In Vivo Bioconcentration, Distribution and Metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dilution Water and Testing Solution Characteristics
2.2. Stability of BP-3 in Water
2.3. BP-3 Water Concentrations
2.4. Exposed Fish Observations
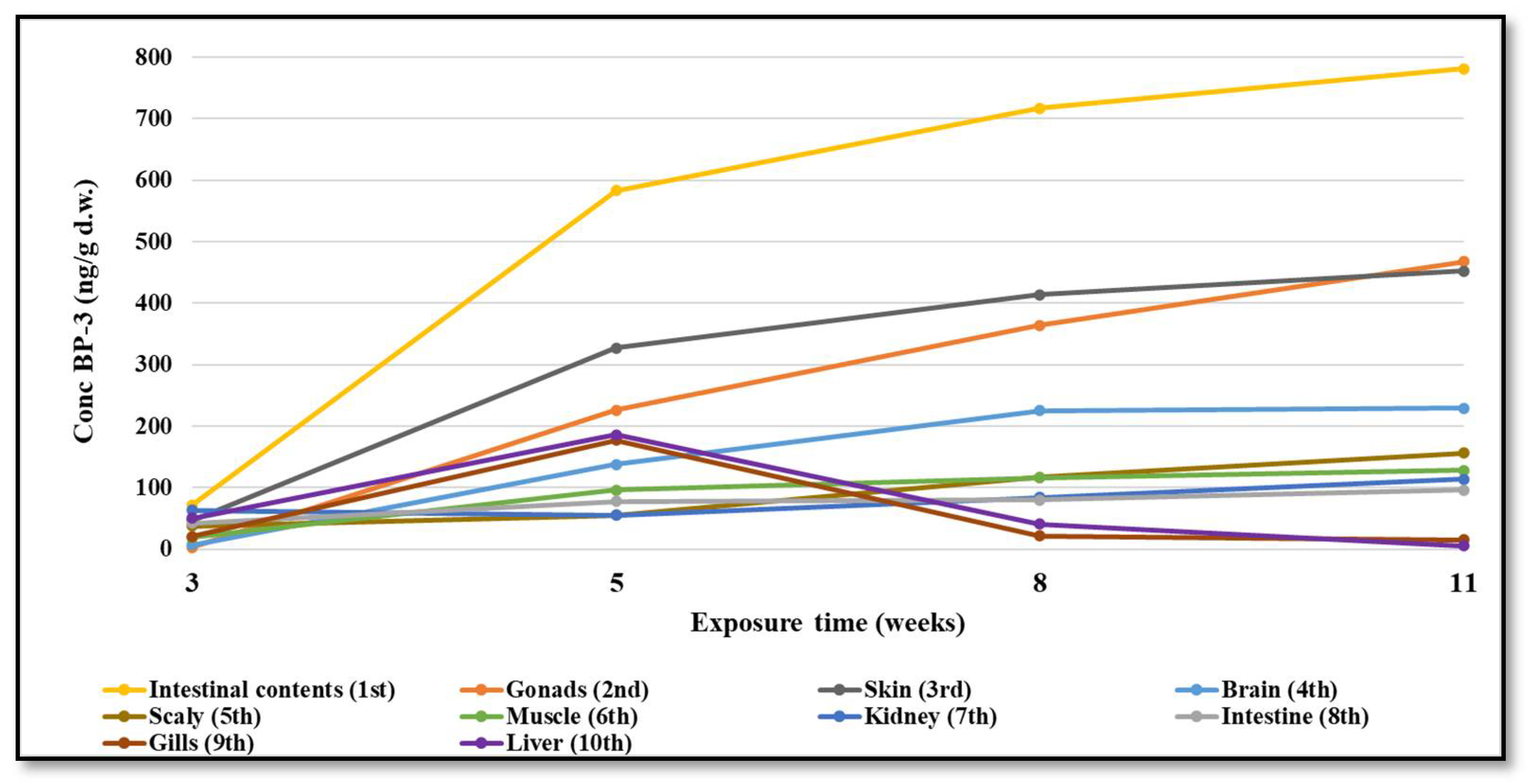
2.5. BP-3 Organs and Tissue Distribution
2.6. Bioconcentration Factors of BP-3
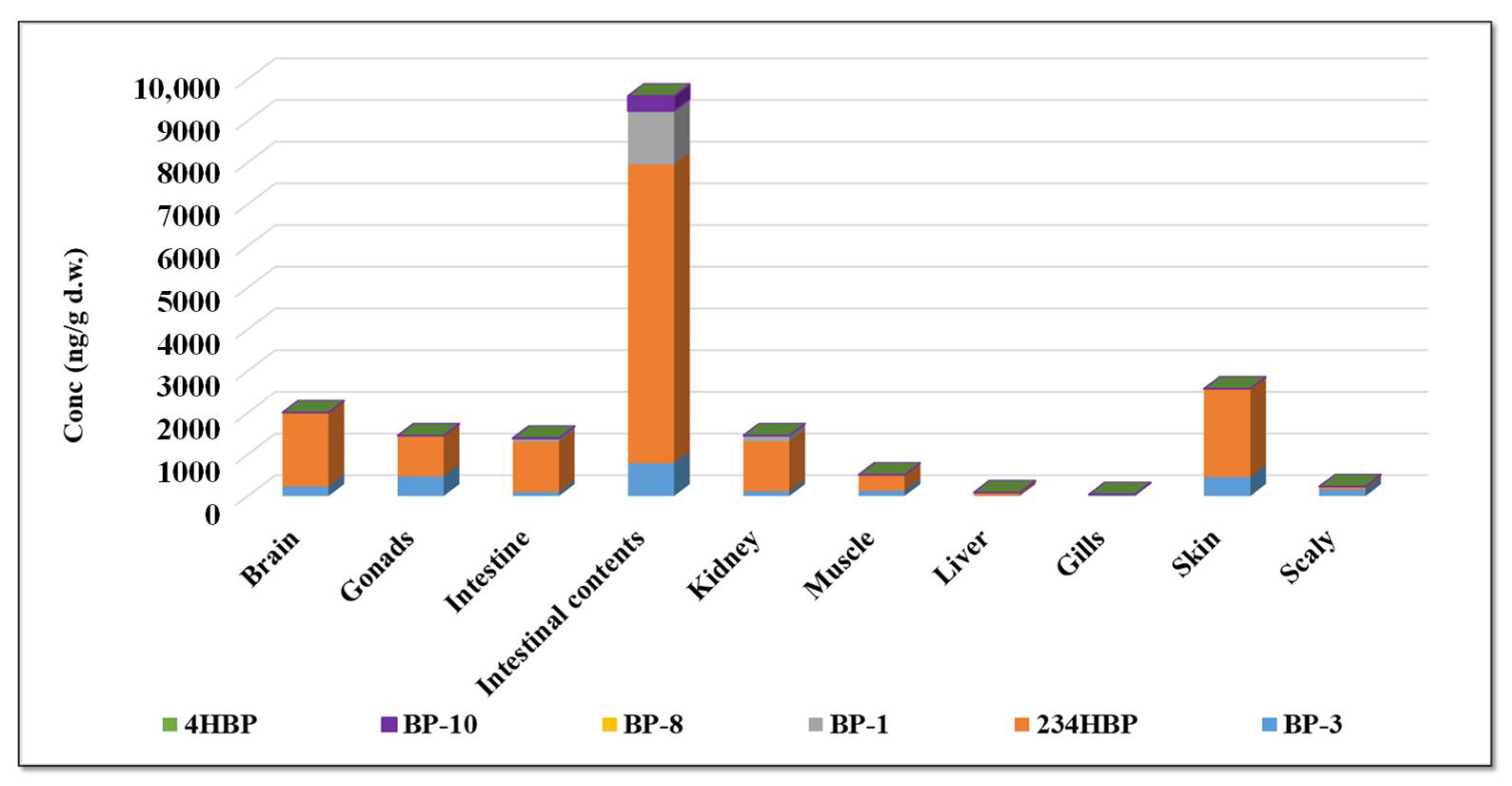
2.7. BP-3 Metabolites in Fish Organs and Tissue
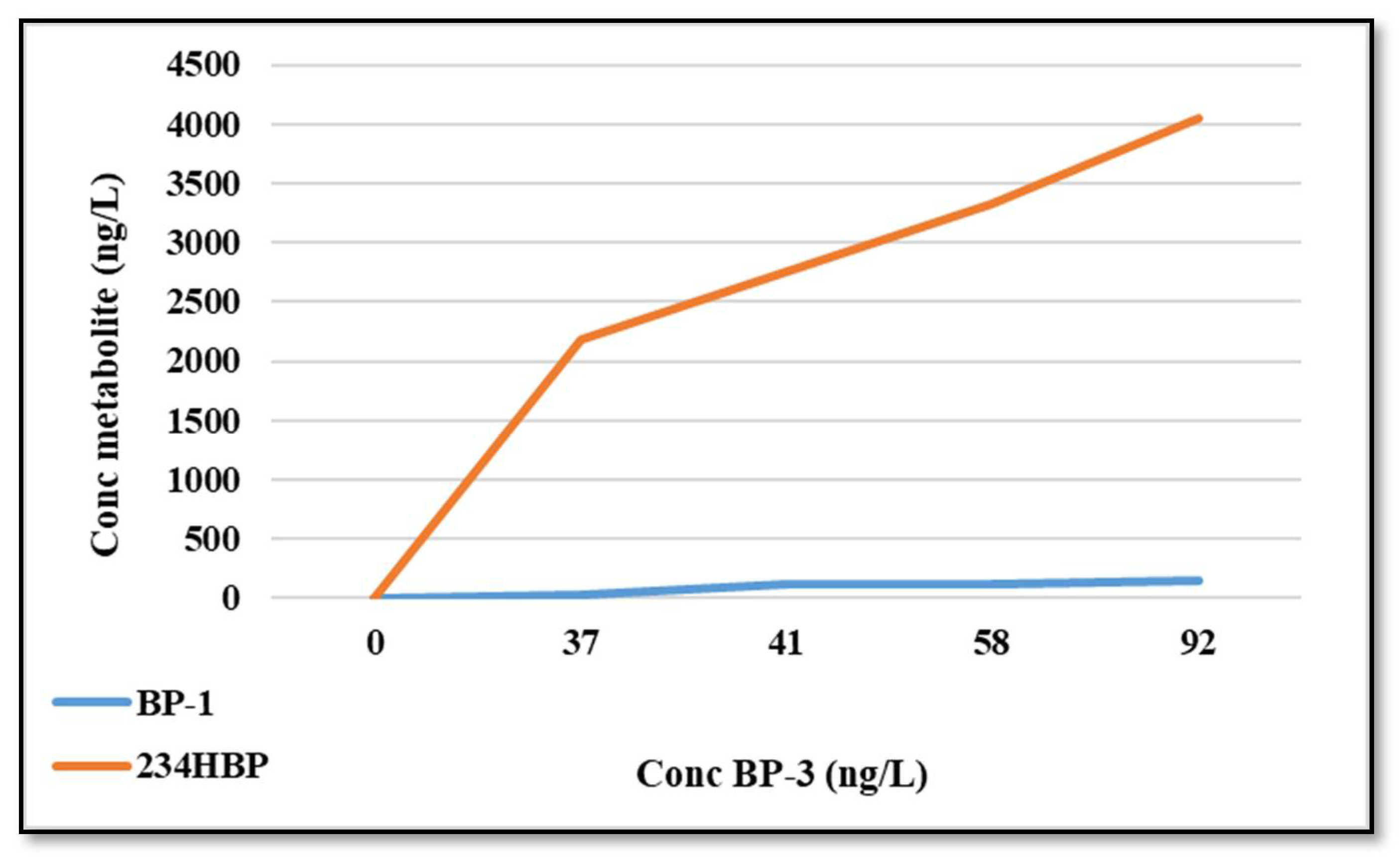
2.8. BP-3 Metabolites in Water
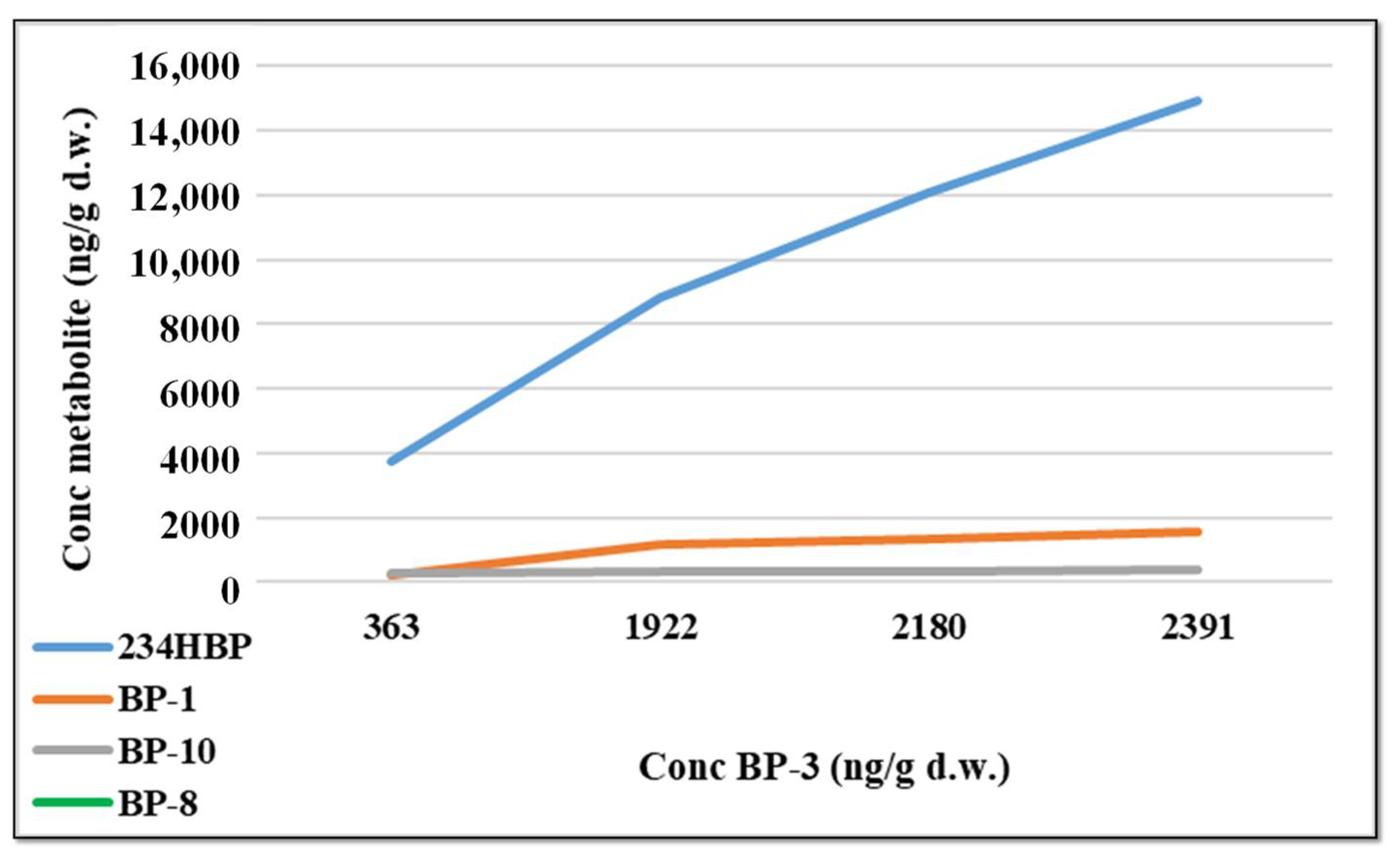
2.9. Relationships between BP-3 and Corresponding Metabolites in Fish Sample
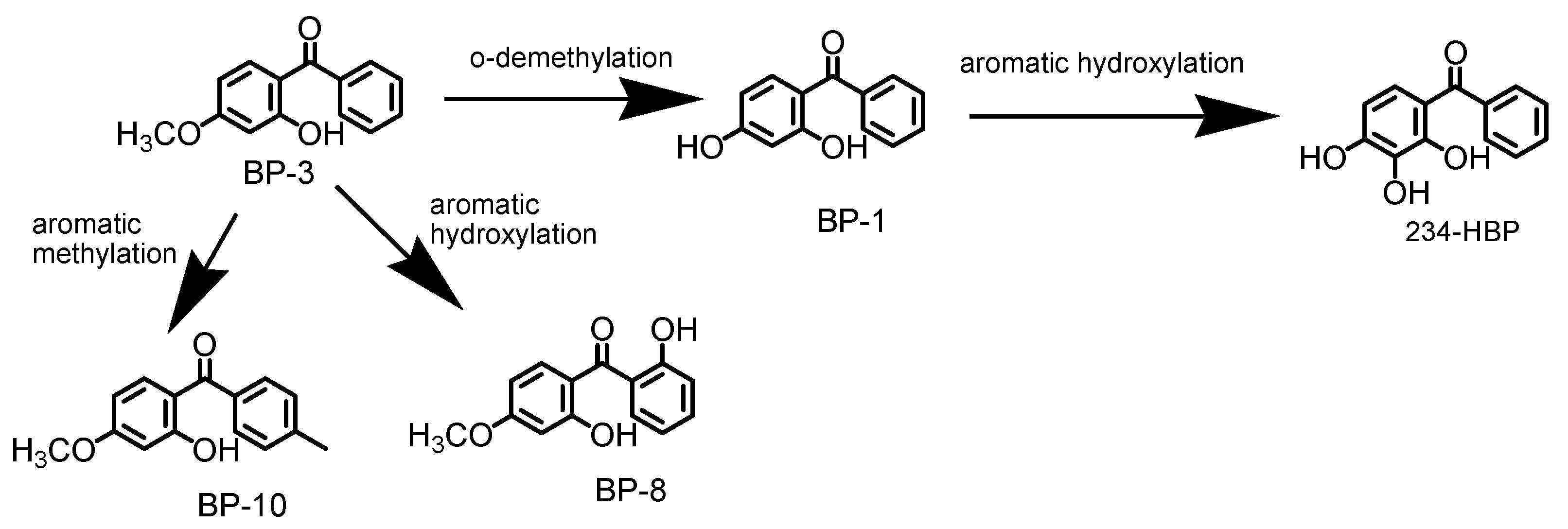
2.10. BP-3 Metabolization Pathway in Fish Organs
3. Materials and Methods
3.1. Chemicals and Materials
3.2. LC-MS/MS Analytical Methods
3.3. Quality Assurance/Quality Control
3.4. Laboratory Conditions
3.4.1. Exposure Condition
3.4.2. Water Analysis
3.4.3. Biota Analysis
3.4.4. Matrix Effects
3.4.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Huang, Y.; Zhao, Y.; Zhou, Y.; Wang, H.; Zhang, L.; Shi, H.; Leung, K.S.-Y.; Zhang, Y. Organic UV filters mixture exposure and childhood adiposity: A prospective follow-up study in China. Environ. Int. 2022, 158, 106912. [Google Scholar] [CrossRef] [PubMed]
- Stien, D.; Clergeaud, F.; Rodrigues, A.M.S.; Lebaron, K.; Pillot, R.; Romans, P.; Fagervold, S.; Lebaron, P. Metabolomics reveal that Octocrylene accumulates in Pocillopora damicornis tissues as fatty acid conjugates and triggers coral cell mitochondrial dysfunction. Anal. Chem. 2019, 91, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Rodil, R.; Quintana, J.B.; Concha-Grana, E.; Lopez-Mahia, P.; Muniategui-Lorenzo, S.; Prada-Rodriguez, D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012, 86, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.E.; Buser, H.-R.; Müller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from swiss lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef] [PubMed]
- ECHA Database. Available online: https://echa.europa.eu/ro/registration-dossier/-/registered-dossier/5515 (accessed on 1 December 2021).
- Paredes, E.; Perez, S.; Rodil, R.; Quintana, J.B.; Beiras, R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014, 104, 44–50. [Google Scholar] [CrossRef]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen UV Filter, Oxybenzone (Benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef]
- Li, M.H. Acute toxicity of benzophenone-type UV filters and paraben preservatives to freshwater planarian, Dugesia japonica. Toxicol. Environ. Chem. 2012, 2094, 566–573. [Google Scholar] [CrossRef]
- Chen, T.H.; Wu, Y.T.; Ding, W.H. UV-filter benzophenone-3 inhibits agonistic behavior in male Siamese fighting fish (Betta splendens). Ecotoxicology 2016, 25, 302–309. [Google Scholar] [CrossRef]
- Kim, S.; Jung, D.; Kho, Y.; Choi, K. Effects of benzophenone-3 exposure on endocrine disruption and reproduction of Japanese medaka (Oryzias latipes)—A two generation exposure study. Aquat Toxicol. 2014, 155, 244–252. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, G.; Sandoval-Gío, J.J.; Arroyo-Silva, A.; Noreña-Barroso, E.; Escalante-Herrera, K.S.; Olvera-Espinosa, F. Evaluation of the estrogenic and oxidative stress effects of the UV filter 3-benzophenone in zebrafish (Danio rerio) eleuthero-embryos. Ecotoxicol. Environ. Saf. 2015, 115, 14–18. [Google Scholar] [CrossRef]
- Ziarrusta, H.; Mijangos, L.; Montes, R.; Rodil, R.; Anakabe, E.; Izagirre, U.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Study of bioconcentration of oxybenzone in gilt-head bream and characterization of its by-products. Chemosphere 2018, 208, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Mastroianni, N.; Diaz-Cruz, M.S.; Barceló, D. Fully automated determination of nine ultraviolet filters and transformation products in natural waters and wastewaters by on-line solid phase extraction–liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1294, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kannan, K. Mass loading and emission of benzophenone-3 (BP-3) and its derivatives in wastewater treatment plants in New York State, USA. Sci. Total Environ. 2017, 579, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Paun, I.; Pirvu, F.; Iancu, V.; Galaon, T. Distribution, removal efficiencies and environmental risk assessment of benzophenone and salicylate UV filters in WWTPs and surface waters from Romania. New J. Chem. 2021, 45, 2478–2487. [Google Scholar] [CrossRef]
- Chiriac, F.L.; Pirvu, F.; Paun, I. Investigation of endocrine disruptor pollutants and their metabolites along the Romanian Black Sea Coast: Occurrence, distribution and risk assessment. Environ. Toxicol. Pharmacol. 2021, 86, 103673. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Oxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Díaz-Cruz, M.S.; Barceló, D. UV filters bioaccumulation in fish from Iberian River basins. Sci. Total Environ. 2015, 518–519, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Klotz, K.; Hof, K.; Hiller, J.; Goen, T.; Drexler, H. Quantification of prominent organic UV filters and their metabolites in human urine and plasma samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1125, 121706. [Google Scholar] [CrossRef]
- Montes-Grajales, D.; Fennix-Agudelo, M.; Miranda-Castro, W. Occurrence of personal care products as emerging chemicals of concern in water resources: A review. Sci. Total Environ. 2017, 595, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Occurrence and environmental hazard of organic UV filters in seawater and wastewater from Gran Canaria Island (Canary Islands, Spain). Environ. Pollut. 2022, 300, 118843. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Linan, L.; Villaverde-de-Saa, E.; Rodil, R.; Quintana, J.B.; Beiras, R. Bioaccumulation of UV filters in Mytilus galloprovincialis mussel. Chemosphere 2018, 190, 267–271. [Google Scholar] [CrossRef]
- Gomez, E.; Bachelot, M.; Boillot, C.; Munaron, D.; Chiron, S.; Casellas, C.; Fenet, H. Bioconcentration of two pharmaceuticals (benzodiazepines) and two personal care products (UV filters) in marine mussels (Mytilus galloprovincialis) under controlled laboratory conditions. Environ. Sci. Pollut. Res. 2012, 19, 2561–2569. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.P.; Tan, C.J.; Ng, K.Y.; Guo, F.W.; Wang, L.H.; Chen, T.H.; Fan, T.Y.; Lam, P.K.S.; Murphy, M.B. Comparative toxicities of four benzophenone ultraviolet filters to two life stages of two coral species. Sci. Total Environ. 2019, 651, 2391–2399. [Google Scholar] [CrossRef]
- Kunz, P.Y.; Galicia, H.F.; Fent, K. Comparison of In Vitro and In Vivo Estrogenic Activity of UV Filters in Fish. Toxicol. Sci. 2006, 90, 349–361. [Google Scholar] [CrossRef]
- Celino-Brady, F.T.; Lerner, D.T.; Seale, A.P. Experimental Approaches for Characterizing the Endocrine-Disrupting Effects of Environmental Chemicals in Fish. Front. Endocrinol. 2021, 11, 619361. [Google Scholar] [CrossRef]
- He, K.; Hain, E.; Timm, A.; Blaney, L. Bioaccumulation of estrogenic hormones and UV-filters in red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 2021, 764, 142871. [Google Scholar] [CrossRef]
- NITE; Chemical Risk Information Platform (CHRIP). Biodegradation and Bioconcentration; National Institute of Technology and Evaluation: Tokyo, Japan, 2017; Available online: https://www.safe.nite.go.jp/english/db.html (accessed on 1 December 2021).
- Franke, C.; Studinger, G.; Berger, G.; Böhling, S.; Bruckmann, U.; Cohors-Fresenborg, D.; Jöhncke, U. The assessment of bioaccumulation. Chemosphere 1994, 29, 1501–1514. [Google Scholar] [CrossRef]
- Peng, X.; Fan, Y.; Jin, J.; Xiong, S.; Liu, J.; Tang, C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ. Pollut. 2017, 225, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Ziarrusta, H.; Mijangos, L.; Picart, S.; Mireia Irazola, A.; Perera-Lluna, A.; Usobiaga, A.; Prieto, A.; Etxebarria, N.; Olivares, M.; Zuloaga, O. Non-targeted metabolomics reveals alterations in liver and plasma of gilt-head bream exposed to oxybenzone. Chemosphere 2018, 211, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Givens, J.; Stien, D.; Matallana-Surget, S.; Lebaron, P. Bioaccumulation and Toxicological Effects of UV-Filters on Marine Species. Sunscreens Coast. Ecosyst. 2020, 94, 85–130. [Google Scholar] [CrossRef]
- Lu, S.; Long, F.; Lu, P.; Lei, B.; Jiang, Z.; Liu, G.; Zhang, J.; Ma, S.; Yu, Y. Benzophenone-UV filters in personal care products and urine of schoolchildren from Shenzhen, China: Exposure assessment and possible source. Sci. Total Environ. 2018, 640–641, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.B.; Ross, J.; Ellwanger, J.; Phrommala, R.M.; Youngblood, H.; Qualley, D.; Williams, J. Sea Anemones Responding to Sex Hormones, Oxybenzone, and Benzyl Butyl Phthalate: Transcriptional Profiling and in Silico Modelling Provide Clues to Decipher Endocrine Disruption in Cnidarians. Front. Genet. 2022, 12, 793306. [Google Scholar] [CrossRef] [PubMed]
- Horricks, R.A.; Tabin, S.K.; Edwards, J.J.; Lumsden, J.S.; Marancik, D.P. Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies. PLoS ONE 2019, 14, e0220280. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Cruz, M.S.; Molins-Delgado, D.; Serra-Roig, M.P.; Kalogianni, E.; Skoulikidis, N.T.; Barceló, D. Personal care products reconnaissance in EVROTAS river (Greece): Water-sediment partition and bioaccumulation in fish. Sci. Total Environ. 2019, 651, 3079–3089. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Schäfer, S.; Buchmeier, G.; Claus, E.; Duester, L.; Heininger, P.; Körner, A.; Mayer, P.; Paschke, A.; Rauert, C.; Reifferscheid, G.; et al. Bioaccumulation in aquatic systems: Methodological approaches, monitoring and assessment. Environ. Sci. Eur. 2015, 27, 5. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, toxicities, and ecological risks of benzophenone-3, a common component of organic sunscreen products: A mini-review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chang, J.-P.; Chen, H.-C.; Liu, X.-R. Fish consumption is an indicator of exposure to benzophenone derivatives: A probabilistic risk assessment in Taiwanese population. Sci. Total Environ. 2022, 812, 152421. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, L.; Beil, S.; Börnick, H.; Stolte, S. Structure-related endocrine-disrupting potential of environmental transformation products of benzophenone-type UV filters: A review. J. Hazard. Mater. 2022, 430, 128495. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Zhu, Q.; Liao, C.; Jiang, G. Several typical endocrine-disrupting chemicals in human urine from general population in China: Regional and demographic-related differences in exposure risk. J. Hazard. Mater. 2022, 424, 127489. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, J.C.; Craig, A.D. Dermatological and environmental toxicological impact of the sunscreen ingredient oxybenzone/benzophenone-3. J. Cosmet. Dermatol. 2018, 17, 15–19. [Google Scholar] [CrossRef]
- Wnuk, A.; Rzemieniec, J.; Lasoń, W.; Krzeptowski, W.; Kajta, M. Apoptosis Induced by the UV Filter Benzophenone-3 in Mouse Neuronal Cells Is Mediated via Attenuation of Erα/Pparγ and Stimulation of Erβ/Gpr30 Signaling. Mol. Neurobiol. 2018, 55, 2362–2383. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-X. Chapter 4—Bioaccumulation and Biomonitoring. In Marine Ecotoxicology; Blasco, J., Chapman, P.M., Campana, O., Hampel, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 99–119. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development. OECD Guidelines for the Testing of Chemicals, Section 3. Test No. 305: Bioaccumulation in Fish: Aqueous and Dietary Exposure. 2012. Available online: https://www.oecd-ilibrary.org/environment/test-no-305-bioaccumulation-in-fish-aqueous-and-dietary-exposure_9789264185296-en (accessed on 1 December 2021).
| Time (h) | Measured Concentration (µg/L) ± SD (n = 3) |
|---|---|
| 0 | 100 ± 1.48 |
| 24 | 95.5 ± 1.41 |
| 48 | 97.7 ± 1.45 |
| 72 | 98.2 ± 1.45 |
| 96 | 99.7 ± 1.47 |
| t120 | 98.5 ± 1.46 |
| Average | 98.3 |
| CV% | 1.48 |
| Time in Days | t0 | 30 min after Fish Exposure | 8:30 a.m. | 13:30 p.m. Post Spike | 15:30 p.m. |
|---|---|---|---|---|---|
| 1 | 99.2 ± 11.9 | 78.3 ± 9.4 | - | - | - |
| 2 | - | - | 7.2 ± 0.9 | 79.3 ± 9.5 | 55.8 ± 8.0 |
| 3 | - | - | 82.9 ± 9.9 * | 80.5 ± 9.7 | 66.6 ± 8.0 |
| 4 | - | - | 9.5 ± 1.1 | 52.3 ± 6.3 | 102.5 ± 12.3 |
| 5 | - | - | 26.2 ± 3.1 | 17.7 ± 2.1 | 64.4 ± 7.7 |
| 6 | - | - | 92.1 ± 11.1 * | 82.8 ± 9.9 | 49.6 ± 6.0 |
| 7 | - | - | 26.3 ± 3.2 | 70.3 ± 8.4 | 50.6 ± 6.1 |
| 8 | - | - | 24.7 ± 3.0 | 81.8 ± 9.8 | 55.3 ± 6.6 |
| 9 | - | - | 86.3 ± 10.4 * | 73.8 ± 8.9 | 17.9 ± 2.1 |
| Exposure Time (Weeks, w) | 3 w | 5 w | 9 w | 11 w | Average BCF |
|---|---|---|---|---|---|
| Intestinal content | 4.04 ± 0.80 | 87.4 ± 17.5 | - | - | 16.9 ± 3.38 |
| Skin | 2.67 ± 0.53 | 49.1 ± 9.82 | 17.3 ± 3.46 | 15.7 ± 3.14 | 16.1 ± 3.22 |
| Gonads | 0.18 ± 0.03 | 33.9 ± 6.78 | 15.3 ± 3.05 | 16.2 ± 3.24 | 13.7 ± 2.75 |
| Liver | 2.84 ± 0.56 | 27.9 ± 5.58 | 1.72 ± 0.34 | 0.20 ± 0.04 | 3.67 ± 0.73 |
| Gills | 1.19 ± 0.23 | 26.6 ± 5.32 | 0.91 ± 0.18 | 0.52 ± 0.10 | 3.04 ± 0.60 |
| Brain | 0.41 ± 0.08 | 20.7 ± 4.14 | 9.45 ± 1.89 | 7.95 ± 1.59 | 7.78 ± 1.55 |
| Muscle | 1.07 ± 0.21 | 14.4 ± 2.88 | 4.88 ± 0.97 | 4.44 ± 0.88 | 4.66 ± 0.93 |
| Intestine | 2.22 ± 0.44 | 11.6 ± 2.32 | 3.37 ± 0.67 | 3.34 ± 0.66 | 3.81 ± 0.76 |
| Kidney | 3.59 ± 0.71 | 8.30 ± 1.66 | 3.51 ± 0.70 | 3.95 ± 0.79 | 4.11 ± 0.82 |
| Scaly | 2.12 ± 0.42 | - | 4.89 ± 0.97 | 5.41 ± 1.08 | 5.37 ± 1.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiriac, F.L.; Lucaciu, I.E.; Paun, I.; Pirvu, F.; Gheorghe, S. In Vivo Bioconcentration, Distribution and Metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp). Foods 2022, 11, 1627. https://doi.org/10.3390/foods11111627
Chiriac FL, Lucaciu IE, Paun I, Pirvu F, Gheorghe S. In Vivo Bioconcentration, Distribution and Metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp). Foods. 2022; 11(11):1627. https://doi.org/10.3390/foods11111627
Chicago/Turabian StyleChiriac, Florentina Laura, Irina Eugenia Lucaciu, Iuliana Paun, Florinela Pirvu, and Stefania Gheorghe. 2022. "In Vivo Bioconcentration, Distribution and Metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp)" Foods 11, no. 11: 1627. https://doi.org/10.3390/foods11111627
APA StyleChiriac, F. L., Lucaciu, I. E., Paun, I., Pirvu, F., & Gheorghe, S. (2022). In Vivo Bioconcentration, Distribution and Metabolization of Benzophenone-3 (BP-3) by Cyprinus carpio (European Carp). Foods, 11(11), 1627. https://doi.org/10.3390/foods11111627







