Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Lipases, Oils and Fatty Acids
2.2. Preparation of Enzyme Solutions
2.3. Lipase Activity Assay
2.4. Chromogenic Plate Assay
2.5. Enzymatic Treatment of Oils in a Liquid Environment
2.6. Extraction of Free Fatty Acids
2.7. GC-MS Analysis of Fatty Acid Methyl Esters
2.8. Determination of Antioxidant Capacity
2.9. Antimicrobial Activity Assays
2.10. Statistical Analysis
3. Results and Discussion
3.1. Screening of Oil Hydrolysis on Chromogenic Plates
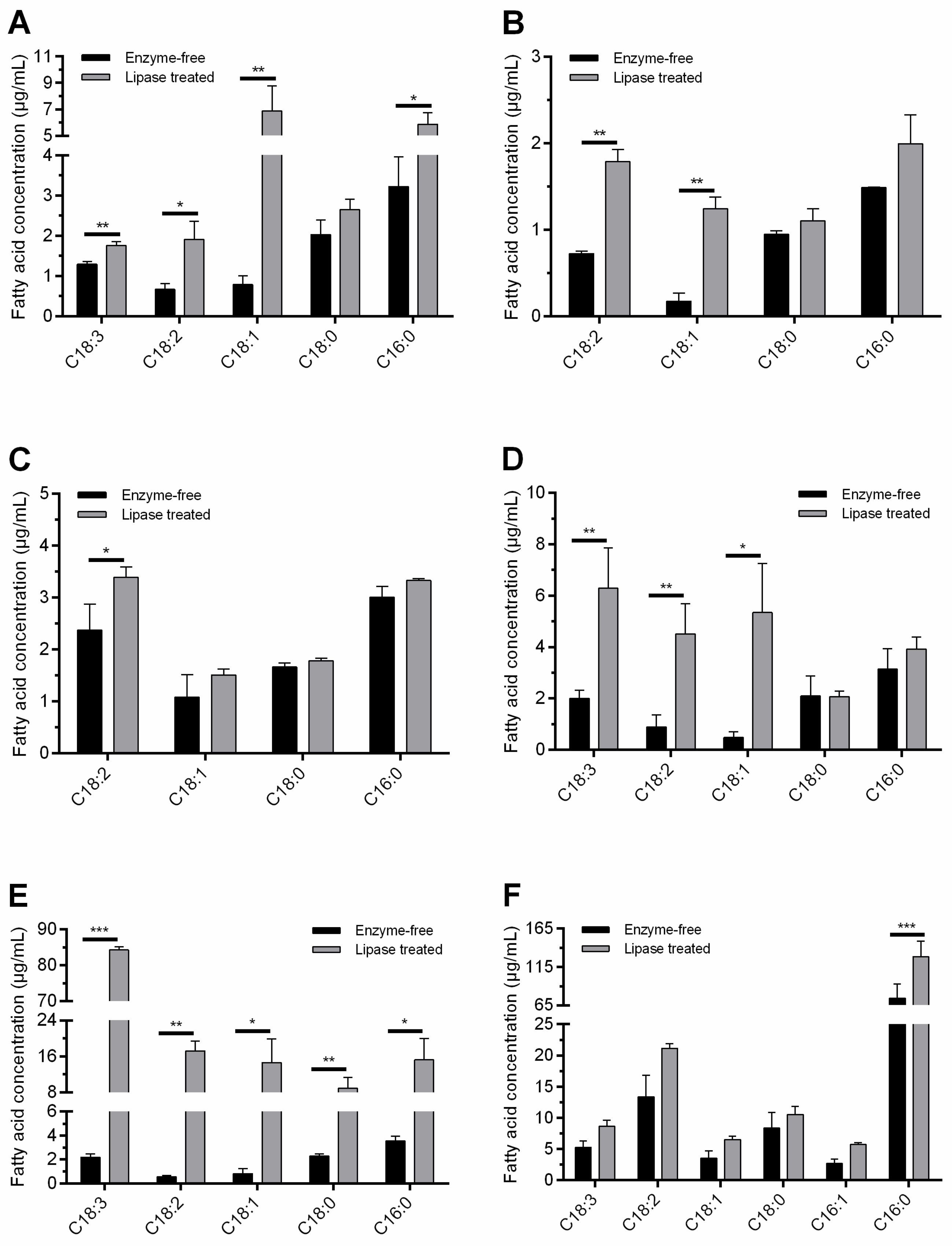
3.2. Lipolysis in Liquid Environment
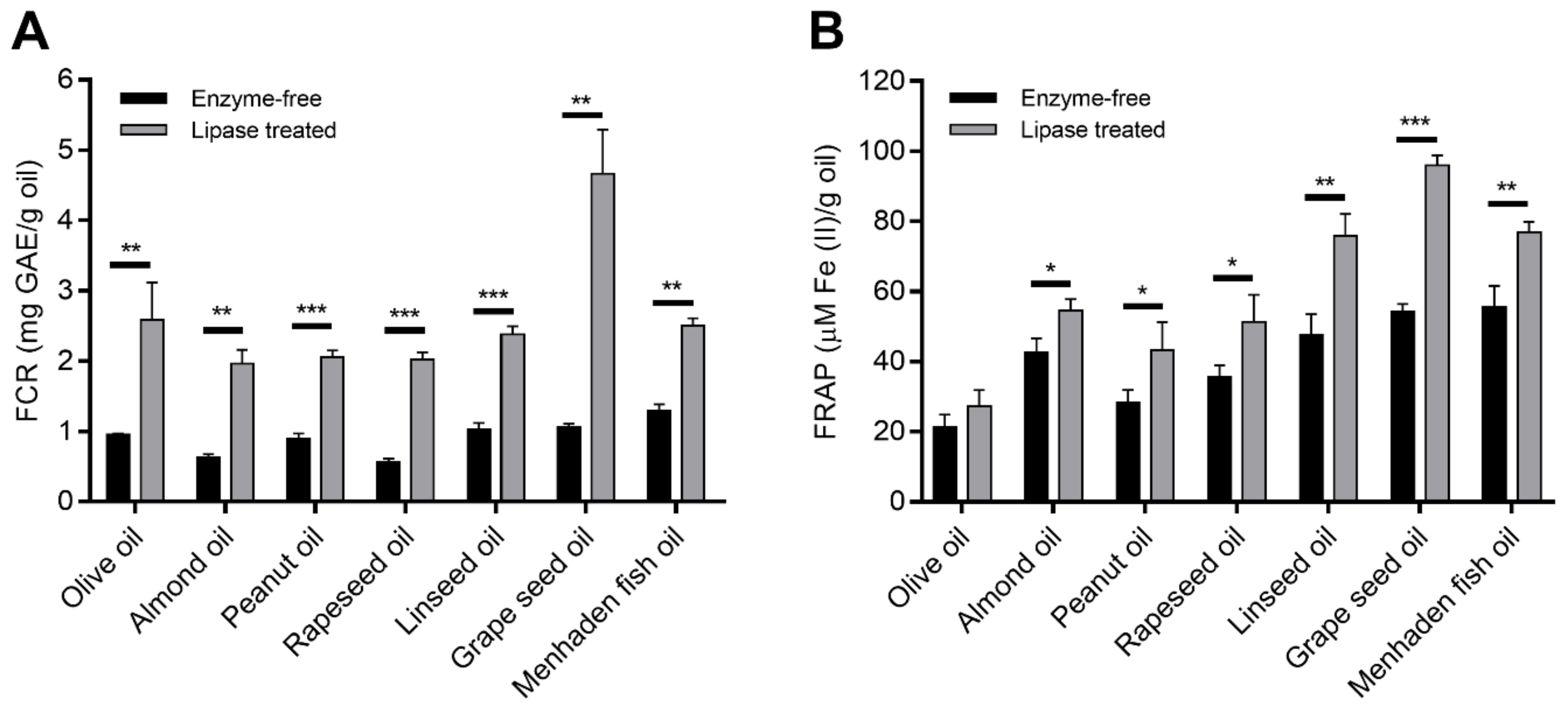
3.3. Bioactive Properties of the Hydrolysates
3.3.1. Antioxidant Capacity
3.3.2. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, R. Important bioactive properties of omega-3 fatty acids. Ital. J. Food Sci. 2015, 27, 129–135. [Google Scholar]
- Szumacher-Strabel, M.; El-Sherbiny, M.; Adam Cieslak, A.; Szczechowiak, J.; Winiarska, H. Bioactive lipid components from ruminant milk and meat: The new face of human health. In Biotechnology of Bioactive Compounds: Sources and Applications; Gupta, V.K., Tuohy, M.G., Eds.; Wiley: Oxford, UK, 2015; pp. 599–629. [Google Scholar]
- Cavaglieri, C.R.; Nishiyama, A.; Fernandes, L.C.; Curi, R.; Miles, E.A.; Calder, P.C. Differential effects of short-chain fatty acids on proliferation and production of pro- and anti-inflammatory cytokines by cultured lymphocytes. Life Sci. 2003, 73, 1683–1690. [Google Scholar] [CrossRef]
- Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Leblanc, J.-C.; et al. Re-evaluation of fatty acids (E 570) as a food additive. EFSA J. 2017, 15, e04785. [Google Scholar] [PubMed]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive oil composition. In Olive Oil: Chemistry and Technology, 2nd ed.; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2006; pp. 41–72. [Google Scholar]
- Shinagawa, F.B.; Santana, F.C.D.; Torres, L.R.O.; Mancini-Filho, J. Grape seed oil: A potential functional Food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Garavaglia, J.; Markoski, M.M.; Oliveira, A.; Marcadenti, A. Grape seed oil compounds: Biological and chemical actions for health. Nutr. Metab. Insights 2016, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) seed oil: A functional food from the winemaking industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Carrín, M.E.; Carelli, A.A. Peanut oil: Compositional data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Ahmad, Z. The uses and properties of almond oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Sharafi, Y.; Majidi, M.M.; Goli, S.A.H.; Rashidi, F. Oil content and fatty acids composition in Brassica species. Int. J. Food Prop. 2015, 18, 2145–2154. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and flaxseed oil: An ancient medicine & modern functional food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar]
- Suchoszek-Łukaniuk, K.; Jaromin, A.; Korycińska, M.; Kozubek, A. Health benefits of peanut (Arachis hypogaea L.) seeds and peanut oil consumption. In Nuts and Seeds in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 873–880. [Google Scholar]
- Lin, L.; Allemekinders, H.; Dansby, A.; Campbell, L.; Durance-Tod, S.; Berger, A.; Jones, P.J.H. Evidence of health benefits of canola oil. Nutr. Rev. 2013, 71, 370–385. [Google Scholar] [CrossRef]
- Gavahian, M.; Khaneghah, A.M.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Tucker, B.W.; Pigott, G.M. Fish oils: Composition and properties. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: London, UK, 2003; pp. 2495–2501. [Google Scholar]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A.; Norulaini, N.A. PUFAs in fish: Extraction, fractionation, importance in health. Compr. Rev. Food Sci. Food Saf. 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Hashim, A.F.; Hamed, S.F.; Hamid, H.A.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Antioxidant and antibacterial activities of omega-3 rich oils/curcumin nanoemulsions loaded in chitosan and alginate-based microbeads. Int. J. Biol. Macromol. 2019, 140, 682–696. [Google Scholar] [CrossRef]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Nicol, M.; Alexandre, S.; Luizet, J.-B.; Skogman, M.; Jouenne, T.; Salcedo, S.P.; Dé, E. Unsaturated fatty acids affect quorum sensing communication system and inhibit motility and biofilm formation of Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 214. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef]
- Peng, M.; Biswas, D. Short chain and polyunsaturated fatty acids in host gut health and foodborne bacterial pathogen inhibition. Crit. Rev. Food Sci. Nutr. 2017, 57, 3987–4002. [Google Scholar] [CrossRef]
- Kumaratilake, L.M.; Robinson, B.S.; Ferrante, A.; Poulos, A. Antimalarial properties of n-3 and n-6 polyunsaturated fatty acids: In vitro effects on Plasmodium falciparum and in vivo effects on P. berghei. J. Clin. Investig. 1992, 89, 961–967. [Google Scholar] [CrossRef]
- Das, U.N. Can essential fatty acids reduce the burden of disease (s)? Lipids Health Dis. 2008, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.E.; Momin, R.A.; Nair, M.G.; Dewitt, D.L. Antioxidant and cyclooxygenase activities of fatty acids found in food. J. Agric. Food Chem. 2002, 50, 2231–2234. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- De Alencar, D.B.; Diniz, J.C.; Rocha, S.A.S.; Pires-Cavalcante, K.M.S.; De Lima, R.L.; De Sousa, K.C.; Freitas, J.O.; Bezerra, R.M.; Baracho, B.M.; Sampaio, A.H.; et al. Fatty acid composition from the marine red algae Pterocladiella capillacea (S. G. Gmelin) Santelices & Hommersand 1997 and Osmundaria obtusiloba (C. Agardh) R. E. Norris 1991 and its antioxidant activity. An. Acad. Bras. Ciênc. 2018, 90, 449–459. [Google Scholar]
- Szabó, É.; Marosvölgyi, T.; Szilágyi, G.; Kőrösi, L.; Schmidt, J.; Csepregi, K.; Márk, L.; Bóna, Á. Correlations between total antioxidant capacity, polyphenol and fatty acid content of native grape seed and pomace of four different grape varieties in Hungary. Antioxidants 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczak, N.; Tańska, M.; Ogrodowska, D. Phenolic compounds in plant oils: A review of composition, analytical methods, and effect on oxidative stability. Trends Food Sci. Technol. 2021, 113, 110–138. [Google Scholar] [CrossRef]
- Zeb, A. A comprehensive review on different classes of polyphenolic compounds present in edible oils. Food Res. Int. 2021, 143, 110312. [Google Scholar] [CrossRef] [PubMed]
- Ghasemifard, S.; Turchini, G.M.; Sinclair, A.J. Omega-3 long chain fatty acid “bioavailability”: A review of evidence and methodological considerations. Prog. Lipid Res. 2014, 56, 92–108. [Google Scholar] [CrossRef]
- Holliday, R.L.; King, J.W.; List, G.R. Hydrolysis of vegetable oils in sub- and supercritical water. Ind. Eng. Chem. Res. 1997, 36, 932–935. [Google Scholar] [CrossRef]
- Satyarthi, J.K.; Srinivas, D.; Ratnasamy, P. Hydrolysis of vegetable oils and fats to fatty acids over solid acid catalysts. Appl. Catal. A Gen. 2011, 391, 427–435. [Google Scholar] [CrossRef]
- Nguyen, T.A.V.; Le, T.D.; Phan, H.N.; Tran, L.B. Hydrolysis activity of virgin coconut oil using lipase from different sources. Scientifica 2018, 2018, 9120942. [Google Scholar] [CrossRef]
- Castiglioni, G.Z.; Bettio, G.; Matte, C.R.; Jacques, R.A.; Dos Santos Polidoro, A.; Rosa, C.A.; Ayub, M.A.Z. Production of volatile compounds by yeasts using hydrolysed grape seed oil obtained by immobilized lipases in continuous packed-bed reactors. Bioprocess Biosyst. Eng. 2020, 43, 1391–1402. [Google Scholar] [CrossRef]
- Mehta, A.; Guleria, S.; Sharma, R.; Gupta, R. The lipases and their applications with emphasis on food industry. In Microbial Biotechnology in Food and Health; Ray, R.C., Ed.; Academic Press: London, UK, 2021; pp. 143–164. [Google Scholar]
- Chandra, P.; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar] [CrossRef]
- Shamel, M.M.; Ramachandran, K.B.; Hasan, M.; Al-Zuhair, S. Hydrolysis of palm and olive oils by immobilised lipase using hollow fibre reactor. Biochem. Eng. J. 2007, 34, 228–235. [Google Scholar] [CrossRef]
- Carvalho, P.D.O.; Campos, P.R.B.; Noffs, M.D.A.; Fregolente, P.B.L.; Fregolente, L.V. Enzymatic hydrolysis of salmon oil by native lipases: Optimization of process parameters. J. Braz. Chem. Soc. 2009, 20, 117–124. [Google Scholar] [CrossRef]
- Goswami, D.; Basu, J.K.; De, S. Lipase applications in oil hydrolysis with a case study on castor oil: A review. Crit. Rev. Biotechnol. 2013, 33, 81–96. [Google Scholar] [CrossRef]
- Alves, J.S.; Vieira, N.S.; Cunha, A.S.; Silva, A.M.; Ayub, M.A.Z.; Fernandez-Lafuente, R.; Rodrigues, R.C. Combi-lipase for heterogeneous substrates: A new approach for hydrolysis of soybean oil using mixtures of biocatalysts. RSC Adv. 2014, 4, 6863–6868. [Google Scholar] [CrossRef]
- Chen, W.; Sun, S.; Liang, S.; Peng, L.; Wang, Y.; Shen, M. Lipase-catalyzed hydrolysis of linseed oil: Optimization using response surface methodology. J. Oleo Sci. 2014, 63, 619–628. [Google Scholar] [CrossRef]
- Aziz, M.; Husson, F.; Kermasha, S. Optimization of the hydrolysis of safflower oil for the production of linoleic acid, used as flavor precursor. Int. J. Food Sci. 2015, 2015, 594238. [Google Scholar] [CrossRef]
- Monié, A.; David, A.; Clemens, K.; Malet-Martino, M.; Balayssac, S.; Perez, E.; Franceschi, S.; Crepin, M.; Delample, M. Enzymatic hydrolysis of rapeseed oil with a non-GMO lipase: A strategy to substitute mono-and diglycerides of fatty acids and improve the softness of sponge cakes. LWT 2021, 137, 110405. [Google Scholar] [CrossRef]
- Huynh, T.; Vörös, M.; Kedves, O.; Turbat, A.; Sipos, G.; Leitgeb, B.; Kredics, L.; Vágvölgyi, C.; Szekeres, A. Discrimination between the two closely related species of the operational group B. amyloliquefaciens based on whole-cell fatty acid profiling. Microorganisms 2022, 10, 418. [Google Scholar] [CrossRef]
- Takó, M.; Kotogán, A.; Papp, T.; Kadaikunnan, S.; Alharbi, N.S.; Vágvölgyi, C. Purification and properties of extracellular lipases with transesterification activity and 1,3-regioselectivity from Rhizomucor miehei and Rhizopus oryzae. J. Microbiol. Biotechnol. 2017, 27, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gupta, N.; Goswami, V.K.; Gupta, R. A simple activity staining protocol for lipases and esterases. Appl. Microbiol. Biotechnol. 2006, 70, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, K.M.; Yoo, Y.; Yu, H.; Lee, C.J.; Jung, H.S.; Kim, K.; Chang, P.S. Catalytic characteristics of a sn-1(3) regioselective lipase from Cordyceps militaris. Biotechnol. Prog. 2019, 35, e2744. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Rajalakshmi, G. Microbial lipases: An overview of screening, production and purification. Biocatal. Agric. Biotechnol. 2019, 22, 101368. [Google Scholar] [CrossRef]
- Schröter, S.; Schnitzlein, K. Enzymatic hydrolysis of rapeseed oil by Thermomyces lanuginosus lipase: Variation of continuous and dispersed phase in a slug flow reactor. Appl. Microbiol. Biotechnol. 2018, 102, 4799–4806. [Google Scholar] [CrossRef]
- Carteret, C.; Jacoby, J.; Blin, J.L. Using factorial experimental design to optimize biocatalytic biodiesel production from Mucor Miehei Lipase immobilized onto ordered mesoporous materials. Microporous Mesoporous Mater. 2018, 268, 39–45. [Google Scholar] [CrossRef]
- Dzurendova, S.; Losada, C.B.; Dupuy-Galet, B.X.; Fjær, K.; Shapaval, V. Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl. Microbiol. Biotechnol. 2022, 106, 101–115. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Fernandez-Lafuente, R. Lipase from Rhizomucor miehei as an industrial biocatalyst in chemical process. J. Mol. Catal. B Enzym. 2010, 64, 1–22. [Google Scholar] [CrossRef]
- Kotogán, A.; Németh, B.; Vágvölgyi, C.; Papp, T.; Takó, M. Screening for extracellular lipase enzymes with transesterification capacity in Mucoromycotina strains. Food Technol. Biotechnol. 2014, 52, 73–82. [Google Scholar]
- Ferreira, M.M.; de Oliveira, G.F.; Basso, R.C.; Mendes, A.A.; Hirata, D.B. Optimization of free fatty acid production by enzymatic hydrolysis of vegetable oils using a non-commercial lipase from Geotrichum candidum. Bioprocess Biosyst. Eng. 2019, 42, 1647–1659. [Google Scholar] [CrossRef]
- Soumanou, M.M.; Edorh, A.P.; Bornscheuer, U.T. Activity and stability of immobilized lipases in lipase-catalyzed modification of peanut oil. Oléagineux Corp. Gras Lipides 2004, 11, 464–468. [Google Scholar] [CrossRef][Green Version]
- Rupani, B.; Kodam, K.; Gadre, R.; Najafpour, G.D. Lipase-mediated hydrolysis of flax seed oil for selective enrichment of α-linolenic acid. Eur. J. Lipid Sci. Technol. 2012, 114, 1246–1253. [Google Scholar] [CrossRef]
- Wanasundara, U.N.; Shahidi, F. Lipase-assisted concentration of n-3 polyunsaturated fatty acids in acylglycerols from marine oils. J. Am. Oil Chem. Soc. 1998, 75, 945–951. [Google Scholar] [CrossRef]
- Rice, K.E.; Watkins, J.; Hill, C.G., Jr. Hydrolysis of menhaden oil by a Candida cylindracea lipase immobilized in a hollow-fiber reactor. Biotechnol. Bioeng. 1999, 63, 33–45. [Google Scholar] [CrossRef]
- Ashjari, M.; Mohammadi, M.; Badri, R. Selective concentration of eicosapentaenoic acid and docosahexaenoic acid from fish oil with immobilized/stabilized preparations of Rhizopus oryzae lipase. J. Mol. Catal. B Enzym. 2015, 122, 147–155. [Google Scholar] [CrossRef]
- Mohammadi, M.; Habibi, Z.; Dezvarei, S.; Yousefi, M.; Ashjari, M. Selective enrichment of polyunsaturated fatty acids by hydrolysis of fish oil using immobilized and stabilized Rhizomucor miehei lipase preparations. Food Bioprod. Process. 2015, 94, 414–421. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C.; Mesa, M.; Wilson, L. Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. Int. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of omega-3 fatty acids by the hydrolysis of fish oil catalyzed by lipases immobilized on hydrophobic supports. J. Am. Oil Chem. Soc. 2011, 88, 1173–1178. [Google Scholar] [CrossRef]
- Na, B.R.; Lee, J.H. In vitro and in vivo digestibility of soybean, fish, and microalgal oils, and their influences on fatty acid distribution in tissue lipid of mice. Molecules 2020, 25, 5357. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.W.; Hu, X.P.; Zhou, D.Y.; Ma, X.C.; Tian, X.G.; Huo, X.K.; Rakariyatham, K.; Shahidi, F.; Zhu, B.W. Evaluation of the stability of tyrosol esters during in vitro gastrointestinal digestion. Food Funct. 2018, 9, 3610–3616. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Biedermann, D.; Valentová, K.; Petrásková, L.; Viktorová, J.; Kuzma, M.; Křen, V. Preparation of retinoyl-flavonolignan hybrids and their antioxidant properties. Antioxidants 2019, 8, 236. [Google Scholar] [CrossRef]
- Plaza, L.; Sánchez-Moreno, C.; de Pascual-Teresa, S.; de Ancos, B.; Cano, M.P. Fatty acids, sterols, and antioxidant activity in minimally processed avocados during refrigerated storage. J. Agric. Food Chem. 2009, 57, 3204–3209. [Google Scholar] [CrossRef]
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef]
- Karaman, M.; Atlagić, K.; Novaković, A.; Šibul, F.; Živić, M.; Stevanović, K.; Pejin, B. Fatty acids predominantly affect anti-hydroxyl radical activity and FRAP value: The case study of two edible mushrooms. Antioxidants 2019, 8, 480. [Google Scholar] [CrossRef]
- Wadhwani, T.; Desai, K.; Patel, D.; Lawani, D.; Bahaley, P.; Joshi, P.; Kothari, V. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. Internet J. Microbiol. 2009, 7, 1. [Google Scholar]
- Desbois, A.P.; Lawlor, K.C. Antibacterial activity of long-chain polyunsaturated fatty acids against Propionibacterium acnes and Staphylococcus aureus. Mar. Drugs 2013, 11, 4544. [Google Scholar] [CrossRef]
- Mi, H.; Wang, D.; Xue, Y.; Zhang, Z.; Niu, J.; Hong, Y.; Drlica, K.; Zhao, X. Dimethyl sulfoxide protects Escherichia coli from rapid antimicrobial-mediated killing. Antimicrob. Agents Chemother. 2016, 60, 5054–5058. [Google Scholar] [CrossRef]
- Lee, S.T.; Ariffin, A.; Son, R.; Ghazali, H.M. Effect of lipase hydrolysis on the antibacterial activity of coconut oil, palm mesocarp oil and selected seed oils against several pathogenic bacteria. Int. Food Res. J. 2015, 22, 46–54. [Google Scholar]
- Hovorková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro antibacterial activity of plant oils containing medium-chain fatty acids against Gram-positive pathogenic and gut commensal bacteria. Czech J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef]
- Pizzolante, G.; Durante, M.; Rizzo, D.; Di Salvo, M.; Tredici, S.M.; Tufariello, M.; De Paolis, A.; Talà, A.; Mita, G.; Alifano, P.; et al. Characterization of two Pantoea strains isolated from extra-virgin olive oil. AMB Express 2018, 8, 113. [Google Scholar] [CrossRef]
- Zullo, B.A.; Maiuro, L.; Ciafardini, G. Survival of coliform bacteria in virgin olive oil. BioMed Res. Int. 2018, 2018, 8490614. [Google Scholar] [CrossRef]
- Cho, S.B.; Chang, W.K.; Kim, Y.J.; Moon, H.I.; Joo, J.W.; Choi, I.S.; Seo, K.H.; Kim, S.K. Effects of plant oils and minerals for the inhibition of lipase activity of Staphylococcus aureus isolated from fermented pork meat. Korean J. Food Sci. Anim. Resour. 2010, 30, 764–772. [Google Scholar] [CrossRef]
- Najee, H.B.; Alkurjia, D.; Almahdawy, O.; Kamerzan, C.; Marutescu, L.; Gheorghe, I.; Popa, M.; Chifiriuc, M.C.; Lazăr, V. Antimicrobial activity of Olea europaea fatty oil against multi-drug resistant and biofilm forming microorganisms. Not. Sci. Biol. 2018, 10, 498–502. [Google Scholar] [CrossRef][Green Version]
- Dabetic, N.M.; Todorovic, V.M.; Djuricic, I.D.; Antic Stankovic, J.A.; Basic, Z.N.; Vujovic, D.S.; Sobajic, S.S. Grape seed oil characterization: A novel approach for oil quality assessment. Eur. J. Lipid Sci. Technol. 2020, 122, 1900447. [Google Scholar] [CrossRef]
- Silva, L.P.; Joanitti, G.A.; Leite, J.R.S.A.; Azevedo, R.B. Comparative study of the antimicrobial activities and mammalian cytotoxicity of 10 fatty acid-rich oils and fats from animal and vegetable. Nat. Prod. J. 2011, 1, 40–46. [Google Scholar] [CrossRef]
- Sutrisno, S.; Hidayah, S.W.; Sukarianingsih, D.; Rachman, I.B.; Retnosari, R. Antibacterial activity of peanut oil (Arachis hypogaea Linn) and its derivatives (K-soap, FFAs, and FAMEs) against Staphylococcus aureus and Escherichia coli. AIP Conf. Proc. 2021, 2353, 030040. [Google Scholar]
- Chanda, W.; Joseph, T.P.; Guo, X.F.; Wang, W.D.; Liu, M.; Vuai, M.S.; Padhiar, A.A.; Zhong, M.T. Effectiveness of omega-3 polyunsaturated fatty acids against microbial pathogens. J. Zhejiang Univ. Sci. B 2018, 19, 253–262. [Google Scholar] [CrossRef]
- Coraça-Huber, D.C.; Steixner, S.; Wurm, A.; Nogler, M. Antibacterial and anti-biofilm activity of omega-3 polyunsaturated fatty acids against periprosthetic joint infections-isolated multi-drug resistant strains. Biomedicines 2021, 9, 334. [Google Scholar] [CrossRef]
- Padmini, N.; Rashiya, N.; Sivakumar, N.; Kannan, N.D.; Manjuladevi, R.; Rajasekar, P.; Prabhu, N.M.; Selvakumar, G. In vitro and in vivo efficacy of methyl oleate and palmitic acid against ESBL producing MDR Escherichia coli and Klebsiella pneumoniae. Microb. Pathog. 2020, 148, 104446. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Senthilkumar, A.; Venkatesalu, V. Antibacterial and antifungal efficacy of fatty acid methyl esters from the leaves of Sesuvium portulacastrum L. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 775–780. [Google Scholar]
| Lipases | Activity (U) | Oil Hydrolysis at 30 °C and 40 °C Temperatures 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Olive Oil | Almond Oil | Rapeseed Oil | Peanut Oil | Linseed Oil | Menhaden Oil | ||||||||
| 30 | 40 | 30 | 40 | 30 | 40 | 30 | 40 | 30 | 40 | 30 | 40 | ||
| C. rugosa | 21.3 | +++ | ++ | ++ | ++ | +++ | ++ | ++++ | +++ | +++ | +++ | ++ | +++ |
| 2.13 | ++ | ND | ND | ND | ++ | ++ | +++ | +++ | +++ | +++ | ND | +++ | |
| R. miehei | 21.5 | ++ | ++++ | ++++ | ++++ | ND | + | +++ | +++ | +++ | +++ | +++++ | +++++ |
| 2.15 | + | +++ | ++ | +++ | ND | ND | +++ | +++ | +++ | +++ | +++ | +++ | |
| A. niger | 13.2 | ++ | +++ | ++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | +++ | ++++ |
| 1.32 | + | ++ | + | ND | + | ++ | +++ | ++ | ND | +++ | ++ | ++ | |
| R. oryzae | 18.8 | ++++ | +++ | +++ | +++ | +++ | ++ | +++ | ++++ | +++ | +++ | +++ | ++++ |
| 1.88 | + | + | ++ | +++ | + | + | ++ | +++ | ++ | ++ | ++ | +++ | |
| R. niveus | 14.8 | +++ | ++ | +++ | + | + | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| 1.48 | ND | ND | +++ | ND | ND | ++ | ND | ++ | ++ | ++ | ++ | ++ | |
| Fatty Acids | Fatty Acid Concentration (µg/mL Reaction Mixture) | |
|---|---|---|
| Enzyme-Free Control | R. miehei Lipase Treated | |
| Palmitic acid (C16:0) | 141.31 ± 7.74 | 278.38 ± 87.68 |
| Palmitoleic acid (C16:1) | 111.96 ± 6.06 | 227.01 ± 61.75 |
| Hexadecanedioic acid (C16:2) | 7.65 ± 1.45 | 33.28 ± 4.77 ** |
| Stearic acid (C18:0) | 29.51 ± 1.73 | 58.96 ± 18.22 |
| OA (C18:1) | 14.58 ± 2.08 | 43.81 ± 12.61 * |
| LA (C18:2) | 4.67 ± 0.32 | 16.93 ± 4.54 * |
| ALA (C18:3) | 9.02 ± 0.57 | 31.58 ± 7.01 * |
| Stearidonic acid (C18:4) | 4.32 ± 0.07 | 8.52 ± 0.99 ** |
| Arachidic acid (C20:0) | 2.31 ± 0.65 | 4.55 ± 1.16 |
| Eicosenoic acid (C20:1) | 9.27 ± 0.39 | 16.27 ± 3.57 |
| Eicosadienoic acid (C20:2) | n. d. 1 | 5.95 ± 0.17 *** |
| Arachidonic acid (C20:4) | 5.41 ± 0.14 | 7.49 ± 0.49 ** |
| EPA (C20:5) | 13.74 ± 1.09 | 52.85 ± 11.5 * |
| Docosapentaenoic acid (C22:5) | n. d. | 12.02 ± 0.68 *** |
| DHA (C22:6) | n. d. | 18.7 ± 2.91 ** |
| Tetracosenoic acid (C24:1) | n. d. | 8.49 ± 1.16 ** |
| Oil Materials | Growth (%) 1 | ||||
|---|---|---|---|---|---|
| B. subtilis | E. coli | P. putida | S. aureus | ||
| Positive Control | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | |
| Olive oil | |||||
| Enzyme-free | 120.8 ± 8.1 bc | 72.4 ± 6.9 abc | 75.9 ± 7.6 bc | 59.5 ± 5.4 bc | |
| Lipase treated | 125.1 ± 5.2 bd | 83.5 ± 14.7 ab | 88.7 ± 8.4 ab | 78.7 ± 4.3 def | |
| Almond oil | |||||
| Enzyme-free | 113.1 ± 6.9 be | 65.9 ± 13.8 bcd | 101.6 ± 12.1 a | 57.3 ± 5.7 bc | |
| Lipase treated | 103.6 ± 4.4 ae | 94.5 ± 3.7 ad | 94.1 ± 3.5 a | 88.3 ± 7.1 ad | |
| Peanut oil | |||||
| Enzyme-free | 106.5 ± 7.8 ae | 60.8 ± 7.8 be | 101.4 ± 2.5 a | 44.9 ± 2.5 b | |
| Lipase treated | 80.9 ± 6.3 f | 72.9 ± 12.1 ade | 89.3 ± 7.1 ab | 72.7 ± 3.8 cdfg | |
| Rapeseed oil | |||||
| Enzyme-free | 135.8 ± 0.6 dg | 57.6 ± 1.2 bce | 100.3 ± 0.4 a | 61.2 ± 5.7 bf | |
| Lipase treated | 128.5 ± 1.2 cd | 97.2 ± 15.1 ad | 86.1 ± 1.9 abd | 93.8 ± 7.1 ae | |
| Linseed oil | |||||
| Enzyme-free | 113.3 ± 2.2 be | 50.5 ± 7.8 ce | 95.3 ± 11.2 a | 55.1 ± 1.7 bg | |
| Lipase treated | 0.0 ± 0.0 h | 56.1 ± 11.2 bce | 48.8 ± 1.4 e | 74.5 ± 7.2 cdf | |
| Grape seed oil | |||||
| Enzyme-free | 45.7 ± 1.5 i | 43.8 ± 10.1 ce | 91.8 ± 6.3 ab | 50.3 ± 9.2 b | |
| Lipase treated | 0.0 ± 0.0 h | 47.1 ± 10.6 ce | 58.6 ± 3.2 ce | 54.8 ± 3.1 bg | |
| Menhaden fish oil | |||||
| Enzyme-free | 147.9 ± 2.2 g | 52.6 ± 6.7 bce | 83.8 ± 3.5 abd | 100.3 ± 11.9 a | |
| Lipase treated | 0.0 ± 0.0 h | 52.3 ± 18.2 bce | 69.2 ± 2.6 cd | 69.2 ± 0.7 cfg | |
| Fatty Acids | B. subtilis | E. coli | P. putida | S. aureus |
|---|---|---|---|---|
| PA (C16:0) | 0.686 | 0.667 | 0.405 | 0.559 |
| SA (C18:0) | 0.613 | 0.562 | 0.345 | 0.364 |
| OA (C18:1) | 0.625 | 0.547 | 0.416 | 0.291 |
| LA (C18:2) | 0.976 ** | 0.911 ** | 0.931 ** | 0.757 * |
| ALA (C18:3) | 0.674 | 0.523 | 0.806 * | 0.171 |
| Fatty Acids | MIC (µg/mL) | |||
|---|---|---|---|---|
| B. subtilis | E. coli | P. putida | S. aureus | |
| ALA (C18:3) | 125 | >1000 | >1000 | >1000 |
| EPA (C20:5) | 62.5 | >1000 | 500 | >1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotogán, A.; Furka, Z.T.; Kovács, T.; Volford, B.; Papp, D.A.; Varga, M.; Huynh, T.; Szekeres, A.; Papp, T.; Vágvölgyi, C.; et al. Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential. Foods 2022, 11, 1711. https://doi.org/10.3390/foods11121711
Kotogán A, Furka ZT, Kovács T, Volford B, Papp DA, Varga M, Huynh T, Szekeres A, Papp T, Vágvölgyi C, et al. Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential. Foods. 2022; 11(12):1711. https://doi.org/10.3390/foods11121711
Chicago/Turabian StyleKotogán, Alexandra, Zsófia Terézia Furka, Tamás Kovács, Bettina Volford, Dóra Anna Papp, Mónika Varga, Thu Huynh, András Szekeres, Tamás Papp, Csaba Vágvölgyi, and et al. 2022. "Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential" Foods 11, no. 12: 1711. https://doi.org/10.3390/foods11121711
APA StyleKotogán, A., Furka, Z. T., Kovács, T., Volford, B., Papp, D. A., Varga, M., Huynh, T., Szekeres, A., Papp, T., Vágvölgyi, C., Mondal, K. C., Kerekes, E. B., & Takó, M. (2022). Hydrolysis of Edible Oils by Fungal Lipases: An Effective Tool to Produce Bioactive Extracts with Antioxidant and Antimicrobial Potential. Foods, 11(12), 1711. https://doi.org/10.3390/foods11121711










