Design of a Signal-Amplified Aptamer-Based Lateral Flow Test Strip for the Rapid Detection of Ochratoxin A in Red Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of AuNPs
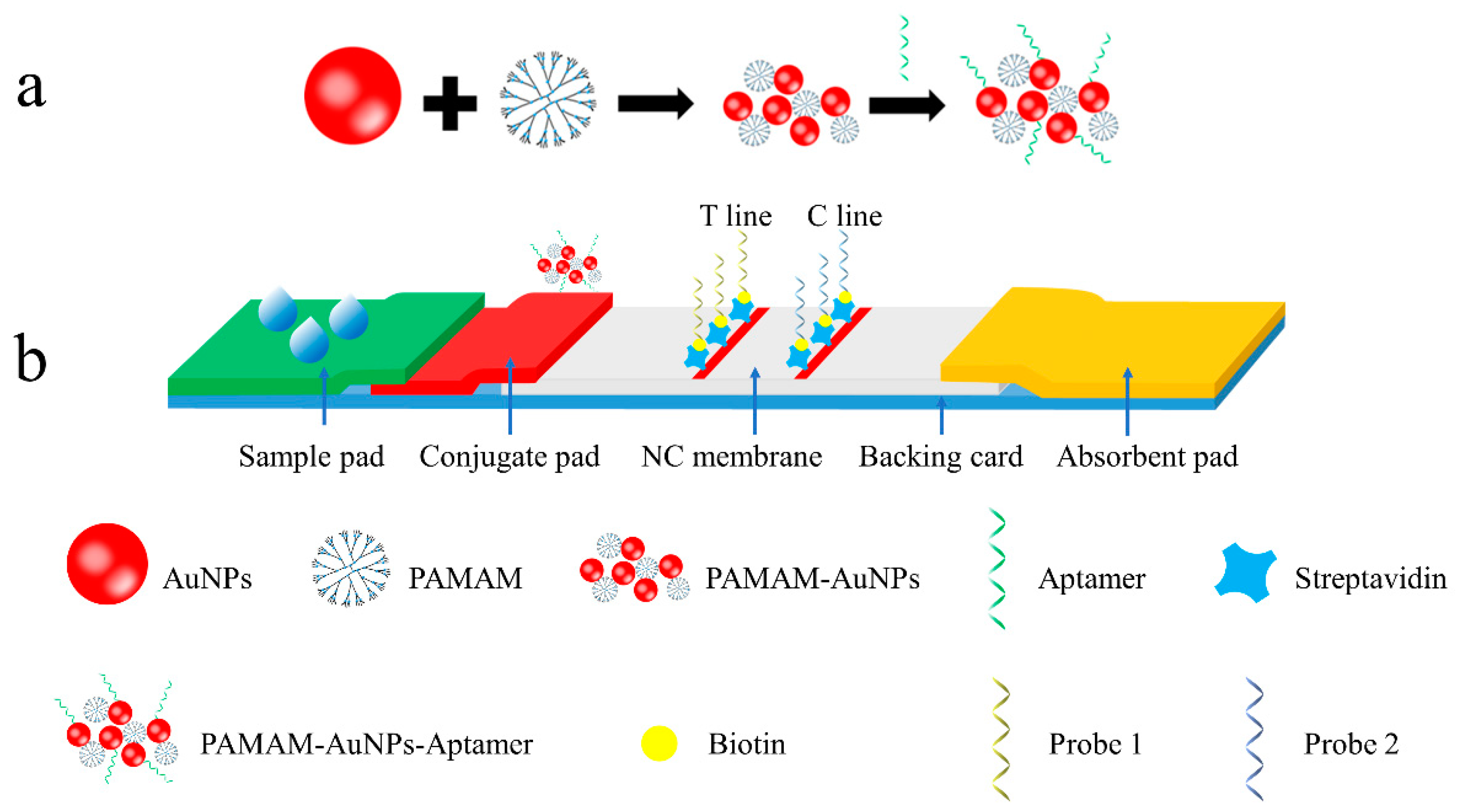
2.3. Preparation of the PAMAM–AuNP–Aptamer Bioconjugates
2.4. Fabrication of the Signal-Amplified Aptamer Strip
2.5. Test Procedure
2.6. Detection of OTA in Red Wine Samples
3. Results and Discussion
3.1. Principle of the Signal-Amplified Aptamer-Based Strip
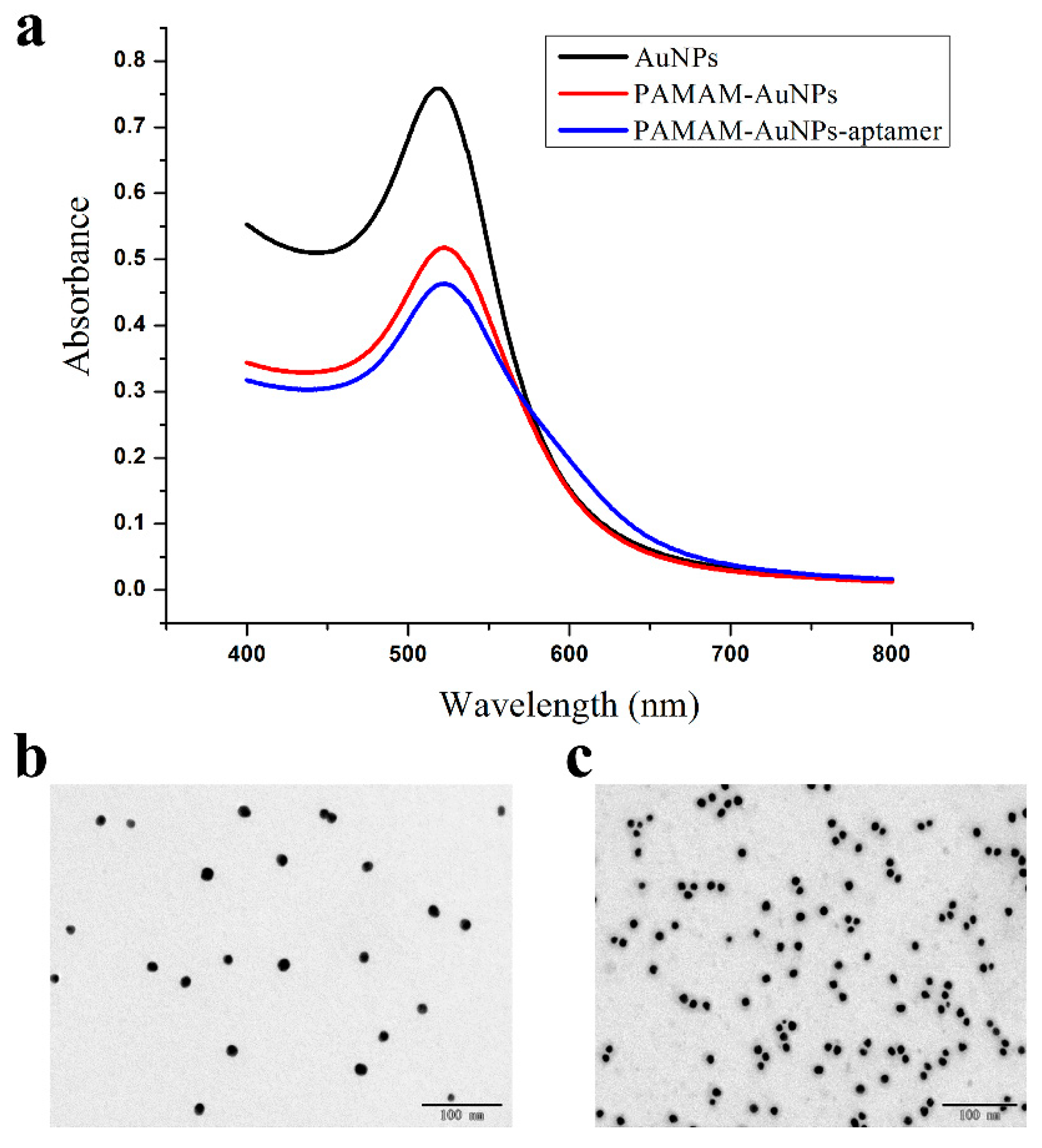
3.2. Characterization of AuNPs, PAMAM–AuNPs and PAMAM–AuNP–Aptamers
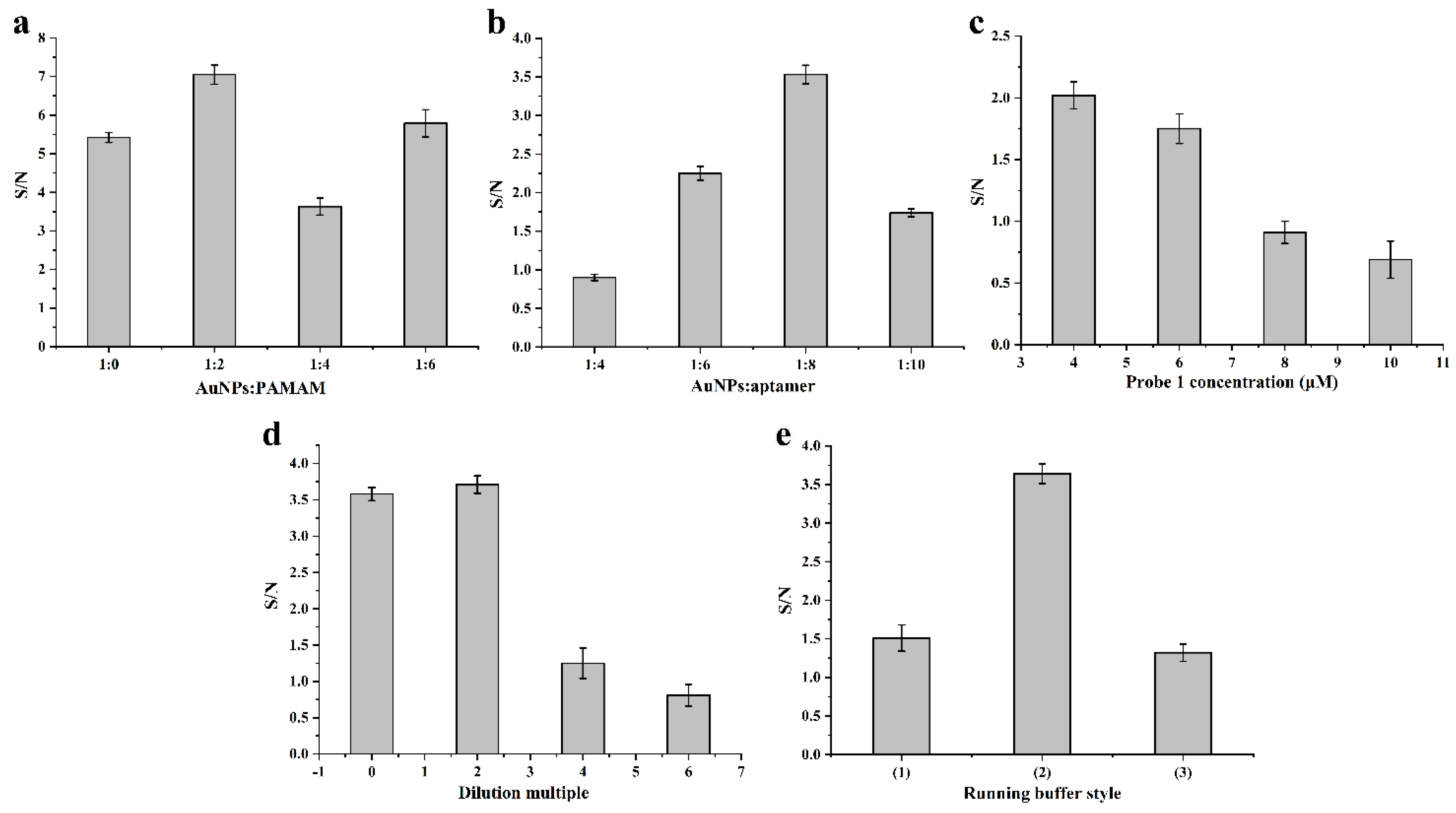
3.3. Optimization of the Experimental Conditions
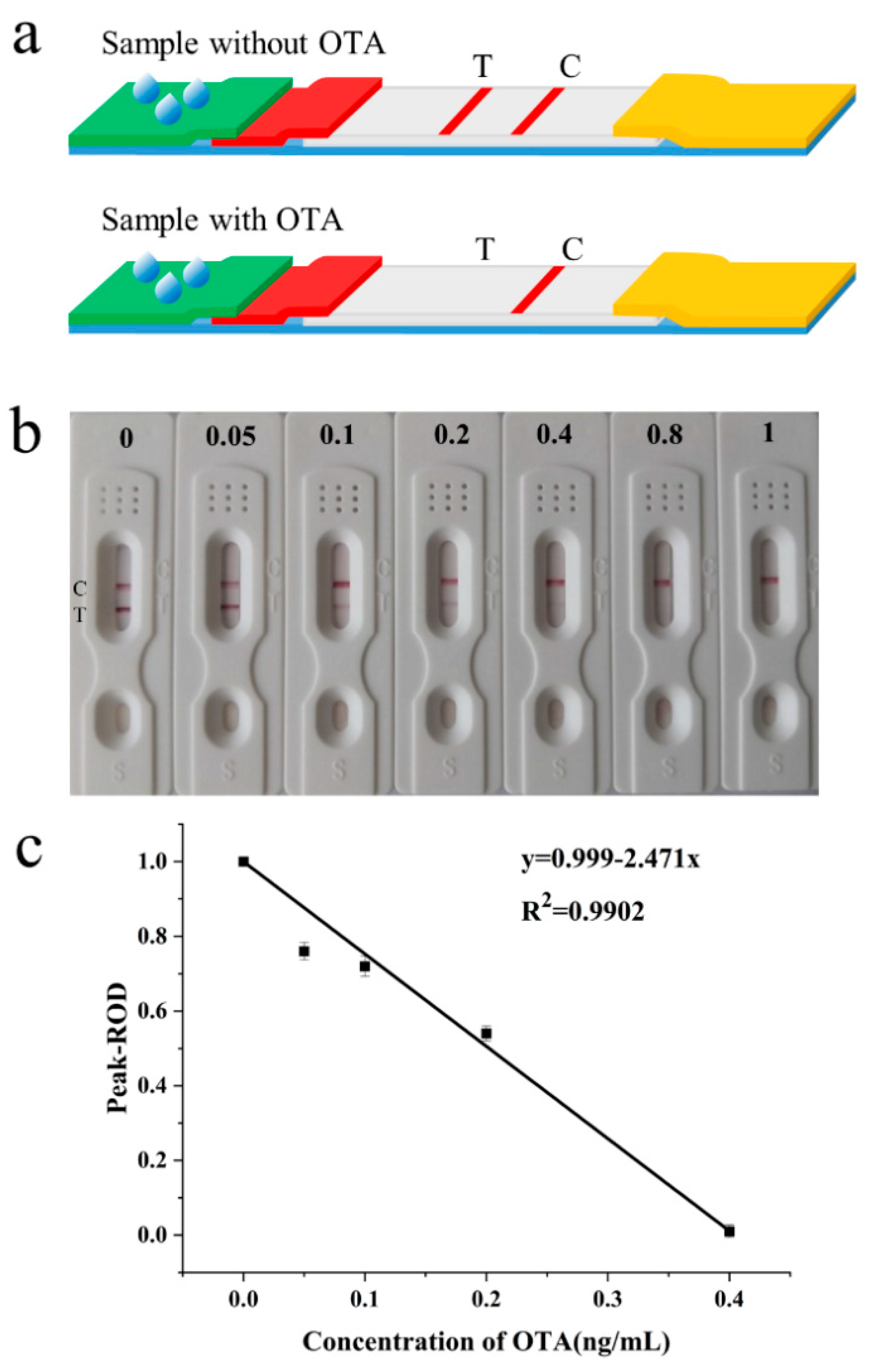
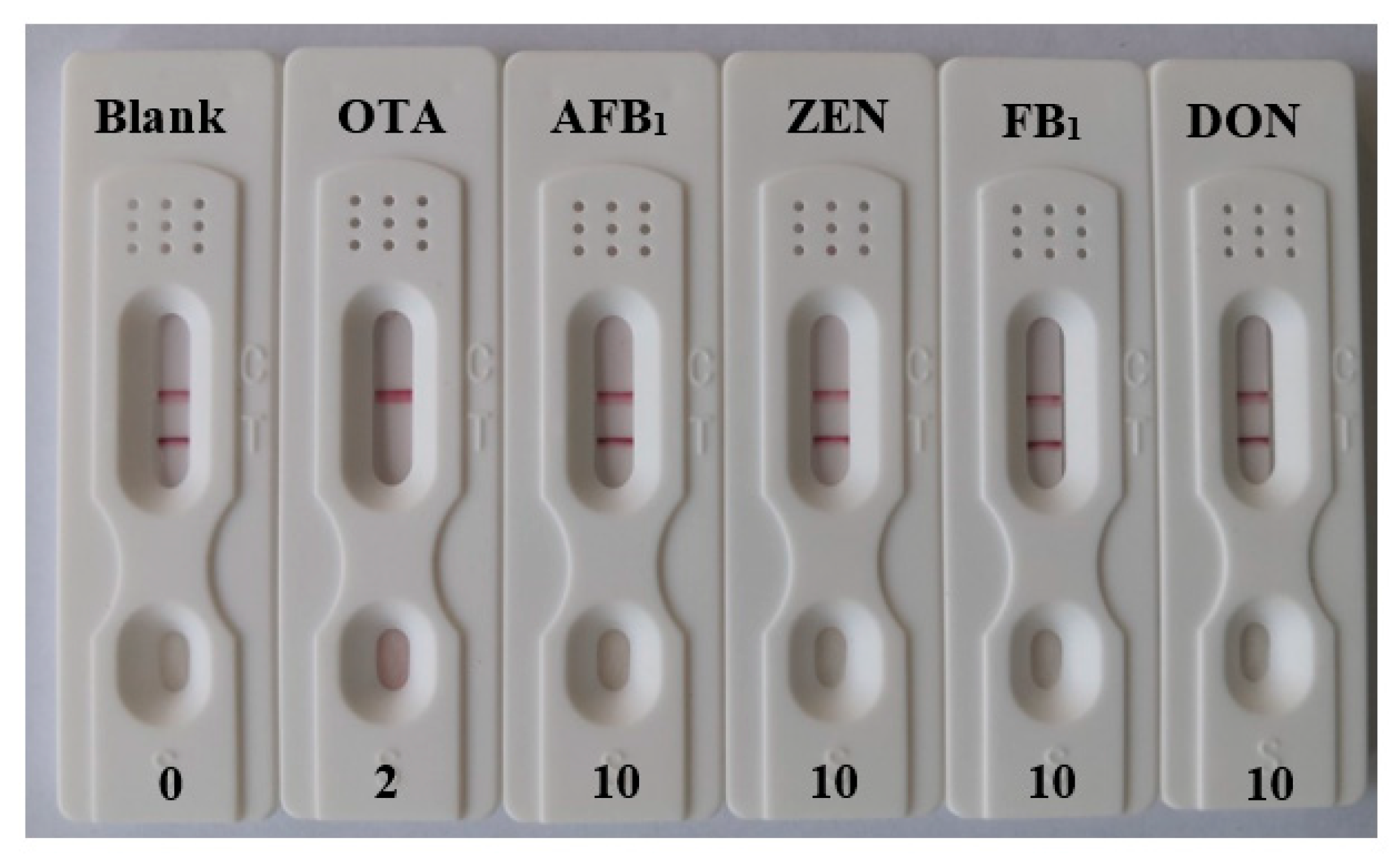
3.4. Analytical Performance of the Strip
3.5. Practical Application of the Test Strip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Guo, Y.; Luo, F.; Rao, P.; Fu, C.; Wang, S. Homogeneous Electrochemical Method for Ochratoxin A Determination Based on Target Triggered Aptamer Hairpin Switch and Exonuclease III-Assisted Recycling Amplification. Food Anal. Methods 2016, 10, 1982–1990. [Google Scholar] [CrossRef]
- Mehri, F.; Esfahani, M.; Heshmati, A.; Jenabi, E.; Khazaei, S. The prevalence of ochratoxin A in dried grapes and grape-derived products: A systematic review and meta-analysis. Toxin Rev. 2022, 41, 347–356. [Google Scholar] [CrossRef]
- Heshmati, A.; Mehri, F.; Nili-Ahmadabadi, A.; Khaneghah, A.M. The fate of ochratoxin A during grape vinegar production. Int. J. Environ. Anal. Chem. 2021, 50, 1–10. [Google Scholar] [CrossRef]
- Badie Bostan, H.; Danesh, N.M.; Karimi, G.; Ramezani, M.; Mousavi Shaegh, S.A.; Youssefi, K.; Charbgoo, F.; Abnous, K.; Taghdisi, S.M. Ultrasensitive detection of ochratoxin A using aptasensors. Biosens. Bioelectron. 2017, 98, 168–179. [Google Scholar] [CrossRef]
- Jiang, C.; Lan, L.; Yao, Y.; Zhao, F.; Ping, J. Recent progress in application of nanomaterial-enabled biosensors for ochratoxin A detection. Trac-Trends Anal. Chem. 2018, 102, 236–249. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Food safety implications of ochratoxin A in animal-derived food products. Vet. J. 2012, 192, 286–292. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, C.; Huang, Y.; Yan, J.; Chen, A. A Lateral Flow Strip Based Aptasensor for Detection of Ochratoxin A in Corn Samples. Molecules 2018, 23, 291. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Chen, C.; Huang, X.; Chen, X.; Wang, L.; Xiong, Y. Phage-free peptide ELISA for ochratoxin A detection based on biotinylated mimotope as a competing antigen. Talanta 2016, 146, 394–400. [Google Scholar] [CrossRef]
- Kong, D.; Liu, L.; Song, S.; Suryoprabowo, S.; Li, A.; Kuang, H.; Wang, L.; Xu, C. A gold nanoparticle-based semi-quantitative and quantitative ultrasensitive paper sensor for the detection of twenty mycotoxins. Nanoscale 2016, 8, 5245–5253. [Google Scholar] [CrossRef]
- Guan, B.; Zhang, X. Aptamers as Versatile Ligands for Biomedical and Pharmaceutical Applications. Int. J. Nanomed. 2020, 15, 1059–1071. [Google Scholar] [CrossRef] [Green Version]
- Song, S.-H.; Gao, Z.-F.; Guo, X.; Chen, G.-H. Aptamer-Based Detection Methodology Studies in Food Safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosens. Bioelectron. 2022, 200, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kong, W.; Dou, X.; Zhao, M.; Ouyang, Z.; Yang, M. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J. Chromatogr. B 2016, 1022, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, W.; Chen, W.; Liu, L.; Ma, W.; Zhu, Y.; Xu, L.; Kuang, H.; Xu, C. An aptamer-based chromatographic strip assay for sensitive toxin semi-quantitative detection. Biosens. Bioelectron. 2011, 26, 3059–3062. [Google Scholar] [CrossRef]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Zhang, R.L.; Gao, B.; Zhang, J.; Cui, H.Z.; Li, D.W. Propagation of PAMAM dendrimers on the carbon fiber surface by in situ polymerization: A novel methodology for fiber/matrix composites. Appl. Surf. Sci. 2015, 359, 812–818. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Senthilkumar, S. Covalent immobilization and enhanced electrical wiring of hemoglobin using gold nanoparticles encapsulated PAMAM dendrimer for electrochemical sensing of hydrogen peroxide. Appl. Surf. Sci. 2019, 495, 143540.143541–143540.143548. [Google Scholar] [CrossRef]
- Shen, G.; Xu, H.; Gurung, A.S.; Yang, Y.; Liu, G. Lateral flow immunoassay with the signal enhanced by gold nanoparticle aggregates based on polyamidoamine dendrimer. Anal. Sci. 2013, 29, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.Y.; Kang, L.C.; Pang, F.Q.; Li, H.M.; Luo, R.F.; Luo, X.L.; Sun, F.X. A signal-enhanced lateral flow strip biosensor for ultrasensitive and on-site detection of bisphenol A. Food Agric. Immunol. 2018, 29, 216–227. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.K.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Lu, S.Y.; Lin, C.; Li, Y.S.; Zhou, Y.; Meng, X.M.; Yu, S.Y.; Li, Z.H.; Li, L.; Ren, H.L.; Liu, Z.S. A screening lateral flow immunochromatographic assay for on-site detection of okadaic acid in shellfish products. Anal. Biochem. 2012, 422, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Giovannoli, C.; Giraudi, G.; Biagioli, F.; Passini, C.; Baggiani, C. A Lateral Flow Immunoassay for the Rapid Detection of Ochratoxin A in Wine and Grape Must. J. Agric. Food Chem. 2012, 60, 11491–11497. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Giovannoli, C.; Passini, C.; Baggiani, C. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal. Bioanal. Chem. 2013, 405, 9859–9867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.M.; Yang, Q.B.; Liang, C.; Chen, Y.M.; Zhang, X.L.; Liu, Z.X.; Wang, A.P. Detection of ochratoxin A by quantum dots-based fluorescent immunochromatographic assay. Anal. Bioanal. Chem. 2021, 413, 183–192. [Google Scholar] [CrossRef]
- Duan, H.; Huang, X.L.; Shao, Y.N.; Zheng, L.Y.; Guo, L.; Xiong, Y.H. Size-Dependent Immunochromatographic Assay with Quantum Dot Nanobeads for Sensitive and Quantitative Detection of Ochratoxin A in Corn. Anal. Biochem. 2017, 89, 7062–7068. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.W.; Chen, J.; Chen, X.R.; Ma, T.T.; Cai, X.X.; Duan, H.; Leng, Y.K.; Huang, X.L.; Xiong, Y.H. A novel magneto-gold nanohybrid-enhanced lateral flow immunoassay for ultrasensitive and rapid detection of ochratoxin A in grape juice. Food Chem. 2021, 336, 127710–127718. [Google Scholar] [CrossRef]
| a Group | Add (ng mL−1) | Found (ng mL−1) | b Recovery (%) | b RSD (%) |
|---|---|---|---|---|
| 1 | 0.05 | 0.050 ± 0.004 | 103 | 8 |
| 2 | 0.1 | 0.095 ± 0.003 | 96 | 3 |
| 3 | 0.4 | 0.42 ± 0.02 | 106 | 4 |
| 4 | 0.5 | 0.49 ± 0.02 | 101 | 3 |
| 5 | 1 | 0.93 ± 0.04 | 93 | 4 |
| Labels | Signal Output | Recognition Probe | LOD (ng mL−1) | Assay Time (min) | Reference |
|---|---|---|---|---|---|
| AuNPs | Colorimetry | Antibody | 1 | 5 | [22] |
| AuNPs/AgNPs | Colorimetry | Antibody | 0.9 | 10 | [23] |
| Quantum dots | Fluorescence | Antibody | 0.07 | 15 | [24] |
| Quantum dot nanobeads | Fluorescence | Antibody | 0.085 | 15 | [25] |
| Magneto–gold nanohybrid | Colorimetry | Antibody | 0.094 | 5 | [26] |
| AuNPs | Colorimetry | Aptamer | 0.18 | 10 | [14] |
| AuNPs | Colorimetry | Aptamer | 1 | 15 | [13] |
| Cy5 | Fluorescence | Aptamer | 0.4 | 10 | [7] |
| AuNPs | Colorimetry | Aptamer | 0.04 | 15 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, D.; Cui, S.; Li, C.; Yun, Z.; Zhang, J.; Sun, F. Design of a Signal-Amplified Aptamer-Based Lateral Flow Test Strip for the Rapid Detection of Ochratoxin A in Red Wine. Foods 2022, 11, 1598. https://doi.org/10.3390/foods11111598
Liu Y, Liu D, Cui S, Li C, Yun Z, Zhang J, Sun F. Design of a Signal-Amplified Aptamer-Based Lateral Flow Test Strip for the Rapid Detection of Ochratoxin A in Red Wine. Foods. 2022; 11(11):1598. https://doi.org/10.3390/foods11111598
Chicago/Turabian StyleLiu, Yinyin, Dan Liu, Shuangshuang Cui, Can Li, Ziguang Yun, Jian Zhang, and Fengxia Sun. 2022. "Design of a Signal-Amplified Aptamer-Based Lateral Flow Test Strip for the Rapid Detection of Ochratoxin A in Red Wine" Foods 11, no. 11: 1598. https://doi.org/10.3390/foods11111598
APA StyleLiu, Y., Liu, D., Cui, S., Li, C., Yun, Z., Zhang, J., & Sun, F. (2022). Design of a Signal-Amplified Aptamer-Based Lateral Flow Test Strip for the Rapid Detection of Ochratoxin A in Red Wine. Foods, 11(11), 1598. https://doi.org/10.3390/foods11111598





