Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Tea Processing and Sample Treatment
2.2.1. Withering
2.2.2. Rolling
2.2.3. Fermenting
2.2.4. Drying
2.3. Tea Sensory Quality Evaluation
2.4. Analysis of the Volatile Metabolites
2.4.1. Sample Preparation
2.4.2. GC-MS/MS Analysis
2.4.3. Qualitative and Quantitative Analyses
2.4.4. OAV Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of STL Additions on the Sensory Qualities of BT
3.2. Effects of STL Addition on BT VMs
3.2.1. Identification of VMs
3.2.2. MEV Analysis
3.2.3. PLS-DA Analysis
3.2.4. Analysis of Differential VMs
3.2.5. OAV Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Qi, R.L.; Mine, Y. The impact of oolong and black tea polyphenols on human health. Food Biosci. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Wang, H.J.; Shen, S.; Wang, J.J.; Jiang, Y.W.; Li, J.; Yang, Y.Q.; Hua, J.J.; Yuan, H.B. Novel insight into the effect of fermentation time on quality of Yunnan Congou black tea. LWT-Food Sci. Technol. 2022, 155, 112939. [Google Scholar] [CrossRef]
- Hua, J.J.; Wang, H.J.; Jiang, Y.W.; Li, J.; Wang, J.J.; Yuan, H.B. Influence of enzyme source and catechins on theaflavins formation during in vitro liquid-state fermentation. LWT-Food Sci. Technol. 2021, 139, 110291. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Hua, J.J.; Yuan, H.B. Status analysis and development prospects of black tea industry in China. China Tea Process. 2018, 4, 5–10. [Google Scholar] [CrossRef]
- Liu, X.G.; Chen, M.C.; Zhu, Y.J.; Zhang, H.F.; Liu, B. Study on the flavor components in Chinese jasmine black tea. Fujian J. Agric. Sci. 2017, 32, 297–298. [Google Scholar] [CrossRef]
- Chen, H.M.; Shi, Z.G.; Di, T.M.; Hu, J.H.; Zhang, Z.Q.; Zhang, X.F. The scenting technology and aroma analysis by HS-SPME/GC-O-MS for Osmanthus black tea. Mod. Food Sci. Technol. 2018, 34, 243–254. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Xu, F.; Tan, L.H.; Zhang, C.L.; Gu, L.F. GC/MS analysis of volatiles in strobilanthes tonkinensis leaf extracted by headspace solid-phase microextraction. Chin. J. Trop. Crops 2015, 36, 603–610. [Google Scholar] [CrossRef]
- Srikun, N. In vitro propagation of the aromatic herb strobilanthes tonkinensis lindau. Agric. Nat. Resour. 2017, 51, 15–19. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, F.K.; Tan, L.H.; Zhu, H.Y. Preliminary study on the nutritional composition of Semmostachya menglaensis. Chin. J. Trop. Crops 2011, 32, 749–751. [Google Scholar] [CrossRef]
- Xu, F.; Tan, L.H.; Chen, P.; Zhu, H.Y.; Hu, R.S.; Zhang, C.L. Determination of amino acids in Strobilanthes tonkinensis Landau leaves by OPA-FMOC pre-column derivatization and RP-HPLC. Chin. J. Trop. Crops 2012, 32, 1482–1486. [Google Scholar] [CrossRef]
- Yang, T.; Wu, Q.; Li, S.Y.; Lv, Z.J.; Hu, B.; Xie, B.J.; Sun, Z.D. Liposoluble compounds with antioxidant activity from strobilanthes tonkinensis. Chem. Nat. Compd. 2014, 49, 1166–1167. [Google Scholar] [CrossRef]
- Tan, L.H.; Yin, G.H.; Zhang, C.H.; Liu, H. Supercritical CO2 Extraction and GC-MS Analysis of Leaf Volatile Constituents in Teucrium manghuaense. Chin. J. Trop. Crops 2008, 29, 530–534. [Google Scholar]
- Li, W.L.; Ma, Y.H.; Zhang, Y.P.; Gao, M.; Fang, J. Study on the constituents of volatile oils of nuomi-scented tea. J. Southwest Univ. (Nat. Sci. Ed.) 2009, 31, 53–56. [Google Scholar] [CrossRef]
- Yin, G.H.; Zhang, C.H.; Shi, H.M.; Li, X.B. Study on the biological activities of ethanol extract of Teucrium Manghuaense. Food Res. Dev. 2009, 30, 18–20. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, H.Y. Classification of Teucrium manghuaense by Using an Electronic Tongue. Chin. J. Trop. Agric. 2011, 31, 73–76. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.N.; Ran, Q.; Huang, Q.W.; Wang, J. Strobilanthes cusia (nees) kuntze, a multifunctional traditional Chinese medicinal plant, and its herbal medicines: A comprehensive review. J. Ethnopharmacol. 2020, 265, 113325. [Google Scholar] [CrossRef]
- Hua, J.J.; Xu, Q.; Yuan, H.B.; Wang, J.J.; Wu, Z.Q.; Li, X.T.; Jiang, Y.W. Effects of novel fermentation method on the biochemical components change and quality formation of congou black tea. J. Food Compos. Anal. 2021, 96, 103751. [Google Scholar] [CrossRef]
- Hua, J.J.; Yuan, H.B.; Wang, W.W.; Jiang, Y.W.; Liu, Q.L.; Chen, G.S.; Wang, F. Effect of withering temperature on dynamic change of main biochemical components and enzymatic activity of tea fresh leaves. J. Tea Sci. 2015, 35, 73–81. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Yin, H.X.; Yuan, H.B.; Jiang, Y.W.; Dong, C.W.; Deng, Y.L. Characterization of the volatile components in green tea by IRAE-HS-SPME/GC-MS combined with multivariate analysis. PLoS ONE 2018, 13, e0193393. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Hua, J.J.; Jiang, Y.W.; Yang, Y.Q.; Wang, J.J.; Yuan, H.B. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Res. Int. 2020, 136, 109479. [Google Scholar] [CrossRef]
- Wang, H.J.; Hua, J.J.; Yu, Q.Y.; Li, J.; Wang, J.J.; Deng, Y.L.; Yuan, H.B.; Jiang, Y.W. Widely targeted metabolomic analysis reveals dynamic changes in non-volatile and volatile metabolites during green tea processing. Food Chem. 2021, 363, 130131. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Hua, J.J.; Wang, B.; Yuan, H.B.; Ma, H.L. Effects of variety, season, and region on theaflavins content of fermented Chinese congou black tea. J. Food Qual. 2018, 2018, 5427302. [Google Scholar] [CrossRef]
- Hua, J.J.; Wang, H.J.; Wang, J.J.; Li, J.; Jiang, Y.W.; Wang, Y.L.; Yuan, H.B. Influences of first-drying methods on the quality of Congou black tea using partial least squares-discrimination analysis. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2020, 36, 260–270. [Google Scholar] [CrossRef]
- Zhang, W.J. Study on New Processing Technology of Teucrium manghuaense Tea. Master’s Thesis, Huazhong Agriculture University, Wuhan, China, 2010; pp. 9–17. [Google Scholar]
- Wang, H.J.; Wang, J.J.; Yuan, H.B.; Shen, S.; Li, J.; Hua, J.J.; Jiang, Y.W. Novel insights into the effect of withering degree on Dianhong Congou black tea quality. Int. J. Food Sci. Technol. 2022, 57, 3713–3726. [Google Scholar] [CrossRef]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.P.; Zhang, Y.; Dai, W.D.; Guo, L.; Tan, J.F.; Peng, Q.H.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Schuwab, W.; Ho, C.T.; Song, C.K.; Wan, X.C. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC–MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Liao, X.L.; Yan, J.N.; Wang, B.; Meng, Q.; Zhang, L.Y.; Tong, H.R. Identification of key odorants responsible for cooked corn-like aroma of green teas made by tea cultivar ‘zhonghuang 1’. Food Res. Int. 2020, 136, 109355. [Google Scholar] [CrossRef]
- Zhu, J.C.; Niu, Y.W.; Xiao, Z.B. Characterization of the key aroma compounds in Laoshan green teas by application of odour activity value (OAV), gas chromatography-mass spectrometry-olfactometry (GC-MS-O) and comprehensive two-dimensional gas chromatography mass spectrometry (GC × GC-qMS). Food Chem. 2021, 339, 128136. [Google Scholar] [CrossRef]
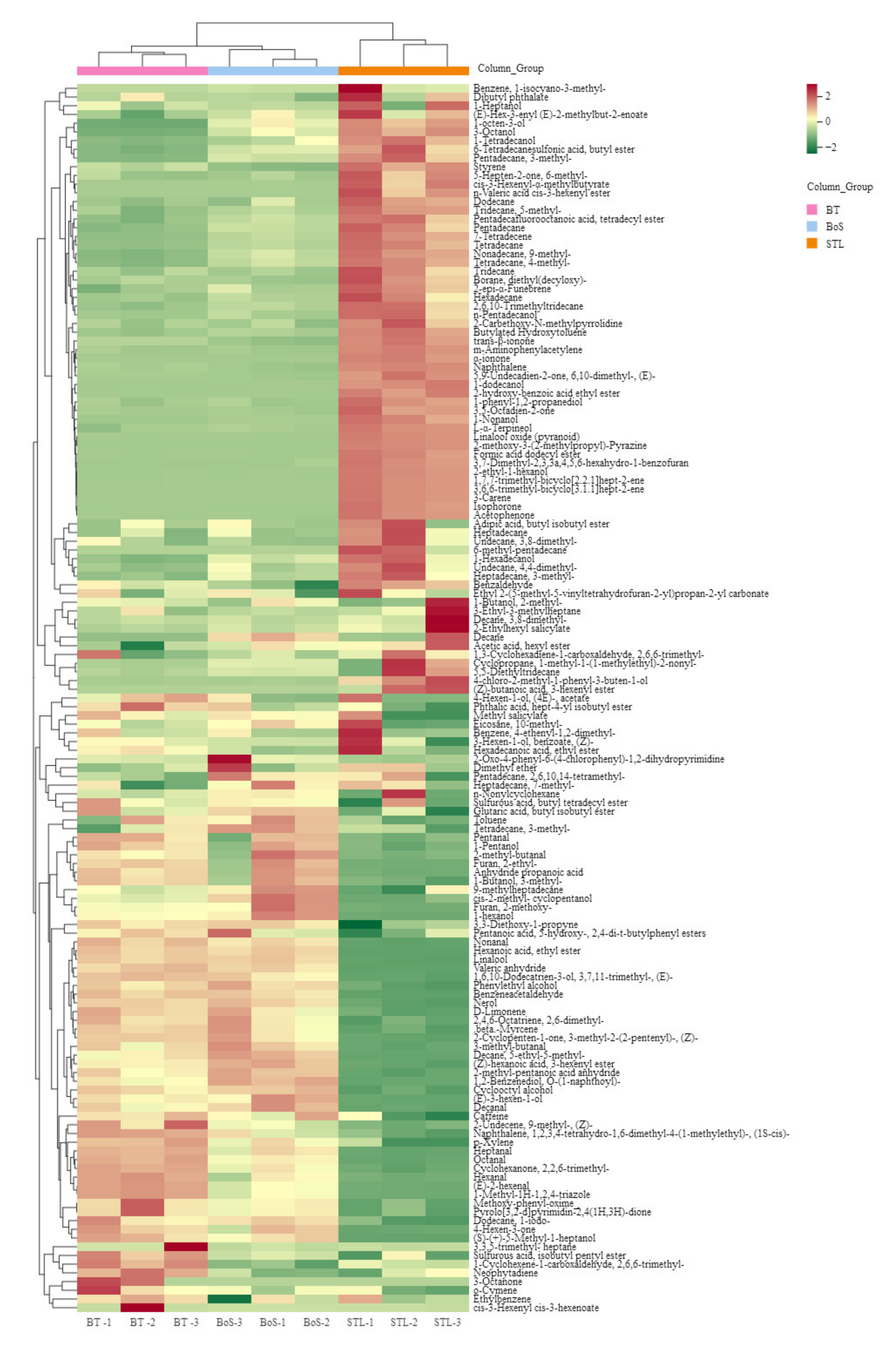
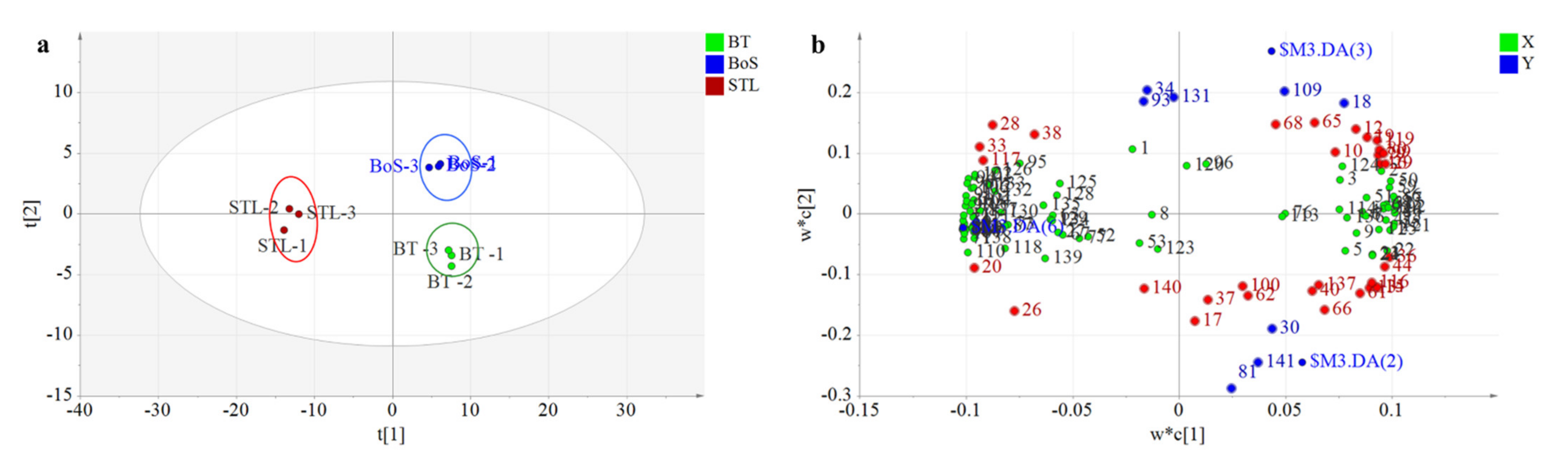
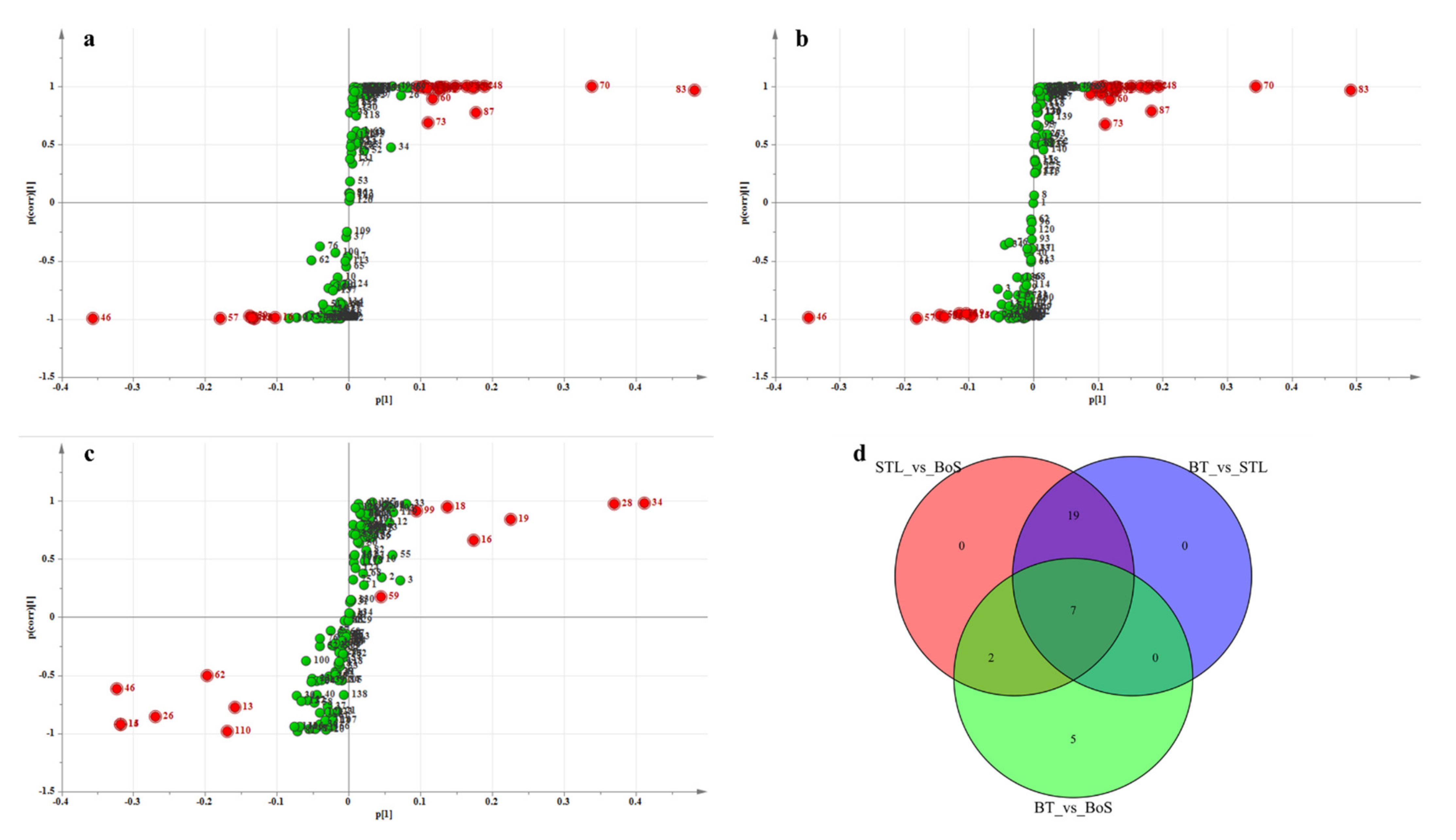
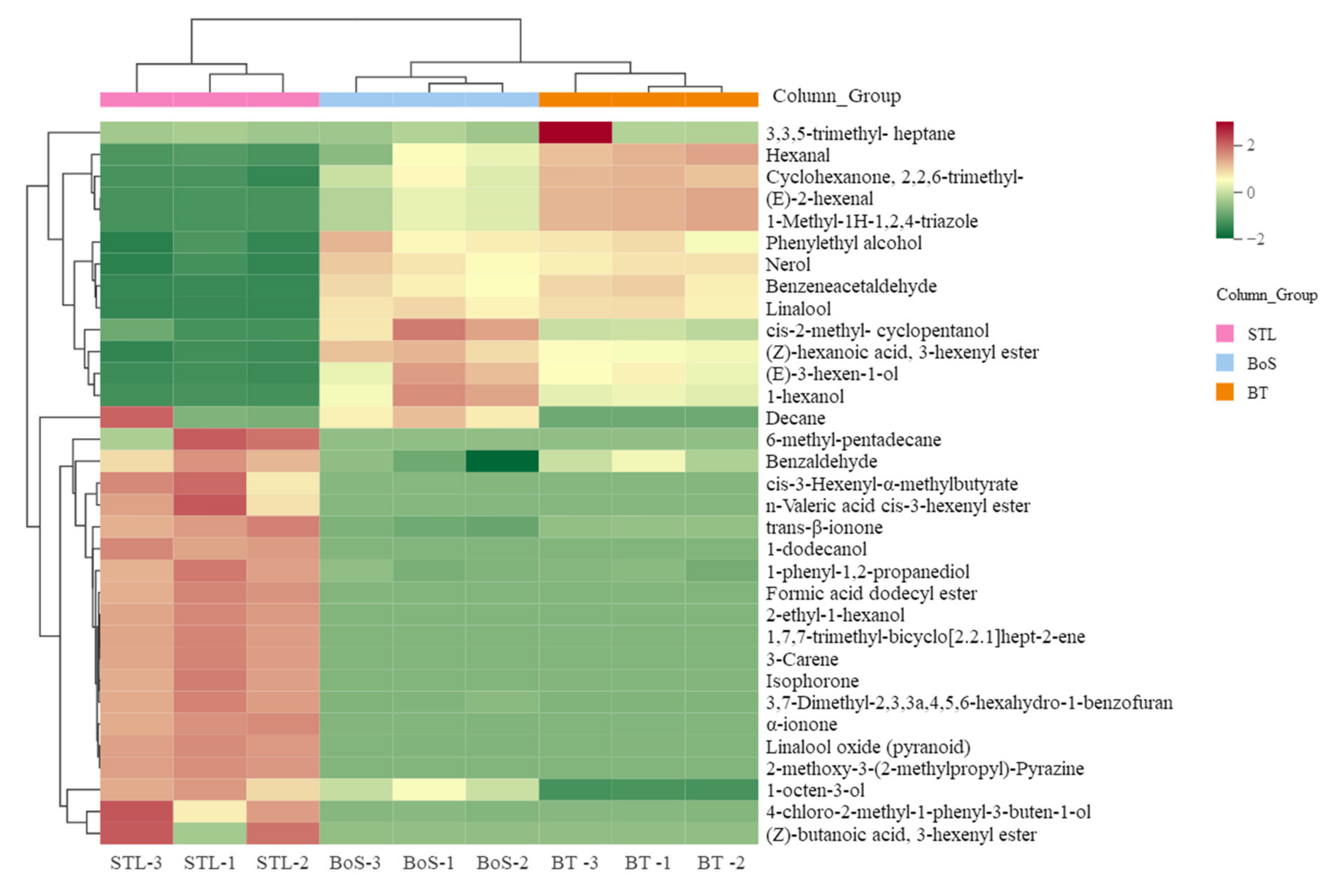
| Added Treatment | Types of Additives during BT Processing | Amounts of Additives (STL/Tea Leaves) |
|---|---|---|
| #1 | STL/withered tea leaves | 20/1500 g/g |
| #2 | STL/rolled tea leaves | 20/1500 g/g |
| #3 | STL/fermented tea leaves | 20/1500 g/g |
| #4 | STL/rolled tea leaves | 10/1500 g/g |
| #5 | STL/rolled tea leaves | 30/1500 g/g |
| Blank control | No additives | - |
| STL Treatment | Appearance (25%) | Liquor Color (10%) | Aroma (25%) | Taste (30%) | Infused Leaf (10%) | Total Score | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Evaluation | Score | Evaluation | Score | Evaluation | Score | Evaluation | Score | Evaluation | Score | ||
| #1 (STL/withered tea leaves with 20/1500) | curly, tight, red, and glossy, uniform | 90.0 ± 0.3 a | red, clarity | 90.2 ± 0.3 a | strong glutinous rice flavor, covering tea aroma | 87.0 ± 0.4 c | strong glutinous rice taste, covering tea taste | 86.8 ± 0.3 c | red bright, stem with buds | 89.9 ± 0.3 a | 88.3 ± 0.3 c |
| #2 (STL/rolled tea leaves with 20/1500) | curly, tight, red, and glossy, uniform | 89.8 ± 0.3 a | red, clarity | 90.0 ± 0.2 a | sweet, with glutinous rice flavor, strong, lasting | 92.5 ± 0.3 a | sweet mellow, thick, smooth | 92.7 ± 0.2 a | red bright, stem with buds | 89.8 ± 0.2 a | 91.4 ± 0.2 a |
| #3 (STL/fermented tea leaves with 20/1500) | curly, tight, red, and glossy, uniform | 90.1 ± 0.2 a | red, clarity | 89.9 ± 0.2 a | sweet, with a little glutinous rice flavor | 90.0 ± 0.4 b | sweet mellow, a little thick | 90.6 ± 0.3 b | red bright, stem with buds | 90.0 ± 0.4 a | 90.2 ± 0.3 b |
| #4 (STL/rolled tea leaves with 10/1500) | curly, tight, red, and glossy, uniform | 90.0 ± 0.3 a | red, clarity | 90.0 ± 0.3 a | sweet, with a little glutinous rice flavor | 90.2 ± 0.3 b | sweet mellow, a little thick | 90.9 ± 0.3 b | red bright, stem with buds | 89.8 ± 0.2 a | 90.3 ± 0.3 b |
| #5 (STL/rolled tea leaves with 30/1500) | curly, tight, red, and glossy, uniform | 89.9 ± 0.3 a | red, clarity | 89.8 ± 0.2 a | strong glutinous rice flavor, covering tea aroma | 86.7± 0.3 c | strong glutinous rice taste, covering tea taste | 86.3 ± 0.4 c | red bright, stem with buds | 89.9 ± 0.3 a | 88.0 ± 0.3 c |
| Blank control (No additives) | curly, tight, red, and glossy, uniform | 90.0 ± 0.2 a | red, clarity | 90.1 ± 0.3 b | sweet, lasting | 89.6 ± 0.5 b | sweet mellow | 90.3 ± 0.5 b | red bright, stem with buds | 89.8 ± 0.3 a | 90.0 ± 0.4 b |
| No. | Name | OTs (µg/kg) A | Aroma Characteristics B | OAV | ||
|---|---|---|---|---|---|---|
| BT | BoS | STL | ||||
| 13 | Hexanal | 10 | Fresh, green, fruity, sweaty | 39.553 ± 2.031 a | 22.318 ± 8.438 b | 4.222 ± 0.743 c |
| 14 | (E)-2-hexenal | 13 | Green, banana, fatty cheesy | 93.353 ± 2.995 a | 50.848 ± 8.973 b | 3.267 ± 0.646 c |
| 16 | (E)-3-hexen-1-ol | 70 | Green, floral, oily, earthy | 11.061 ± 0.892 b | 13.756 ± 3.272 a | 0.823 ± 0.162 c |
| 19 | 1-hexanol | 500 | Ethereal, fruity, sweet, green | 0.978 ± 0.038 b | 1.502 ± 0.383 a | 0.040 ± 0.010 c |
| 26 | Benzaldehyde | 3 | Almond, caramel flavor | 390.533 ± 34.965 b | 265.668 ± 77.463 c | 515.784 ± 36.185 a |
| 28 | 1-octen-3-ol | 1 | Mushroom, green, oily | 169.18 ± 9.072 c | 836.963 ± 130.754 b | 1366.109 ± 150.229 a |
| 42 | 2-ethyl-1-hexanol | 270,000 | Citrus, fresh, floral, oily, sweet | 0.000 ± 0.000 b | 0.000 ± 0.000 b | 0.008 ± 0.001 a |
| 46 | Benzeneacetaldehyde | 4 | Woody, sweet, honey | 2289.967 ± 158.374 a | 2125.484 ± 188.864 a | 139.284 ± 23.827 b |
| 48 | Isophorone | 2 | Woody, sweet, green, tobacco | 20.439 ± 0.611 b | 12.556 ± 0.807 c | 1211.616 ± 126.046 a |
| 57 | Linalool | 6 | Citrus, floral, sweet, woody | 374.445 ± 19.218 a | 371.961 ± 22.024 a | 15.894 ± 3.487 b |
| 59 | Phenylethyl alcohol | 750 | Sweet, floral, fresh | 2.380 ± 0.19 a | 2.516 ± 0.296 a | 0.615 ± 0.152 b |
| 70 | Linalool oxide (pyranoid) | 320 | Floral, honey | 0.600 ± 0.054 b | 0.545 ± 0.085 b | 24.518 ± 1.156 a |
| 72 | 2-methoxy-3-(2-methylpropyl)-pyrazine | 0.015 | Green, pea flavor, galbanum | 186.988 ± 8.858 b | 205.408 ± 6.493 b | 40984.985 ± 1488.308 a |
| 75 | 1-dodecanol | 0.066 | Waxy, floral, honey, coconut | 15.519 ± 13.684 b | 20.815 ± 18.117 b | 30780.489 ± 2020.076 a |
| 86 | Nerol | 300 | Sweet, floral, neroli, magnolia | 4.699 ± 0.146 a | 4.751 ± 0.452 a | 0.566 ± 0.235 b |
| 105 | α-ionone | 0.4 | Sweet, woody, floral | 71.301 ± 1.292 b | 67.817 ± 6.416 b | 1819.192 ± 135.53 a |
| 110 | trans-β-ionone | 0.007 | Violet-like, floral, raspberry-like | 59942.082 ± 444.755 b | 40619.259 ± 5716.759 c | 177451.466 ± 13572.469 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, J.; Li, J.; Ouyang, W.; Wang, J.; Yuan, H.; Jiang, Y. Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content. Foods 2022, 11, 1678. https://doi.org/10.3390/foods11121678
Hua J, Li J, Ouyang W, Wang J, Yuan H, Jiang Y. Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content. Foods. 2022; 11(12):1678. https://doi.org/10.3390/foods11121678
Chicago/Turabian StyleHua, Jinjie, Jia Li, Wen Ouyang, Jinjin Wang, Haibo Yuan, and Yongwen Jiang. 2022. "Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content" Foods 11, no. 12: 1678. https://doi.org/10.3390/foods11121678
APA StyleHua, J., Li, J., Ouyang, W., Wang, J., Yuan, H., & Jiang, Y. (2022). Effect of Strobilanthes tonkinensis Lindau Addition on Black Tea Flavor Quality and Volatile Metabolite Content. Foods, 11(12), 1678. https://doi.org/10.3390/foods11121678







