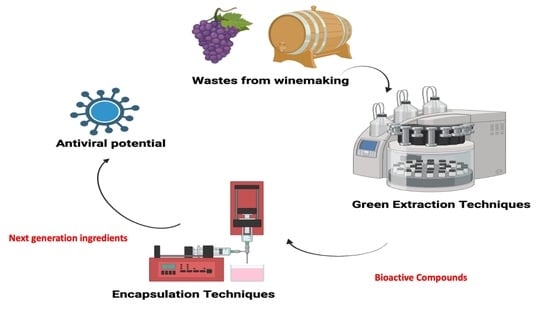
Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties
Abstract
:1. Introduction
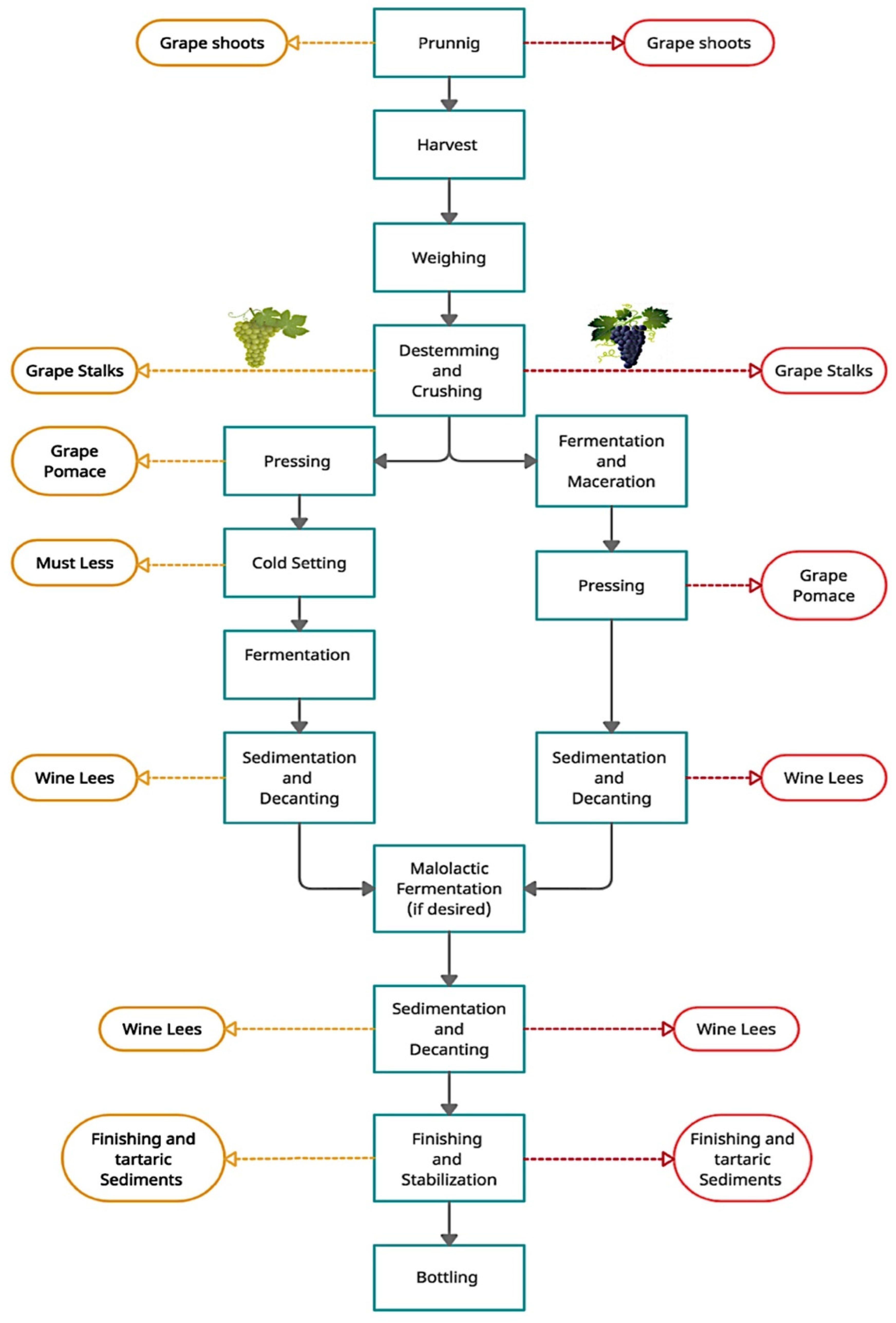
| By-Products | Bioactive Compounds | Current Use | Reference |
|---|---|---|---|
| Grape pomace waste | Organic matter content, polyphenols (anthocyanins and tannins), flavonol content, ethanol precipitate | Alternative source of antioxidant compounds and dietary fiber for yogurt | [7,8] |
| Energy source | [9] | ||
| To extend shelf life of lamb meat | [10] | ||
| To reduce acrylamide formation | [11] | ||
| To neutralize the production of reactive oxygen | [12] | ||
| To reduce cholesterol level | [13] | ||
| Stable delivery system, protecting resveratrol | [14] | ||
| Biomethane | [15] | ||
| Cosmetic formulation (skin aging) | [16] | ||
| Dietary fiber supplement, human food supplement | [17] | ||
| Grape seed | Flavanol content. Lignocellulosic content | To modify the formulation of meat products | [18] |
| Energy production, biodiesel | [19] | ||
| Direct inclusion of natural antioxidants | [20] | ||
| Skin moisturizer (gel formulation) | [21] | ||
| Animal feed (rainbow trout) | [22] | ||
| Extraction with supercritical CO2 | [23,24] | ||
| Wastewater | Tartaric acid and malic acid content | Acidulant compound in soft drinks | [9] |
| Vine shoot and stems | Phenolic compounds | Biodegradable packaging | [25] |
| Energy production, biomethane | [26] |
2. Updating of Bioactive Compounds Extracted from Winemaking By-Products
3. Development of Ingredients of Products Based on Winemaking Products
| Raw Material | Technology | Process Variable/Formulation | Encapsulation Agent | Main Result | References |
|---|---|---|---|---|---|
| Dry grape residue pressed | Microcapsulation. Buchi B-290 spray drying (Buchi Labortechnic AG, Switzerland). | Spray drying with the main chamber of 165 mm diameter, 600 mm cylindrical height, and 1.5 mm nozzle diameter at four air inlet temperatures (120, 140, 160, 180 °C). The pump power was kept at 40% to maintain feed flow rate as 12 mL min−1, and air flow rate as 35 m3 h−1. During drying processes, the temperature of the feed mixture was 25 °C | Maltodextrin and gum arabic as coating material. Two different core: coating material ratios (1:1 and 1:2), three different maltodextrin: gum arabic ratios (10:0, 8:2, and 6:4) | Encapsulation efficiency 98.8% and 99.1% for core: coating ratios of 1:1 and 1:2. Highest yield (64.9%) MD:GA ratio 10:0, at temperature 180 °C | [20] |
| Agiorgitiko (Vitis vinifera) grape pomace | Spray drying (Buchi, B-191, Buchi Laboratoriums-Technik, Flawil, Switzerland) | Ratio of wall-to-core material of 8.8, an inlet air temperature of 189 °C, a drying air flow rate of 65% | Maltodextrin:skim milk powder (50:50) | Optimum values of encapsulation efficiency (92.49%) and yield (37.28%) | [21] |
| Dry grape residue pressed | Spray drying process Buchi B-290 equipped with a 1.5 mm nozzle diameter and 600 mm × 165 mm main spray chamber | Peristaltic pump set to 40% power, 12 mL min−1 feed flow rate, and 35 m3 h−1 air flow rate. The temperature of the feed mixture kept constant at 25 °C during drying process. | Maltodextrin dextrose equivalents (MDDE4-7 and MDDE17-20) and gum Arabic (G9752) | The microcapsules obtained under optimal conditions were stored at two different relative humidities (33% and 52%) during 75 days. | [22] |
| Byproducts (seeds and peels) of Bordo red grapes (V. labrusca) | Pilot spray drying model MSD 5.0; freeze-drying in the proper equipment model LC 1500 | Used a 2 mm nozzle and air flow of 40 L/min. The compressor air pressure was 0.2 MPa and the feed rate of the mixture 44 mL/min, performed by a peristaltic pump. Variables tested were inlet air temperature (130, 150, and 170 °C) | The carrier agent used in the atomization process was maltodextrin MOR-REX® 1910 | Bordo grape extracts using maltodextrin produced powders with low moisture content, low hygroscopicity, high solubility, and stable color. | [23] |
4. New Insights against Disease and Viruses
5. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Compounds of Interest | Grape Pomace (Skin and Seed) | Grape Skin | Grape Seed |
|---|---|---|---|
| Gallic acid | 1090.1 μg g−1 of extract (RP-HPLC) [69] | 122 μg g−1 of extract (HPLC–UV) [70] | 9.8 mg kg−1 of fresh grape (HPLC-DAD-FLV) [71] |
| 397.67 μg mL−1 of extract (HPLC-DAD) [72] | 8.76 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | 30.3 mg kg−1 dw (RP-HPLC/UV) [74] | |
| 252.8 μg g−1 of extract (HPLC-MWD) [8] | 1.19 mg kg−1 of grape (HPLC-DAD) [75] | 136.74 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | |
| 95.36 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | 1.92 mg kg−1 of grape (HPLC-DAD) [75] | ||
| 260.92 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| Syringic acid | 1731.7 μg g−1 of extract (HPLC-MWD) [8] | ||
| Caffeic acid | 16.0 μg g−1 of extract (HPLC-MWD) [8] | 0.54 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | 1.06 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] |
| 438.43 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | |||
| p-Coumaric acid | 64.6 μg g−1 of extract (HPLC-MWD) [8] | 1.96 mg kg−1 of grape (HPLC-DAD) [75] | |
| 214.55 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | |||
| Ferulic acid | 24.1 μg g−1 of extract (HPLC-MWD) [8] | 2.12 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | 2.17 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] |
| 1.33 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | |||
| Caftaric acid | 1.80 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | ||
| Trans-resveratrol | 36.0 μg g−1 of extract (HPLC-MWD) [8] | 5.64 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | |
| 20.66 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | 1.43 mg kg−1 of grape (HPLC-DAD) [73] | ||
| Cyanidin 3-O-glucoside | 870 μg g−1 of extract (HPLC-MWD) [8] | 528 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 6.99 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| Myricetin | 36.77 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | 1.8 μmol kg−1 of grape (HPLC-DAD/FLD) [81] | 2.42 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] |
| 452 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | 2.1 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | ||
| 2.45 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Rutin | 998.5 μg g−1 of extract (RP-HPLC) [69] | 57.04 mg kg−1 dw (HPLC-DAD) [83] | 9.05 mg kg−1 dw (HPLC-DAD) [83] |
| 112.96 μg mL−1 of extract (HPLC-DAD) [72] | 223 μg g−1 of extract (HPLC–UV) [70] | 30.7 mg kg−1 dw (RP-HPLC/UV) [74] | |
| Delphinidin 3-O-acetylglucoside | 1043 μg g−1 of extract (HPLC-MWD) [8] | ||
| 9.79 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| (+)-Catechin | 5083 μg g−1 of extract (RP-HPLC) [69] | 13.20 mg kg−1 dw (HPLC-DAD) [83] | 117 mg kg−1 dw (HPLC-DAD) [83] |
| 89.73 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | 628 μg g−1 of extract (HPLC–UV) [70] | 270 mg kg−1 of fresh grape (HPLC-DAD-FLV) [73] | |
| 275.09 μg mL−1 of extract (HPLC-DAD) [72] | 49.38 mg kg−1 of grape (HPLC–DAD–ESI-MS/MS) [84] | 21.1 mg kg−1 dw (RP-HPLC/UV) [74] | |
| 3387.5 μg g-1 of extract (HPLC-MWD) [8] | 7.47 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | 86.73 mg kg−1 of grape (HPLC–DAD–ESI-MS/MS) [84] | |
| 11.45 mg kg−1 of grape (HPLC-DAD) [75] | 270.26 mg kg−1 dw (UHPLC-DAD-MS/MS) [71] | ||
| 25 mg kg−1 of fresh grape (HPLC-DAD-FLV) [71] | 106.5 mg kg−1 of grape (HPLC-DAD) [75] | ||
| (-)-Epicatechin | 192.8 μg g−1 of extrac (RP-HPLC) [69] | 323 μg g−1 of extract (HPLC–UV) [70] | 210 mg kg−1 of fresh grape (HPLC-DAD-FLV) [71] |
| 1763.4 μg g−1 of extract (HPLC-MWD) [8] | 13.55 mg kg−1 of grape (HPLC–DAD–ESI-MS/MS) [84] | 38.1 mg kg−1 dw (RP-HPLC/UV) [74] | |
| 112.72 mg kg−1 dw (HPLC-PDA-ESI-MS/MS (Lingua, 2016 #242) | 3.56 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | 6.81 mg kg−1 of grape (HPLC–DAD–ESI-MS/MS) [84] | |
| 2.67 mg kg−1 of grape (HPLC-DAD) [75] | 223.08 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | ||
| 13 mg kg−1 of fresh grape (HPLC-DAD-FLV) [71] | 77.51 mg kg−1 of grape (HPLC-DAD) [75] | ||
| 47.50 mg kg−1 dw (HPLC-DAD) [83] | |||
| Kaempferol | 346.8 μg g−1 of extract (RP-HPLC) [69] | 34.2 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 28.53 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | 0.41 μmol kg−1 of grape (HPLC-PDA-ESI-MS/MS) [78] | ||
| 2.37 mg kg−1 dw (HPLC-UV-DAD) [80] | 8.93 mg kg−1 dw (HPLC-DAD/FLD) [81] | ||
| 34.23 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | 14.89 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | ||
| 1.53 mg kg−1 dw (UPLC-DAD-MS) [79] | |||
| Quercetin 3-glucuronide | 130 mg kg−1 dw (HPLC-UV-DAD) [80] | 22 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 81.42 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | 0.98 mg 100g−1 (HPLC-DAD) [75] | ||
| 990 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| Peonidin 3-O-glucoside | 0,15 mg g−1 of extract (HPLC-UV-DAD) [85] | 551 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 2460 μg g−1 of extract (HPLC-MWD) [8] | |||
| 18.31 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 1591 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 18.70 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| 0.97 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Malvidin 3-O-glucoside | 5,70 mg g−1 of extract (HPLC-UV-DAD) [85] | 2489 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 26,658 μg g−1 of extract (HPLC-MWD) [8] | |||
| 955.85 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 12182 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 64.6 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| 142.22 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Quercetin | 650.2 μg g−1 of extract (RP-HPLC–DAD) [69] | 316 μg g−1 of extract (HPLC–UV) [70] | 1009.4 mg kg−1 dw (RP-HPLC/UV) [74] |
| 159.60 μg mL−1 of extract (HPLC-DAD) [72] | 40.03 mg kg−1 dw (HPLC-DAD) [83] | 11.72 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | |
| 557.3 μg g−1 of extract (HPLC-MWD) [8] | 0.53 μmol kg−1 of grape (HPLC-DAD/FLD) [81] | ||
| 26.25 mg kg−1 dw (HPLC-ESI/MS/MS) [76] | 121.94 mg kg−1 dw (UHPLC-DAD-MS/MS) [73] | ||
| 382.93 mg L−1 of extract (HPLC-PDA-MS) [77] | 1043 mg kg−1 dw (UPLC-DAD-MS) [79] | ||
| 0.54 mg g−1 of extract (HPLC-UV-DAD) [85] | 3.68 mg kg−1 dw (HPLC-DAD) [83] | ||
| 392 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 15.30 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| 251.06 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Delphinidin 3-O-glucoside | 0,16 mg g−1 of extract (HPLC-UV-DAD) [85] | 870 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 4581 μg g−1 of extract (HPLC-MWD) [8] | |||
| 4.47 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 775 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 3.73 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| Petunidin 3-O-acetylglucoside | 1424 μg g−1 of extract (HPLC-MWD) [8] | ||
| 72.13 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 0.86 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Malvidin 3-O-acetylglucoside | 2,02 mg g−1 of extract (HPLC-UV-DAD) [85] | 486 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 4021 μg g−1 of extract (HPLC-MWD) [8] | |||
| 1718.92 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 937 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 0.96 mg kg−1 dw (HPLC-UV-DAD) [80] | |||
| 195.01 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Cyanidin 3-O-p-coumaroylglucoside | 1886 μg g−1 of extract (HPLC-MWD) [8] | 327 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 3.99 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| Petunidin 3-O-p-coumaroylglucoside | 2481 μg g−1 of extract (HPLC-MWD) [8] | 339 mg kg−1 dw (UPLC-DAD-MS) [79] | |
| 29.95 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 765 ppm of dry extract (HPLC-DAD-ESI-MS/MS) [82] | |||
| 72.95 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] | |||
| Peonidin 3-O-acetylglucoside | 1902 μg g−1 of extract (HPLC-MWD) [8] | ||
| 32.64 mg L−1 of extract (HPLC-PDA-MS) [77] | |||
| 1.83 mg kg−1 dw (HPLC-PDA-ESI-MS/MS) [78] |
References
- Matos, C.; Pirra, A. Water to wine in wineries in Portugal Douro Region: Comparative study between wineries with different sizes. Sci. Total Environ. 2020, 732, 139332. [Google Scholar] [CrossRef] [PubMed]
- OIV. Wine Production First Estimates. 27 October 2020. 2020. Available online: https://www.greatwinecapitals.com/wp-content/uploads/2021/02/OIV-2020-World-Wine-Production-First-Estimates-presentation-pdf.pdf (accessed on 14 June 2021).
- Bharathiraja, B.; Iyyappan, J.; Jayamuthunagai, J.; Kumar, R.P.; Sirohi, R.; Gnansounou, E.; Pandey, A. Critical review on bioconversion of winery wastes into value-added products. Ind. Crops Prod. 2020, 158, 112954. [Google Scholar] [CrossRef]
- Dwyer, K.; Hosseinian, F.; Rod, M.R. The market potential of grape waste alternatives. J. Food Res. 2014, 3, 91. [Google Scholar] [CrossRef]
- de Campos, L.M.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Kekelidze, I.; Ebelashvili, N.; Japaridze, M.; Chankvetadze, B.; Chankvetadze, L. Phenolic antioxidants in red dessert wine produced with innovative technology. Ann. Agrar. Sci. 2018, 16, 34–38. [Google Scholar] [CrossRef]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; da Silva, L.P.; Penna, N.G. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds From Grape Pomace: A Review. Food Bioprocess Technol. 2021, 1–25. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2013, 236, 47–56. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Perkowski, J. Phenolic acids in cereal grain: Occurrence, biosynthesis, metabolism and role in living organisms. Crit. Rev. Food Sci. Nutr. 2019, 59, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 5638. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef] [Green Version]
- Bakowska-Barczak, A.M.; Kolodziejczyk, P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011, 34, 1301–1309. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Delgado-Torre, M.P.; Ferreiro-Vera, C.; Priego-Capote, F.; Pérez-Juan, P.M.; Luque de Castro, M.a.D. Comparison of accelerated methods for the extraction of phenolic compounds from different vine-shoot cultivars. J. Agric. Food Chem. 2012, 60, 3051–3060. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Tolun, A.; Artik, N.; Altintas, Z. Effect of different microencapsulating materials and relative humidities on storage stability of microencapsulated grape pomace extract. Food Chem. 2020, 302, 125347. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.B.; Thomazini, M.; de Carvalho Balieiro, J.C.; Fávaro-Trindade, C.S. Effect of spray drying on the physicochemical properties and color stability of the powdered pigment obtained from vinification byproducts of the Bordo grape (Vitis labrusca). Food Bioprod. Processing 2015, 93, 39–50. [Google Scholar] [CrossRef]
- Gençdağ, E.; Özdemir, E.E.; Demirci, K.; Görgüç, A.; Yılmaz, F.M. Copigmentation and stabilization of anthocyanins using organic molecules and encapsulation techniques. Curr. Plant Biol. 2022, 29, 100238. [Google Scholar] [CrossRef]
- Randazzo, W.; Fabra, M.J.; Falcó, I.; López-Rubio, A.; Sánchez, G. Polymers and biopolymers with antiviral activity: Potential applications for improving food safety. Compr. Rev. Food Sci. Food Saf. 2018, 17, 754–768. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mascaraque, L.G.; Sanchez, G.; López-Rubio, A. Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Rani, J.; Rautela, A.; Kumar, S. Chapter 4—Biovalorization of winery industry waste to produce value-added products. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Krishnaraj Rathinam, N., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 63–85. [Google Scholar]
- Xia, E.-Q.; Deng, G.-F.; Guo, Y.-J.; Li, H.-B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Spinelli, R.; Nati, C.; Pari, L.; Mescalchin, E.; Magagnotti, N. Production and quality of biomass fuels from mechanized collection and processing of vineyard pruning residues. Appl. Energy 2012, 89, 374–379. [Google Scholar] [CrossRef]
- Duranay, N.D.; Akkuş, G. Solid fuel production with torrefaction from vineyard pruning waste. Biomass Convers. Biorefinery 2019, 1–12. [Google Scholar] [CrossRef]
- Friedman, M. Antibacterial, Antiviral, and Antifungal Properties of Wines and Winery Byproducts in Relation to Their Flavonoid Content. J. Agric. Food Chem. 2014, 62, 6025–6042. [Google Scholar] [CrossRef]
- Royo, C.; Ferradás, Y.; Martínez-Zapater, J.M.; Motilva, M.-J. Characterization of Tempranillo negro (VN21), a high phenolic content grapevine Tempranillo clone, through UHPLC-QqQ-MS/MS polyphenol profiling. Food Chem. 2021, 360, 130049. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, Y.; Lu, J. Phenolic Contents and Compositions in Skins of Red Wine Grape Cultivars among Various Genetic Backgrounds and Originations. Int. J. Mol. Sci. 2012, 13, 3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Liu, X.; Yan, Q.; Yuan, F.; Gao, Y. A novel copigment of quercetagetin for stabilization of grape skin anthocyanins. Food chem. 2015, 166, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkowska, A.; Ambroziak, W.; Czyzowska, A.; Adamiec, J. Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11. [Google Scholar] [CrossRef] [Green Version]
- Gessner, D.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Yadav, K.; Bajaj, R.K.; Mandal, S.; Mann, B. Encapsulation of grape seed extract phenolics using whey protein concentrate, maltodextrin and gum arabica blends. J. Food Sci. Technol. 2020, 57, 426–434. [Google Scholar] [CrossRef]
- Prado-Alvarez, M.; Lynch, S.; Kane, A.; Darmody, G.; Pardo, B.; Martínez, P.; Cotterill, J.; Wontner-Smith, T.; Culloty, S. Oral immunostimulation of the oyster Ostrea edulis: Impacts on the parasite Bonamia ostreae. Fish Shellfish. Immunol. 2015, 45, 43–51. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Processing 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Q.; Xi, B.; Dai, H. Study on the regulation of anthocyanin biosynthesis by exogenous abscisic acid in grapevine. Sci. Hortic. 2019, 250, 294–301. [Google Scholar] [CrossRef]
- Yilmaz, E.E.; Özvural, E.B.; Vural, H. Extraction and identification of proanthocyanidins from grape seed (Vitis Vinifera) using supercritical carbon dioxide. J. Supercrit. Fluids 2011, 55, 924–928. [Google Scholar] [CrossRef]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.-L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, F.A.; Mahrose, K.M.; Basyony, M.M. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch. Anim. Nutr. 2016, 70, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.B.; Hwang, J.-W.; Kim, Y.-S.; Kim, E.-K.; Park, P.-J. Ocular promoting activity of grape polyphenols—A review. Environ. Toxicol. Pharmacol. 2017, 50, 83–90. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Cheng, V.J.; McConnell, M.; Zhao, J.H.; Sedcole, R.; Harrison, R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011, 129, 837–845. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Bekhit, A.E.-D.A.; Cheng, V.J.; Zhang, H.; Mros, S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Bekhit, A.A.; McConnell, M. Effect of extraction system and grape variety on anti-influenza compounds from wine production residue. Food Control 2019, 99, 180–189. [Google Scholar] [CrossRef]
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273. [Google Scholar] [CrossRef]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef]
- Zannella, C.; Giugliano, R.; Chianese, A.; Buonocore, C.; Vitale, G.A.; Sanna, G.; Sarno, F.; Manzin, A.; Nebbioso, A.; Termolino, P.; et al. Antiviral Activity of Vitis vinifera Leaf Extract against SARS-CoV-2 and HSV-1. Viruses 2021, 13, 1253. [Google Scholar] [CrossRef]
- Vázquez-Armenta, F.; Silva-Espinoza, B.; Cruz-Valenzuela, M.; González-Aguilar, G.; Nazzaro, F.; Fratianni, F.; Ayala-Zavala, J. Antibacterial and antioxidant properties of grape stem extract applied as disinfectant in fresh leafy vegetables. J. Food Sci. Technol. 2017, 54, 3192–3200. [Google Scholar] [CrossRef] [PubMed]
- Silva Soares, S.C.; de Lima, G.C.; Carlos Laurentiz, A.; Féboli, A.; dos Anjos, L.A.; de Paula Carlis, M.S.; da Silva Filardi, R.; da Silva de Laurentiz, R. In vitro anthelmintic activity of grape pomace extract against gastrointestinal nematodes of naturally infected sheep. Int. J. Vet. Sci. Med. 2018, 6, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, S.S.; Su, X.; D’Souza, D.H. Antiviral effects of grape seed extract against feline calicivirus, murine norovirus, and hepatitis A virus in model food systems and under gastric conditions. Food Microbiol. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, Q.; Chen, Y.; Duan, M.; Tian, G.; Deng, X.; Sun, Y.; Zhou, T.; Zhang, G.; Chen, W. Inhibition of proanthocyanidin A2 on porcine reproductive and respiratory syndrome virus replication in vitro. PLoS ONE 2018, 13, e0193309. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Murador, D.C.; de Souza Mesquita, L.M.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxidative Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef] [Green Version]
- Pauletto, M.; Elgendy, R.; Ianni, A.; Marone, E.; Giantin, M.; Grotta, L.; Ramazzotti, S.; Bennato, F.; Dacasto, M.; Martino, G. Nutrigenomic effects of long-term grape pomace supplementation in dairy cows. Animals 2020, 10, 714. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Kishi, Y.; Oishi, K.; Hirooka, H.; Kumagai, H. Effects of feeding polyphenol-rich winery wastes on digestibility, nitrogen utilization, ruminal fermentation, antioxidant status and oxidative stress in wethers. Anim. Sci. J. 2015, 86, 260–269. [Google Scholar] [CrossRef]
- Biscarini, F.; Palazzo, F.; Castellani, F.; Masetti, G.; Grotta, L.; Cichelli, A.; Martino, G. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE 2018, 13, e0205670. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos, K.; Stagos, D.; Mitsagga, C.; Giavasis, I. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2017, 101, e108–e121. [Google Scholar] [CrossRef] [PubMed]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Martinez-Gutierrez, M.; Sousa, D.P.d. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konowalchuk, J.; Speirs, J.I. Virus inactivation by grapes and wines. Appl. Environ. Microbiol. 1976, 32, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Messina, G.; Polito, R.; Monda, V.; Cipolloni, L.; Di Nunno, N.; Di Mizio, G.; Murabito, P.; Carotenuto, M.; Messina, A.; Pisanelli, D. Functional role of dietary intervention to improve the outcome of COVID-19: A hypothesis of work. Int. J. Mol. Sci. 2020, 21, 3104. [Google Scholar] [CrossRef]
- Roshanravan, N.; Seif, F.; Ostadrahimi, A.; Pouraghaei, M.; Ghaffari, S. Targeting Cytokine Storm to Manage Patients with COVID-19: A Mini-Review. Arch. Med. Res. 2020, 51, 608–612. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Ozkan, G.; Yetim, H.; Ekici, L.; Yilmaz, M.T. RP-HPLC–DAD analysis of phenolic compounds in pomace extracts from five grape cultivars: Evaluation of their antioxidant, antiradical and antifungal activities in orange and apple juices. Food Chem. 2011, 126, 1749–1758. [Google Scholar] [CrossRef]
- Farhadi, K.; Esmaeilzadeh, F.; Hatami, M.; Forough, M.; Molaie, R. Determination of phenolic compounds content and antioxidant activity in skin, pulp, seed, cane and leaf of five native grape cultivars in West Azerbaijan province, Iran. Food Chem. 2016, 199, 847–855. [Google Scholar] [CrossRef]
- Montealegre, R.R.; Peces, R.R.; Vozmediano, J.C.; Gascueña, J.M.; Romero, E.G. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 2006, 19, 687–693. [Google Scholar] [CrossRef]
- Ribeiro, L.; Ribani, R.; Francisco, T.; Soares, A.; Pontarolo, R.; Haminiuk, C. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.M.; Zagorac, D.Č.D.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Ouerghemmi, I.; Wannes, W.A.; Ksouri, R.; Zemni, H.; Marzouk, B.; Kchouk, M.E. Valorization of three varieties of grape. Ind. Crops Prod. 2009, 30, 292–296. [Google Scholar] [CrossRef]
- Di Lecce, G.; Arranz, S.; Jáuregui, O.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Lamuela-Raventós, R.M. Phenolic profiling of the skin, pulp and seeds of Albariño grapes using hybrid quadrupole time-of-flight and triple-quadrupole mass spectrometry. Food Chem. 2014, 145, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Lopez, L.; McGlynn, W.; Goad, C.; DeWitt, C.M. Simultaneous determination of phenolic compounds in Cynthiana grape (Vitis aestivalis) by high performance liquid chromatography–electrospray ionisation–mass spectrometry. Food Chem. 2014, 149, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Trikas, E.D.; Melidou, M.; Papi, R.M.; Zachariadis, G.A.; Kyriakidis, D.A. Extraction, separation and identification of anthocyanins from red wine by-product and their biological activities. J. Funct. Foods 2016, 25, 548–558. [Google Scholar] [CrossRef]
- Lingua, M.S.; Fabani, M.P.; Wunderlin, D.A.; Baroni, M.V. In vivo antioxidant activity of grape, pomace and wine from three red varieties grown in Argentina: Its relationship to phenolic profile. J. Funct. Foods 2016, 20, 332–345. [Google Scholar] [CrossRef]
- Harsha, P.S.; Gardana, C.; Simonetti, P.; Spigno, G.; Lavelli, V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Bioresour. Technol. 2013, 140, 263–268. [Google Scholar] [CrossRef]
- Amico, V.; Napoli, E.; Renda, A.; Ruberto, G.; Spatafora, C.; Tringali, C. Constituents of grape pomace from the Sicilian cultivarNerello Mascalese’. Food Chem. 2004, 88, 599–607. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A comparative study on different extraction techniques to recover red grape pomace polyphenols from vinification byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Phenolic composition of the berry parts of hybrid grape cultivar BRS Violeta (BRS Rubea× IAC 1398-21) using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]

| Bioactive Ingredient Extract | Disease and Virus | Reference |
|---|---|---|
| Grape seed and grape marc meal extract | Gut morphology, apparent digestibility of nutrients, microbial composition in faeces, and the expression of pro-inflammatory genes in the intestine of pigs. | [48] |
| Extraction from wine production waste (seeds, skin, and pomace) from Pinot noir and Pinot meunier | Anti-influenza activity | [49] |
| Polyphenols extraction from Cabernet Sauvignon grape pomace | Effect of different classes of antibiotics against Staphylococcus aureus and Escherichia coli, especially against multi-drug resistant clinical isolates | [50] |
| Oligostilbenoids isolated from extracts of Vitis vinifera L. Pinot Noir grape canes | Antiproliferative activity on four different cell lines (MCR-5, AGS, SK-MES-1, and J82) determined by means of the MTT reduction assay. | [51] |
| Leaf extract Vitis vinifera var. Paulsen 1103 | Antiviral activity against two human viruses: The Herpes simplex virus type 1 (HSV-1) and widespread severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). | [52] |
| Phenolic extract from grape stems (Vitis vinifera var. Red Globe) | Inhibit the growth of Listeria monocytogenes, Staphylococcus aureus, Salmonella enterica subsp. enterica serovar Typhimurium, and Escherichia coli O157 | [53] |
| Hydroalcoholic extract from grape pomace var. Máximo IAC 138-22 | Ovicidal and larvicidal activity against gastrointestinal nematodes of sheep. | [54] |
| Grape seed extract | Antiviral activities against hepatitis A virus (HAV) and human norovirus surrogates (feline calicivirus (FCV-F9) and murine norovirus (MNV-1)). | [55] |
| Grape seed-extracted proanthocyanidin | Inhibition of porcine reproductive and respiratory syndrome virus (PRRSV) | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, G.; López, M.D.; Vargas, M.; Aranda, M.; Cañumir, J.A. Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties. Foods 2022, 11, 1604. https://doi.org/10.3390/foods11111604
Pascual G, López MD, Vargas M, Aranda M, Cañumir JA. Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties. Foods. 2022; 11(11):1604. https://doi.org/10.3390/foods11111604
Chicago/Turabian StylePascual, Guillermo, María Dolores López, Marisol Vargas, Mario Aranda, and Juan Antonio Cañumir. 2022. "Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties" Foods 11, no. 11: 1604. https://doi.org/10.3390/foods11111604
APA StylePascual, G., López, M. D., Vargas, M., Aranda, M., & Cañumir, J. A. (2022). Next Generation Ingredients Based on Winemaking By-Products and an Approaching to Antiviral Properties. Foods, 11(11), 1604. https://doi.org/10.3390/foods11111604