to be induced by inducers [41,42], but pgalU could efficiently express the galU gene in a lactose medium, even without the inducers, and the colonies formed were larger and moister. This might be attributed to two reasons: one is that lactose, as an inducer, is influenced by the lactose-specific element lacZ gene, leading to galU expression [43,44]; the other is that ΔgalU is more inclined to transcribe DNA damage-repair genes, particularly when encountering adverse environments [45,46,47]. Thus, the galU expression in ΔgalU was stronger. Further studies are warranted to explore the pertinent mechanisms.
In this paper, we show that the ΔgalU strain can serve as an efficient expression system, with the expression vector containing galU. Using lactose agar, strains with positive expression can be screened, even in the absence of inducers in the growth medium. This screening method is simple and does not involve the use of antibiotics. We aim to further study the knockout strain ΔgalU to validate its safety and expression mechanism, and we expect ΔgalU to become a food-grade high-efficiency expression system of L. acidophilus.
Author Contributions
Z.Z.: Carrying out the experiments, acquisition of data, data curation, revision of the manuscript, and methodology. X.Z.: Conceptualization, project administration, funding acquisition, validation, and supervision. Y.G.: Writing—review and editing, data curation, and formal analysis. Z.W.: Conceptualization, writing—review and editing, data curation, formal analysis, investigation, and project administration. Z.C.: Writing—original draft, data curation, investigation, methodology, and formal analysis. D.P.: Resources and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (32072195, 41406165, 41641052, 31972093), the Science and Technology Department of Zhejiang Province (LGN19C200011, 2019C02085) and the New talent plan of Zhejiang Province (2021R405054).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Altermann, E.; Russell, W.; Azcarate, M.; Barrangou, R.; Buck, B.; Mcauliffe, O.; Souther, N.; Dobson, A.; Duong, T.; Callanan, M.; et al. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc. Natl. Acad. Sci. USA 2005, 102, 3906–3912. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, C.; Tiihonen, K.; Saarinen, M.; Putaala, H.; Rautonen, N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: Intestinal and immune parameters. Br. J. Nutr. 2008, 101, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Christel, R.; Xavier, T.; Agathe, G.; Nicolas, B.; Christel, N.; Laurent, D.; Caroline, D.; Emilie, M.; Karen, G.; Mathias, C. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat. Med. 2007, 13, 35–37. [Google Scholar] [CrossRef]
- Konstantinov, S.; Hauke, S.; Vos, W.; Bruijns, S.; Satwinder, S.; Florence, V.; Daniel, M.; Sylvie, L.; Eric, A.; Klaenhammer, T.; et al. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 19474–19479. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, S.; Larsen, N.; Pedersen-Skovsgaard, T.; Berg, R.; Meller, K.; Svendsen, K.; Jakobsen, M.; Pedersen, B. Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br. J. Nutr. 2010, 104, 1831–1838. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Pisanu, F.; Baldo, D.; Parolin, C.; Filippini, G.; Vitali, B.; Lanciotti, R.; Patrignani, F. Unravelling the functional and technological potential of soy milk based microencapsulated Lactobacillus crispatus and Lactobacillus gasseri. J. Funct. Foods 2021, 87, 1756–4646. [Google Scholar] [CrossRef]
- Delphine, M.A.; Jennifer, K.; Glenn, G.; James, V. Mechanisms of probiosis and prebiosis: Considerations for enhanced functional foods. Curr. Opin. Biotechnol. 2009, 20, 135–141. [Google Scholar] [CrossRef]
- Canducci, F.; Armuzzi, A.; Cremonini, F.; Cammarota, G.; Gasbarrini, A. A lyophilized and inactivated culture of Lactobacillus acidophilus increases Helicobacter pylori eradication rates. Aliment. Pharmacol. Ther. 2001, 14, 1625–1629. [Google Scholar] [CrossRef]
- Giulio, B.D.; Orlando, P.; Barba, G.; Coppola, R.; Rosa, M.D.; Sada, A.; Prisco, P.P.D.; Nazzaro, F. Use of alginate and cryo-protective sugars to improve the viability of lactic acid bacteria after freezing and freeze-drying. World J. Microbiol. Biotechnol. 2005, 21, 736–749. [Google Scholar] [CrossRef]
- Bosca, S.; Barresi, A.; Fissore, D. On the use of model-based tools to optimize in-line a pharma ceuticals freeze-drying process. Dry. Technol. 2016, 37, 937–955. [Google Scholar] [CrossRef]
- Mingxue, L.; Xiaoqun, Z.; Yating, H.; Chaoran, X.; Lu, C.; Zhen, W.; Hangzhen, L.; Daodong, P. iTRAQ-based quantitative proteomic analysis of the effect of heat shock on freeze-drying of Lactobacillus acidophilus ATCC4356. Int. J. Food Sci. Technol. 2021, 56, 5569–5580. [Google Scholar] [CrossRef]
- Wiley, H.; Leveille, A. Influence of Periodicity of Eating on the Activity of Adipose Tissue and Muscle Glycogen Synthesizing Enzymes in the Rat. J. Nutr. 1970, 100, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Alberto, A.; Jack, P. Bacterial glycogen and plant starch biosynthesis. Biochem. Educ. 1992, 20, 196–203. [Google Scholar] [CrossRef]
- Cools, F.; Torfs, E.; Vanhoutte, B.; Bidart, D.; Bonofiglio, L.; Mollerach, M.; Maes, L.; Caljon, G. Streptococcus pneumoniae galU gene mutation has a direct effect on biofilm growth, adherence and phagocytosis in vitro and pathogenicity in vivo. Pathog. Dis. 2018, 76, 1120–1178. [Google Scholar] [CrossRef] [PubMed]
- Munch, C.; Petersen, A.; Nygaard, P.; Jespersen, K. Mutants constitutive for nucleoside-catabolizing enzymes in Escherichia coli K12 isolation, charactrization and mapping. Eur. J. Biochem. 2010, 27, 208–215. [Google Scholar] [CrossRef]
- Sulek, K.; Smedsgaard, J.; Skov, T.; Wilcks, A.; Licht, T. Metabolic footprint of Lactobacillus acidophilus NCFM at different pH. Metabolomics 2012, 8, 244–252. [Google Scholar] [CrossRef]
- Kim, H.; Shim, J.E.; Shin, J.; Lee, I. Ecolinet: A database of cofunctional gene network for Escherichia coli. Database 2015, 2015, bav001. [Google Scholar] [CrossRef]
- Choonia, H.S.; Saptarshi, S.D.; Lele, S.S. Release of intracellular β-galactosidase from Lactobacillus acidophilus and L-asparaginase from Pectobacterium carotovorum by high-pressure homogenization. Chem. Eng. Commun. 2013, 200, 1415–1424. [Google Scholar] [CrossRef]
- Guimont, C.; Gaillard, J. Comparative study of the protein compositionof three strains of Streptococcus thermophilus grown either in M17 medium or in milk. Dairy Sci. Technol. 2002, 82, 645–656. [Google Scholar] [CrossRef][Green Version]
- Lee, B.; Chen, J.; Du, G.; Li, H.; Zhang, J.; Dong, Z. Codon and propeptide optimizations to improve the food-grade expression of bile salt hydrolase in Lactococcus lactis. Protein Pept. Lett. 2015, 22, 727–735. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Gou, K.; Luo, Y. Heterologous expression of stearoyl-CoA desaturase-1 in Lactococcus lactis NZ3900. Chin. J. Biotechnol. 2012, 28, 1106–1117. [Google Scholar] [CrossRef]
- Guo, M.; Yi, S.; Guo, Y.; Zhang, S.; Niu, J.; Wang, K.; Hu, G. Construction of a recombinant Lactococcus lactis strain expressing a variant porcine epidemic diarrhea virus S1 gene and its immunogenicity analysis in mice. Viral Immunol. 2019, 141, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhu, J.; Liu, L.; Cong, Y.; Hu, F.; Li, J.; Yu, X. Functional identification of a putative β-galactosidase gene in the special lac gene cluster of Lactobacillus acidophilus. Curr. Microbiol. 2010, 60, 172–180. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, R.; Duan, G.; Shi, J. Food-grade expression of helicobacter pylori UreB subunit in Lactococcus lactis and its Immunoreactivity. Curr. Microbiol. 2011, 62, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Mcconville, K.; Mcreynolds, C.; Collins, M. Electrotransformation of Lactobacillus plantarum using linearized plasmid DNA. Lett. Appl. Microbiol. 2007, 25, 419–425. [Google Scholar] [CrossRef]
- Thu-Ha, N.; Barbara, S.; Stanimira, K.; Wolfgang, K.; Kulbe, D.; Christina, D.; Dietmar, H. Characterization and molecular cloning of a heterodimeric β-galactosidase from the probiotic strain Lactobacillus acidophilus R22. Fems Microbiol. Lett. 2007, 269, 136–144. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhang, R.G.; Duan, G.C.; Fan, Q.T. Expression of Helicobacter pylori ureB gene in Lactococcus lactis NZ3900 and its immunoreactivity. Chin. J. Zoonoses 2011, 79, 699–703. [Google Scholar] [CrossRef]
- Song, J.; Jiaping, L.; Wang, Z.; Zhang, L.; Zhang, S.; Xianbao, H. Effect of centrifugation conditions on the survival rate of freeze-drying culture. Sci. Technol. Food Ind. 2009, 30, 82–84. [Google Scholar] [CrossRef]
- Hood, S.; Zottola, A. Electron microscopic study of the adherence properties of Lactobacillus acidophilus. J. Food Sci. 2006, 52, 791–792. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Zhen, N.; Zeng, X.; Wang, H.; Yu, J.; Guo, Y. Effects of heat shock treatment on the survival rate of Lactobacillus acidophilus after freeze-drying. Food Res. Int. 2020, 136, 109507. [Google Scholar] [CrossRef] [PubMed]
- Carević, M.; Vukašinović-Sekulić, M.; Ćorović, M.; Rogniaux, H.; Ropartz, D.; Veličković, D.; Bezbradica, D. Evaluation of β-galactosidase from Lactobacillus acidophilus as biocatalyst for galacto-oligosaccharides synthesis: Product structural characterization and enzyme immobilization. J. Biosci. Bioeng. 2018, 86, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Beerthuyzen, M.; Vaughan, E.; Vos, W.; Kuipers, P. Controlled gene expression systems for lactic acid bacteria: Transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 1997, 63, 4581–4584. [Google Scholar] [CrossRef] [PubMed]
- Broach, B.; Gu, X.; Bar-Peled, M. Biosynthesis of UDP-glucuronic acid and UDP-galacturonic acid in Bacillus cereus subsp. cytotoxis NVH 391-98. Febs J. 2012, 279, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Geiger, B.; Nguyen, H.-M.; Wenig, S.; Lorenz, C.; Kittl, R.; Mathiesen, G.; Eijsink, V.G.; Haltrich, D.; Nguyen, T.-H. From by-product to valuable components: Efficient enzymatic conversion of lactose in whey using β-galactosidase from Streptococcus thermophilus. Biochem. Eng. J. 2016, 96, 1024–1037. [Google Scholar] [CrossRef]
- Liang, X.; Sun, Z.; Zhong, J.; Zhang, Q.; Huan, L. Adverse effect of nisin resistance protein on nisin-induced expression system in Lactococcus lactis. Microbiol. Res. 2010, 165, 458–465. [Google Scholar] [CrossRef]
- Platteeuw, C.; van Alen-Boerrigter, I.N.; van Schalkwijk, S.A.; De Vos, W.M. Food-grade cloning and expression system for Lactococcus lactis. Appl. Environ. Microbiol. 1996, 62, 1008–1013. [Google Scholar] [CrossRef]
- Cao, H.; Wang, H.; Yang, X.; Zhang, A.; Yang, F. Lactococcus lactis Anchoring avian infectious bronchitis virus multi-epitope peptide epiC induced specific immune responses in chickens. J. Agric. Chem. Soc. Jpn. 2013, 77, 1831–1839. [Google Scholar] [CrossRef]
- Wang, P.; Baiyuan, L.; Li, B.; Cai, X.; Zeng, Z.; Chen, X.; Wang, X. Development of an efficient conjugation-based genetic manipulation system for Pseudoalteromonas. Microb. Cell Factories 2015, 32, 156–170. [Google Scholar] [CrossRef]
- Guo, Y.; Sagaram, U.; Kim, J.; Wang, N. Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in planta of Xanthomonas citri, subspecies citri. Appl. Environ. Microbiol. 2010, 76, 2234–2242. [Google Scholar] [CrossRef]
- Zechel, L.; Withers, S. Glycosidase mechanisms: Anatomy of a finely tuned catalyst. Acc. Chem. Res. 2000, 33, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Belogurov, A.; Artsimovitch, I. Regulation of transcript elongation. Annu. Rev. Microbiol. 2015, 69, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gao, Y.; Gao, G.; Lou, Y. Oral administration of recombinant Lactococcus lactis expressing the cellulase gene increases digestibility of fiber in geese. Curr. Microbiol. 2015, 71, 693–698. [Google Scholar] [CrossRef]
- Yi, Z.; Saixiang, F.; Chenggang, X.; Bin, Z.; Suming, Z.; Lingyun, Z.; Xianhui, H.; Jingyi, L.; Zhen, Y.; Ming, L. The role of galU and galE of Haemophilus parasuis SC096 in serum resistance and biofilm formation. Vet. Microbiol. 2013, 162, 278–284. [Google Scholar] [CrossRef]
- Kulkarni, R.; Thomas, R.A.; Tucker, J.D. Expression of DNA repair and apoptosis genes in mitochondrial mutant and normal cells following exposure to ionizing radiation. Environ. Mol. Mutagenes. 2011, 52, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Post, A.E.M.; Bussink, J.; Sweep, F.C.G.J.; Span, P.N. A Changes in DNA damage repair gene expression and cell cycle gene expression do not explain radioresistance in tamoxifen-resistant breast cancer. Oncol. Res. 2019, 43, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, H.; Miller, J.; Shih, D.; Hicks, G.; Xie, J. Cells deficient in oxidative DNA damage repair genes Myhand Ogg1are sensitive to oxidants with increased G2/M arrest and multinucleation. Carcinogenesis 2008, 55, 456–458. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).