Catechin Mediates Ferroptosis to Exert an Anti-Inflammatory Effect on RAW 264.7 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anti-Inflammatory Effect
2.2.1. Cell Culture
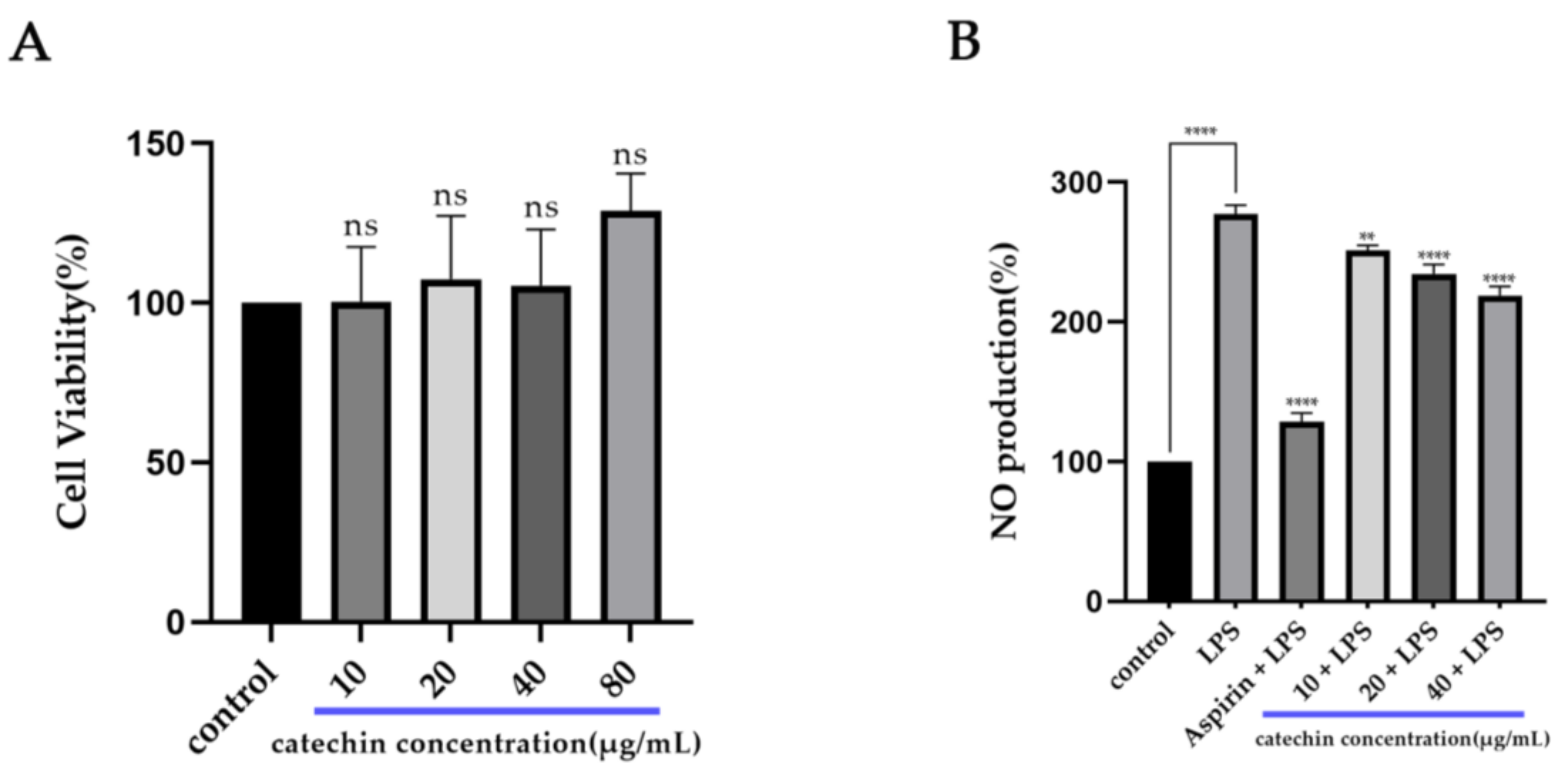
2.2.2. CCK-8 Assay
2.2.3. NO Assay
2.3. Network Pharmacology Analysis
2.4. Metabolomic Analysis
2.5. Molecular Docking
2.6. Statistical Analysis
3. Results
3.1. Anti-Inflammatory Activity of Catechin
3.2. Potential Targets and Pathways of Catechin against Inflammation
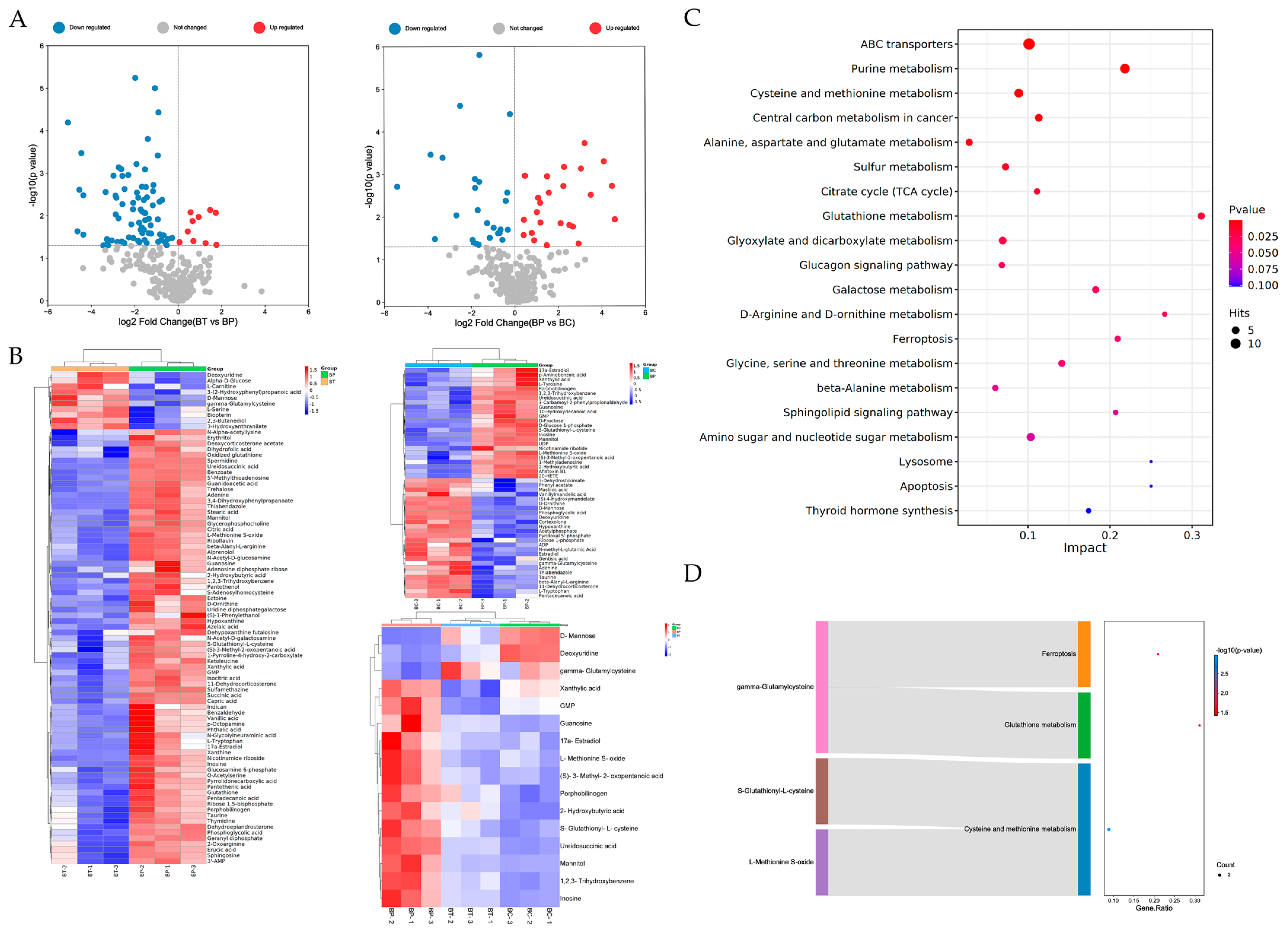
3.3. Potential Different Metabolites and Related Metabolic Pathways
3.4. Binding Modes of Catechin with Related Proteins
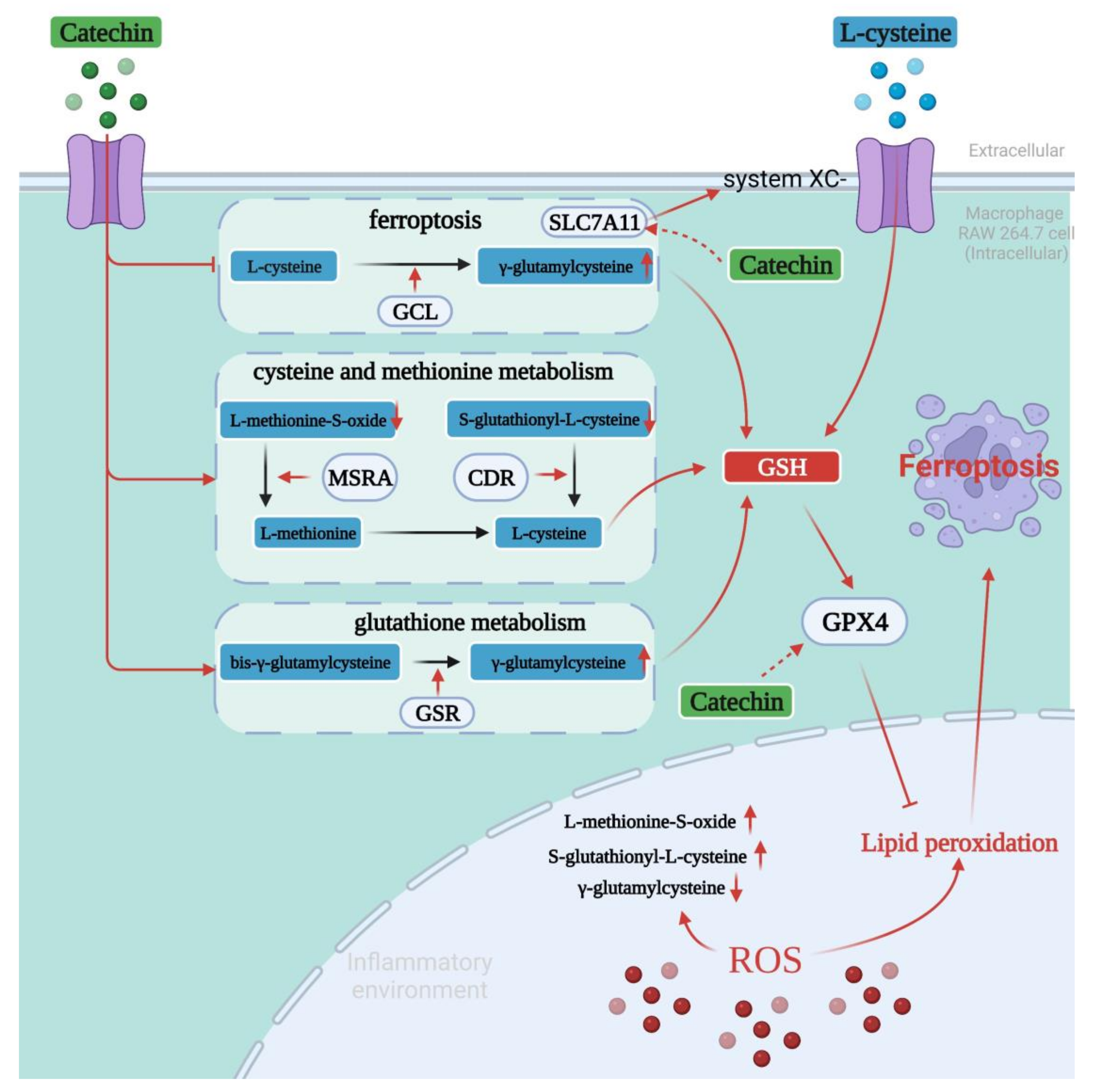
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diab. Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2018, 133, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Kuprash, D.V.; Nedospasov, S.A. Molecular and Cellular Mechanisms of Inflammation. Biochemistry 2016, 81, 1237–1239. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Sun, Y.T.; Chen, P.; Zhai, B.T.; Zhang, M.M.; Xiang, Y.; Fang, J.H.; Xu, S.N.; Gao, Y.F.; Chen, X.; Sui, X.B.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef]
- Wang, W.M.; Green, M.; Choi, J.E.; Gijon, M.; Kennedy, P.D.; Johnson, J.K.; Liao, P.; Lang, X.T.; Kryczek, I.; Sell, A.; et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019, 569, 270–274. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [Green Version]
- Mao, H.M.; Zhao, Y.H.; Li, H.X.; Lei, L. Ferroptosis as an emerging target in inflammatory diseases. Prog. Biophys. Mol. Bio. 2020, 155, 20–28. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xu, Z.; Zheng, W.M. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [Green Version]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Siebert, D.A.; Paganelli, C.J.; Queiroz, G.S.; Alberton, M.D. Anti-inflammatory activity of the epicuticular wax and its isolated compounds catechin and gallocatechin from Eugenia brasiliensis Lam. (Myrtaceae) leaves. Nat. Prod. Res. 2021, 35, 4720–4723. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, T.; Yan, F.F.; Zhu, X.L.; Lu, Q.; Liu, R. Synergistic inhibitory effects of procyanidin B2 and catechin on acrylamide in food matrix. Food Chem. 2019, 296, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, X.; Yang, S.J.; Cheng, M.; Zhou, Y.; Zhou, M.; Ye, Z.; Qiu, W.H.; He, H.; Cen, X.Z.; et al. Acrylamide exposure and pulmonary function reduction in general population: The mediating effect of systemic inflammation. Sci. Total. Environ. 2021, 778, 146304. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.W.; Cai, S.; Zhao, T.S.; Li, M.; Tian, Y. Green tea derivative (-)-epigallocatechin-3-gallate (EGCG) confers protection against ionizing radiation-induced intestinal epithelial cell death both in vitro and in vivo. Free Radic. Biol. Med. 2020, 161, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.L.; Han, Y.; Yang, L.H.; Hu, L.M.; Duan, X.W.; Yang, X.; Huang, R.M. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macromol. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Sohn, S.H.; Kim, T.S.; Kim, J.W.; Yoo, S.M.; Jo, W.M. Anti-thrombotic and anti-inflammatory activity of sulodexide compared to aspirin in the rat model. Clin. Hemorheol. Microcirc. 2021, 77, 435–442. [Google Scholar] [CrossRef]
- Chen, M.M.; Yang, F.F.; Yang, X.M.; Lai, X.M.; Gao, Y.X. Systematic Understanding of Mechanisms of a Chinese Herbal Formula in Treatment of Metabolic Syndrome by an Integrated Pharmacology Approach. Int. J. Mol. Sci. 2016, 17, 2114. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.Q.; Luo, L.X.; Lin, H.W.; Li, X.L.; Huang, R.M. Potential therapeutic targets and molecular details of anthocyan-treated inflammatory bowel disease: A systematic bioinformatics analysis of network pharmacology. RSC Adv. 2021, 11, 8239–8249. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zhang, Y.Q.; Liu, Z.M.; Chen, T.; Lv, C.Y.; Tang, S.H.; Zhang, X.B.; Zhang, W.; Li, Z.Y.; Zhou, R.R.; et al. ETCM: An encyclopaedia of traditional Chinese medicine. Nucleic Acids Res. 2019, 47, D976–D982. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.H.; Lv, R.L.; Qian, H.B.; Chen, X.Y.; Yang, C.F. Study on the Multitarget Mechanism and Key Active Ingredients of Herba Siegesbeckiae and Volatile Oil against Rheumatoid Arthritis Based on Network Pharmacology. Evid. Based Complement. Alternat. Med. 2019, 2019, 8957245. [Google Scholar] [CrossRef]
- Guo, H.H.; Guo, H.X.; Zhang, L.; Tang, Z.M.; Yu, X.M.; Wu, J.F.; Zeng, F.C. Metabolome and Transcriptome Association Analysis Reveals Dynamic Regulation of Purine Metabolism and Flavonoid Synthesis in Transdifferentiation during Somatic Embryogenesis in Cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Tian, G.L.; Qin, J.W.; Wu, B.Q.; Tan, L.; Xu, L.; Wu, S.Z.; Yang, J.T.; Jiang, J.H.; Yu, R.Q. Coupling bootstrap with synergy self-organizing map-based orthogonal partial least squares discriminant analysis: Stable metabolic biomarker selection for inherited metabolic diseases. Talanta 2020, 219, 121370. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [Green Version]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Duarte, J.M.; Dutta, S.; Fayazi, M.; Feng, Z.; et al. RCSB Protein Data Bank: Celebrating 50 years of the PDB with new tools for understanding and visualizing biological macromolecules in 3D. Protein Sci. 2022, 31, 187–208. [Google Scholar] [CrossRef]
- Guruvayoorappan, C.; Kuttan, G. (+)-Catechin inhibits tumour angiogenesis and regulates the production of nitric oxide and TNF-alpha in LPS-stimulated macrophages. Innate Immun. 2008, 14, 160–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaldan, S.; Bush, A.I.; Devos, D.; Rolland, A.S.; Moreau, C. Striking while the iron is hot: Iron metabolism and ferroptosis in neurodegeneration. Free Radical. Bio. Med. 2019, 133, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.K.; Li, P.; Wu, W.Z.; Wang, Q.Z.; Qian, B.; Li, X.; Shen, M.L. Roles of ferroptosis in urologic malignancies. Cancer Cell Int. 2021, 21, 676. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Li, M.X.; Liu, Y.F.; Qiao, Z.T.; Wang, Z.W. Inhibition of ferroptosis alleviates atherosclerosis through attenuating lipid peroxidation and endothelial dysfunction in mouse aortic endothelial cell. Free Radic. Biol. Med. 2020, 160, 92–102. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Wang, J.M.; Hu, W.W.; Feng, Z.H. The Regulation of Ferroptosis by Tumor Suppressor p53 and its Pathway. Int. J. Mol. Sci. 2020, 21, 8387. [Google Scholar] [CrossRef] [PubMed]
- Boulesteix, A.L.; Strimmer, K. Partial least squares: A versatile tool for the analysis of high-dimensional genomic data. Brief. Bioinform. 2007, 8, 32–44. [Google Scholar] [CrossRef] [Green Version]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Jaune-Pons, E.; Vasseur, S. Role of amino acids in regulation of ROS balance in cancer. Arch. Biochem. Biophys. 2020, 689, 108438. [Google Scholar] [CrossRef]
- Kim, Y.K.; Shin, Y.J.; Lee, W.H.; Kim, H.Y.; Hwang, K.Y. Structural and kinetic analysis of an MsrA-MsrB fusion protein from Streptococcus pneumoniae. Mol. Microbiol. 2009, 72, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Cho, I.J.; Kim, D.; Kim, E.O.; Jegal, K.H.; Kim, J.K.; Park, S.M.; Zhao, R.J.; Ki, S.H.; Kim, S.C.; Ku, S.K. Cystine and Methionine Deficiency Promotes Ferroptosis by Inducing B-Cell Translocation Gene 1. Antioxidants 2021, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Sha, W.X.; Hu, F.; Xi, Y.; Chu, Y.D.; Bu, S.Z. Mechanism of Ferroptosis and Its Role in Type 2 Diabetes Mellitus. J. Diabetes Res. 2021, 2021, 9999612. [Google Scholar] [CrossRef] [PubMed]
- Sabens Liedhegner, E.A.; Gao, X.H.; Mieyal, J.J. Mechanisms of altered redox regulation in neurodegenerative diseases—Focus on S—Glutathionylation. Antioxid. Redox. Signal. 2012, 16, 543–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imber, M.; Pietrzyk-Brzezinska, A.J.; Antelmann, H. Redox regulation by reversible protein S-thiolation in Gram-positive bacteria. Redox Biol. 2019, 20, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Tung, Q.N.; Linzner, N.; Loi, V.V.; Antelmann, H. Application of genetically encoded redox biosensors to measure dynamic changes in the glutathione, bacillithiol and mycothiol redox potentials in pathogenic bacteria. Free Radical. Bio. Med. 2018, 128, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, T.; Sato, M.; Oh-Hashi, K.; Furuta, K.; Hirata, Y. Oxindole-curcumin hybrid compound enhances the transcription of gamma-glutamylcysteine ligase. Eur. J. Pharmacol. 2021, 896, 173898. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Li, B.; Yao, H.; Zarka, M.; Welch, J.; Sachdev, P.; Bridge, W.; Braidy, N. Supplementation with gamma-glutamylcysteine (gamma-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 2021, 144, 104931. [Google Scholar] [CrossRef]
- Kim, J.; Copley, S.D. The orphan protein bis-gamma-glutamylcystine reductase joins the pyridine nucleotide disulfide reductase family. Biochemistry 2013, 52, 2905–2913. [Google Scholar] [CrossRef] [Green Version]
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta. Med. Indones. 2017, 49, 158–165. [Google Scholar]
- Hussain, T.; Tan, B.; Yin, Y.L.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Venkataramani, V. Iron Homeostasis and Metabolism: Two Sides of a Coin. Adv. Exp. Med. Biol. 2021, 1301, 25–40. [Google Scholar] [CrossRef]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Tarafdar, S.; Kim, G.; Levine, R.L. Drosophila methionine sulfoxide reductase A (MSRA) lacks methionine oxidase activity. Free Radical. Bio. Med. 2019, 131, 154–161. [Google Scholar] [CrossRef]
- Sheng, L.L.; Luo, Q.; Chen, L.G. Amino Acid Solute Carrier Transporters in Inflammation and Autoimmunity. Drug Metab. Dispos. 2022, DMD-AR-2021-000705. [Google Scholar] [CrossRef]
- Habib, E.; Linher-Melville, K.; Lin, H.X.; Singh, G. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox. Biol. 2015, 5, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Pfau, J.C.; Seib, T.; Overocker, J.J.; Roe, J.; Ferro, A.S. Functional expression of system x(c)- is upregulated by asbestos but not crystalline silica in murine macrophages. Inhal. Toxicol. 2012, 24, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Li, H.H.; Shen, Y.J.; Xiao, H.; Sun, W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef]
- Quintana-Cabrera, R.; Fernandez-Fernandez, S.; Bobo-Jimenez, V.; Escobar, J.; Sastre, J.; Almeida, A.; Bolanos, J.P. gamma-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat. Commun. 2012, 3, 718. [Google Scholar] [CrossRef] [Green Version]


| Gene Name | UniProt ID | Protein Name | Degree Centrality (DC) |
|---|---|---|---|
| Akt1 | P31750 | RAC-alpha serine/threonine-protein kinase | 11 |
| Hsp90aa1 | P07901 | Heat shock protein HSP 90-alpha | 10 |
| Esr1 | P19785 | Estrogen receptor | 10 |
| Hsp90ab1 | P11499 | Heat shock protein HSP 90-beta | 9 |
| Esr2 | O08537 | Estrogen receptor beta | 6 |
| Nos3 | P70313 | Nitric oxide synthase, endothelial | 5 |
| Itgb1 | P09055 | Integrin beta-1 | 5 |
| Cdk1 | P11440 | Cyclin-dependent kinase 1 | 5 |
| Ncoa1 | P70365 | Nuclear receptor coactivator 1 | 5 |
| Ahr | P30561 | Aryl hydrocarbon receptor | 4 |
| Chuk | Q60680 | Inhibitor of nuclear factor kappa-B kinase subunit alpha | 4 |
| Cdk2 | P97377 | Cyclin-dependent kinase 2 | 4 |
| Chek1 | O35280 | Serine/threonine-protein kinase Chk1 | 4 |
| Hspa2 | P17156 | Heat shock-related 70 kDa protein 2 | 3 |
| Actb | P60710 | Actin, cytoplasmic 1 | 3 |
| Differential Metabolites | VIP (VIP > 1) | p-Value (p < 0.05) | Fold Change (FC) | BP vs. BC Trend | BT vs. BP Trend |
|---|---|---|---|---|---|
| 17a-Estradiol | 1.23640275 | 0.02890117 | 0.63 | up 1 | down 2 |
| Xanthylic acid | 1.378648746 | 0.007098714 | 0.3 | up | down |
| Porphobilinogen | 1.228633844 | 0.041716502 | 0.66 | up | down |
| 1,2,3-Trihydroxybenzene | 1.408122971 | 0.004251857 | 0.33 | up | down |
| Ureidosuccinic acid | 1.479845722 | 5.71205 × 10−6 | 0.25 | up | down |
| Guanosine | 1.381268976 | 0.041686027 | 0.29 | up | down |
| GMP | 1.387077366 | 0.002758109 | 0.1 | up | down |
| S-Glutathionyl-l-cysteine | 1.350657845 | 0.011718718 | 0.39 | up | down |
| Inosine | 1.429164585 | 0.00206919 | 0.31 | up | down |
| L-Methionine s-oxide | 1.411921836 | 0.003672009 | 0.37 | up | down |
| (S)-3-Methyl-2-oxopentanoic acid | 1.226482998 | 0.033345036 | 0.24 | up | down |
| 2-Hydroxybutyric acid | 1.251116465 | 0.037188342 | 0.58 | up | down |
| Mannitol | 1.449246459 | 0.001098845 | 0.2 | up | down |
| Deoxyuridine | 1.242857502 | 0.041777372 | 1.04 | down | up |
| D-Mannose | 1.332811288 | 0.008474664 | 3.32 | down | up |
| γ-Glutamylcysteine | 1.350096846 | 0.007306674 | 2.77 | down | up |
| Compound | Protein | Amino Acid Residues | Docking Score | Binding Energy (kcal/mol) |
|---|---|---|---|---|
| Catechin | MSRA | Lys79, Asn138, Asp149, Asp150 | −5.587 | −31.47 |
| Catechin | CDR | Asp35, Asn42, Arg112, Asp112, Ser112, Ala115 | −8.428 | −55.50 |
| Catechin | GSR | Ser52, Cys80, Val83, Cyt85, Lys88, Ser199 | −6.949 | −52.12 |
| Catechin | GCL | Arg185, Asn247, Arg296, Ser403, Trp406, Arg410, Arg427 | −5.950 | −42.67 |
| Catechin | SLC7A11 | Val57, Ser60, Gly61, Ile134, Arg135, Tyr244, Tye251, Ser393 | −6.229 | −44.74 |
| Catechin | GPX4 | Gly47, Lys48, Trp136 | −3.744 | −33.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuang, W.; Yang, J.; Liu, Z.; Zeng, J.; Xia, X.; Chen, X.; Zhong, S.; Huang, R. Catechin Mediates Ferroptosis to Exert an Anti-Inflammatory Effect on RAW 264.7 Cells. Foods 2022, 11, 1572. https://doi.org/10.3390/foods11111572
Kuang W, Yang J, Liu Z, Zeng J, Xia X, Chen X, Zhong S, Huang R. Catechin Mediates Ferroptosis to Exert an Anti-Inflammatory Effect on RAW 264.7 Cells. Foods. 2022; 11(11):1572. https://doi.org/10.3390/foods11111572
Chicago/Turabian StyleKuang, Weiyang, Jiajia Yang, Zhiyuan Liu, Jinzi Zeng, Xuewei Xia, Xiaodan Chen, Saiyi Zhong, and Riming Huang. 2022. "Catechin Mediates Ferroptosis to Exert an Anti-Inflammatory Effect on RAW 264.7 Cells" Foods 11, no. 11: 1572. https://doi.org/10.3390/foods11111572
APA StyleKuang, W., Yang, J., Liu, Z., Zeng, J., Xia, X., Chen, X., Zhong, S., & Huang, R. (2022). Catechin Mediates Ferroptosis to Exert an Anti-Inflammatory Effect on RAW 264.7 Cells. Foods, 11(11), 1572. https://doi.org/10.3390/foods11111572








