Detection of Resistant and Enterotoxigenic Strains of Staphylococcus warneri Isolated from Food of Animal Origin
Abstract
1. Introduction
2. Materials and Methods
2.1. Detection of the Phenotypic Properties of the Isolates of Isolation of Strains
2.2. Isolation DNA from Staphylococcal Isolates
2.3. Genotypic Analysis of Staphylococcal Isolates
2.4. Detection of Genes Encoding Staphylococcal Enterotoxins
2.5. Detection of Antimicrobial Resistance
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azimi, T.; Mirzadeh, M.; Sabour, S.; Nasser, A.; Fallah, F.; Pourmand, M.R. Coagulase-negative staphylococci (CoNS) meningitis: A narrative review of the literature from 2000 to 2020. New Microbes New Infect. 2020, 37, 100755. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, B.; Bhatnagar, U.B.; Conaway, D.G. An Unusual Presentation of Native Valve Endocarditis Caused by Staphylococcus warneri. Rev. Cardiovasc. Med. 2016, 17, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Seng, R.; Kitti, T.; Thummeepak, R.; Kongthai, P.; Leungtongkam, U.; Wannalerdsakun, S.; Sitthisak, S. Biofilm formation of methicillin-resistant coagulase negative staphylococci (MR-CoNS) isolated from community and hospital environments. PLoS ONE 2017, 12, e0184172. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Peitler, D.; Karakulska, J. Staphylococci isolated from ready-toeat meat-identification, antibiotic resistance and toxin gene profile. Int. J. Food Microbiol. 2016, 238, 113–120. [Google Scholar] [CrossRef]
- Bhargava, K.; Zhang, Y. Characterization of methicillin-resistant coagulasenegative staphylococci (MRCoNS) in retail meat. Food Microbiol. 2014, 42, 56–60. [Google Scholar] [CrossRef]
- Incani, R.N.; Hernández, M.; Cortez, J.; González, M.E.; Dorel Salazar, Y. Staphylococcus warneri meningitis in a patient with Strongyloides stercoralis hyperinfection and lymphoma: First report of a case. Rev. Inst. Med. Trop. São Paulo 2010, 52, 169–170. [Google Scholar] [CrossRef]
- Musharrafieh, R.; Tacchi, L.; Trujeque, J.; LaPatra, S.; Salinas, I. Staphylococcus warneri, a resident skin commensal of rainbow trout (Oncorhynchus mykiss) with pathobiont characteristics. Vet. Microbiol. 2014, 169, 80–88. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Bai, Y.; Xu, X.; Zhou, G. Pathogenicity and antibiotic resistance of coagulase-negative staphylococci isolated from retailing chicken meat. LWT 2018, 90, 152–156. [Google Scholar] [CrossRef]
- El-Ashker, M.; Gwida, M.; Monecke, S.; Ehricht, R.; Elsayed, M.; El-Gohary, F.; Reißig, A.; Müller, E.; Paul, A.; Igbinosa, E.O.; et al. Microarray-based detection of resistance genes in coagulase-negative staphylococci isolated from cattle and buffalo with mastitis in Egypt. Trop. Anim. Health Prod. 2020, 52, 3855–3862. [Google Scholar] [CrossRef]
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic potential of coagulase-negative staphylococci from ready-to-eat food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, N.; Ceniti, C.; Santoro, A.; Clausi, M.T.; Casalinuovo, F. Foodborne pathogen assessment in raw milk cheeses. Int. J. Food Sci. 2020, 2020, 3616713. [Google Scholar] [CrossRef] [PubMed]
- Hleba, L.; Kačániová, M. Antibiotická Rezistencia Čeľade Enterobacteriaceae vo Vzťahu k Potravinám; Slovenská Poľnohospodárska Univerzita v Nitre: Nitra, Slovenska, 2015; p. 93. ISBN 978-80-552-1408-5. [Google Scholar]
- Wright, G.D. The antibiotic resistome: The nexus of chemical and genetic diversity. Nat. Rev. Microbiol. 2007, 5, 175–186. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Schlüter, A.; Szczepanowski, R.; Pühler, A.; Top, E.M. Genomics of IncP-1 antibiotic resistance plasmids isolated from wastewater treatment plants provides evidence for a widely accessible drug resistance gene pool. FEMS Microbiol. Rev. 2007, 31, 449–477. [Google Scholar] [CrossRef]
- Deyno, S.; Fekadu, S.; Seyfe, S. Prevelance and antimicrobial resistance of coagulase staphylococci clinical isolates from Ethiopia: A meta analysis. BMC Microbiol. 2018, 18, 43. [Google Scholar] [CrossRef]
- Szczuka, E.; Krzyminska, S.; Kaznowski, A. Clonality, virulance and the occurrence of genes encoding antibiotic resistance among Staphylococcus warneri from bloodstream infections. J. Med. Microbiol. 2016, 65, 828–836. [Google Scholar] [CrossRef]
- Chaves, R.D.; Pradella, F.; Turatti, M.A.; Amaro, E.C.; da Silva, A.R.; dos Santos Farias, A.; Pereira, J.S.; Khaneghah, A.M. Evaluation of Staphylococcus spp. in food and kitchen premises of Campinas, Brazil. Food Control. 2018, 84, 463–470. [Google Scholar] [CrossRef]
- Owens, J.J. The egg yolk reaction produced by several species of bacteria. J. Appl. Bacteriol. 1974, 37, 137–148. [Google Scholar] [CrossRef]
- Sharma, N.K.; Rees, C.E.; Dodd, C.E. Development of a single-reaction multiplex PCR toxin typing assay for Staphylococcus aureus strains. Appl. Environ. Microbiol. 2000, 66, 1347–1353. [Google Scholar] [CrossRef]
- Iwase, T.; Seki, K.; Shinji, H.; Mizunoe, Y.; Masuda, S. Development of a real-time PCR assay for the detection and identification of Staphylococcus capitis, Staphylococcus haemolyticus and Staphylococcus warneri. J. Med. Microbiol. 2007, 56, 1346–1349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CLSI Document VET01-S2 Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013; pp. 1–168.
- EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; pp. 1–43.
- Poulsen, A.B.; Skov, R.; Pallesen, L.V. Detection of methicillin resistance in coagulase-negative staphylococci and in staphylococci directly from simulated blood cultures using the EVIGENE MRSA Detection Kit. J. Antimicrob. Chemother. 2003, 51, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Hoveida, L.; Ataei, B.; Amirmozafari, N.; Noormohammadi, Z. Species diversity and molecular analysis of Staphylococcus in confectioneries of a developing country, Iran. Infez. Med. 2018, 26, 148–154. [Google Scholar] [PubMed]
- Hammad, A.M.; Watanabe, W.; Fujii, T.; Shimamoto, T. Occurrence and characteristics of methicillin-resistant and -susceptible Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci from Japanese retail ready-to-eat raw fish. Int. J. Food Microbiol. 2016, 156, 286–289. [Google Scholar] [CrossRef]
- Vandenesch, F.; Lina, G.; Henry, T. Staphylococcus aureus hemolysins, bi-component leukocidins, and cytolytic peptides: A redundant arsenal of membrane-damaging virulence factors. Front. Cell. Infect. Microbiol. 2012, 2, 12. [Google Scholar] [CrossRef]
- Noumi, E.; Merghni, A.; Alreshidi, M.; Del Campo, R.; Adnan, M.; Haddad, O.; De Feo, V.; Snoussi, M. Phenotypic and Genotypic Characterization with MALDI-TOF-MS Based Identification of Staphylococcus spp. Isolated from Mobile Phones with their Antibiotic Susceptibility, Biofilm Formation, and Adhesion Properties. Int. J. Environ. Res. Public Health 2020, 17, 3761. [Google Scholar] [CrossRef]
- Fisher, K.; Phillips, C. The ecology, epidemiology and virulence of Enterococcus. Microbiology 2009, 155, 1749–1757. [Google Scholar] [CrossRef]
- LEPIDI, A. Chapter 12—Staphylococcal Lipases. In Pet-to-Man Travelling Staphylococci; Academic Press: Cambridge, MA, USA, 2018; pp. 147–159. ISBN 978-0-12-813-547-1. [Google Scholar]
- Gundogan, N.; Ataol, O.; Torlak, F.O. Determination of Some Virulence Factors in Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium Isolated from Meat and Milk Products. J. Food Saf. 2012, 33, 387–393. [Google Scholar] [CrossRef]
- Gundogan, N.; Ataol, O. Biofilm, protease and lipase properties and antibiotic resistance profiles of staphylococci isolated from various foods. Afr. J. Microbiol. Res. 2013, 33, 3582–3588. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Production, purification and biochemical characterisation of a novel lipase from a newly identified lipolytic bacterium Staphylococcus caprae NCU S6. J. Enzym. Inhib. Med. Chem. 2021, 36, 248–256. [Google Scholar] [CrossRef]
- Poyart, C.; Quesne, G.; Boumaila, C.; Trieu-Cuot, P. Rapid and accurate species-level identification of coagulase-negative staphylococci by using the sodA gene as a target. J. Clin. Microbiol. 2001, 39, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Staphylococcal enterotoxins. Toxins 2010, 2, 2177–2197. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, I.; Soriano, J.M.; Mañes, J. Enterotoxinomics: The omic sciences in the study of staphylococcal toxins analyzed in food matrices. Food Res. Int. 2013, 54, 1052–1060. [Google Scholar] [CrossRef]
- De Freitas Guimarães, F.; Nóbrega, D.B.; Richini-Pereira, V.B.; Marson, P.M.; de Figueiredo Pantoja, J.C.; Langoni, H. Enterotoxin genes in coagulase-negative and coagulase-positive staphylococci isolated from bovine milk. J. Dairy Sci. 2013, 96, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, S.; Wałecka-Zacharska, E.; Schubert, J.; Tabiś, A.; Król, J.; Stefaniak, T.; Węsierska, E.; Bania, J. Staphylococcal Enterotoxin Genes in Coagulase-Negative Staphylococci—Stability, Expression, and Genomic Context. Int. J. Mol. Sci. 2022, 23, 2560. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.L.; Li, S.; Fang, R.; Ono, H.K. Update on molecular diversity and multipathogenicity of staphylococcal superantigen toxins. Anim. Dis. 2021, 1, 7. [Google Scholar] [CrossRef]
- Hoveida, L.; Ataei, B.; Amirmozafari, N.; Noormohammadi, Z. Species variety, antibiotic susceptibility patterns and prevalence of enterotoxin genes in Staphylococci isolated from foodstuff in Central Iran. Iran. J. Public Health 2020, 49, 96. [Google Scholar] [CrossRef]
- Saber, H.; Jasni, A.S.; Jamaluddin, T.Z.M.T.; Ibrahim, R. A review of staphylococcal cassette chromosome mec (SCCmec) types in coagulase-negative staphylococci (CoNS) species. Malays. J. Med. Sci. 2017, 24, 7–18. [Google Scholar] [CrossRef]
- Ballhausen, B.; Kriegeskorte, A.; Schleimer, N.; Peters, G.; Becker, K. The mecA homolog mecC confers resistance against-lactams in Staphylococcus aureus irrespective of the genetic strain background. Antimicrob. Agents Chemother. 2014, 58, 3791–3798. [Google Scholar] [CrossRef]
- Humphries, R.M.; Magnano, P.; Burnham, C.A.D.; Dien Bard, J.; Dingle, T.C.; Callan, K.; Westblade, L.F. Evaluation of surrogate tests for the presence of mecA-mediated methicillin resistance in Staphylococcus capitis, Staphylococcus haemolyticus, Staphylococcus hominis, and Staphylococcus warneri. J. Clin. Microbiol. 2020, 59, e02290-20. [Google Scholar] [CrossRef]
- Al-Saadi, D.A.A.; Abd Al-Mayahi, F.S. Antibiogram Susceptibility Patterns of Staphylococcus Aureus Harboring of MecA Gene and Prevalence Aminoglycoside Modifying Enzymes (AMEs) Genes in Iraq. IOP Conf. Ser. Earth Environ. Sci. 2021, 923, 012049. [Google Scholar] [CrossRef]
- Perumal, N.; Murugesan, S.; Krishnan, P. Distribution of genes encoding aminoglycoside-modifying enzymes among clinical isolates of methicillin-resistant staphylococci. Indian J. Med. Microbiol. 2016, 34, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, C.; Sundsfjord, A.; Rønnestad, A.; Mikalsen, J.; Gaustad, P.; Flægstad, T. Phenotypic and genotypic aminoglycoside resistance in blood culture isolates of coagulase-negative staphylococci from a single neonatal intensive care unit, 1989–2000. J. Antimicrob. Chemother. 2004, 54, 889–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ardic, N.; Sareyyupoglu, B.; Ozyurt, M.; Haznedaroglu, T.; Ilga, U. Investigation of aminoglycoside modifying enzyme genes in methicillin-resistant staphylococci. Microbiol. Res. 2006, 161, 49–54. [Google Scholar] [CrossRef]
- Emaneini, M.; Taherikalani, M.; Eslampour, M.A.; Sedaghat, H.; Aligholi, M.; Jabalameli, F.; Shahsavan, S.; Sotoudeh, N. Phenotypic and genotypic evaluation of aminoglycoside resistance in clinical isolates of staphylococci in Tehran, Iran. Microb. Drug Resist. 2009, 15, 129–132. [Google Scholar] [CrossRef]
- Martineau, F.; Picard, F.J.; Lansac, N.; Ménard, C.; Roy, P.H.; Ouellette, M.; Bergeron, M.G. Correlation between the resistance genotype determined by multiplex PCR assays and the antibiotic susceptibility patterns of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob. Agents Chemother. 2000, 44, 231–238. [Google Scholar] [CrossRef]
- Chaudhury, A.; Kumar, A.G. In vitro activity of antimicrobial agents against oxacillin resistant staphylococci with special reference to Staphylococcus haemolyticus. Indian J. Med. Microbiol. 2007, 25, 50–52. [Google Scholar] [CrossRef]
- Goudarzi, G.; Tahmasbi, F.; Anbari, K.; Ghafarzadeh, M. Distribution of genes encoding resistance to macrolides among staphylococci isolated from the nasal cavity of hospital employees in Khorramabad, Iran. Iran. Red Crescent Med. J. 2016, 18, e25701. [Google Scholar] [CrossRef]
- Roberts, M.C.; Sutcliffe, J.; Courvalin, P.; Jensen, L.B.; Rood, J.; Seppala, H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 1999, 43, 2823–2830. [Google Scholar] [CrossRef]
- Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 2002, 34, 482–492. [Google Scholar] [CrossRef]
- Szemraj, M.; Czekaj, T.; Kalisz, J.; Szewczyk, E.M. Differences in distribution of MLS antibiotics resistance genes in clinical isolates of staphylococci belonging to species: S. epidermidis, S. hominis, S. haemolyticus, S. simulans and S. warneri. BMC Microbiol. 2019, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Munier, A.L.; de Lastours, V.; Barbier, F.; Chau, F.; Fantin, B.; Ruimy, R. Comparative dynamics of the emergence of fluoroquinolone resistance in staphylococci from the nasal microbiota of patients treated with fluoroquinolones according to their environment. Int. J. Antimicrob. Agents 2015, 46, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Pegues, D.A.; Colby, C.; Hibberd, P.L.; Cohen, L.G.; Ausubel, F.M.; Calderwood, S.B.; Hooper, D.C. The epidemiology of resistance to ofloxacin and oxacillin among clinical coagulase-negative staphylococcal isolates: Analysis of risk factors and strain types. Clin. Infect. Dis. 1998, 26, 72–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Persson-Waller, K.; Aspán, A.; Nyman, A.; Persson, Y.; Grönlund Andersson, U. CNS species and antimicrobial resistance in clinical and subclinical bovine mastitis. Vet. Microbiol. 2011, 152, 112–116. [Google Scholar] [CrossRef]
- Nunes, R.S.C.; Del Aguila, E.M.; Paschoalin, V.M.F. Safety evaluation of the coagulase-negative staphylococci microbiota of salami: Superantigenic Toxin Production and antimicrobial resistance. BioMed Res. Int. 2015, 2015, 483–548. [Google Scholar] [CrossRef]
- Thomas, D.Y.; Jarraud, S.; Lemercier, B.; Cozon, G.; Echasserieau, K.; Etienne, J.; Gougeon, M.L.; Lina, G.; Vandenesch, F. Staphylococcal enterotoxin-like toxins U2 and V, two new staphylococcal superantigens arising from recombination within the enterotoxin gene cluster. Infect. Immun. 2006, 74, 4724–4734. [Google Scholar] [CrossRef]
- Seng, P.; Boushab, B.M.; Romain, F.; Gouriet, F.; Bruder, N.; Martin, C.; Papazian, L. Emerging role of Raoultella ornithinolytica in human infections: A series of cases and review of the literature. Int. J. Infect. Dis. 2016, 45, 65–71. [Google Scholar] [CrossRef]
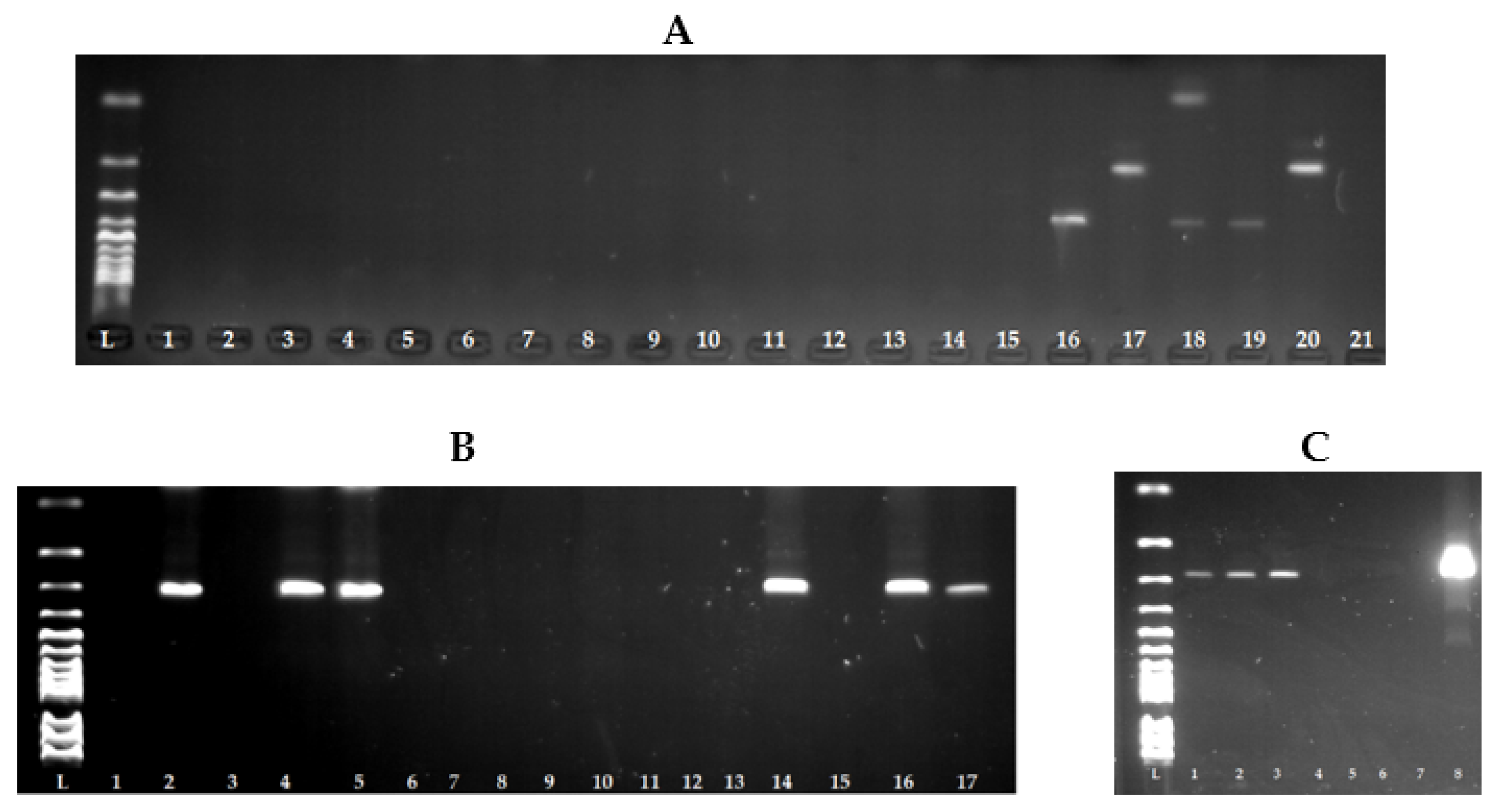
| Gene | Primer | Nucleotide Sequence 5′-3′ | Product Size (bp) |
|---|---|---|---|
| universal | Sauni-F | TGTATGTATGGAGGTGTAAC | |
| sea | SAA -R | ATTAACCGAAGGTTCTGT | 270 |
| seb | SAB -R | ATAGTGACGAGTTAGGTA | 165 |
| sec | SAC -R | AAGTACATTTTGTAAGTTCC | 102 |
| sed | SAD -R | TTCGGGAAAATCACCCTTAA | 306 |
| see | SAE -R | GCCAAAGCTGTCTGAG | 213 |
| Number of Strains | Pigment | Hemolysis | Lecithinase | Lipase | Nuclease | ||||
|---|---|---|---|---|---|---|---|---|---|
| White (without Pigment) | Gray | Gray-White | Yellow-White | ||||||
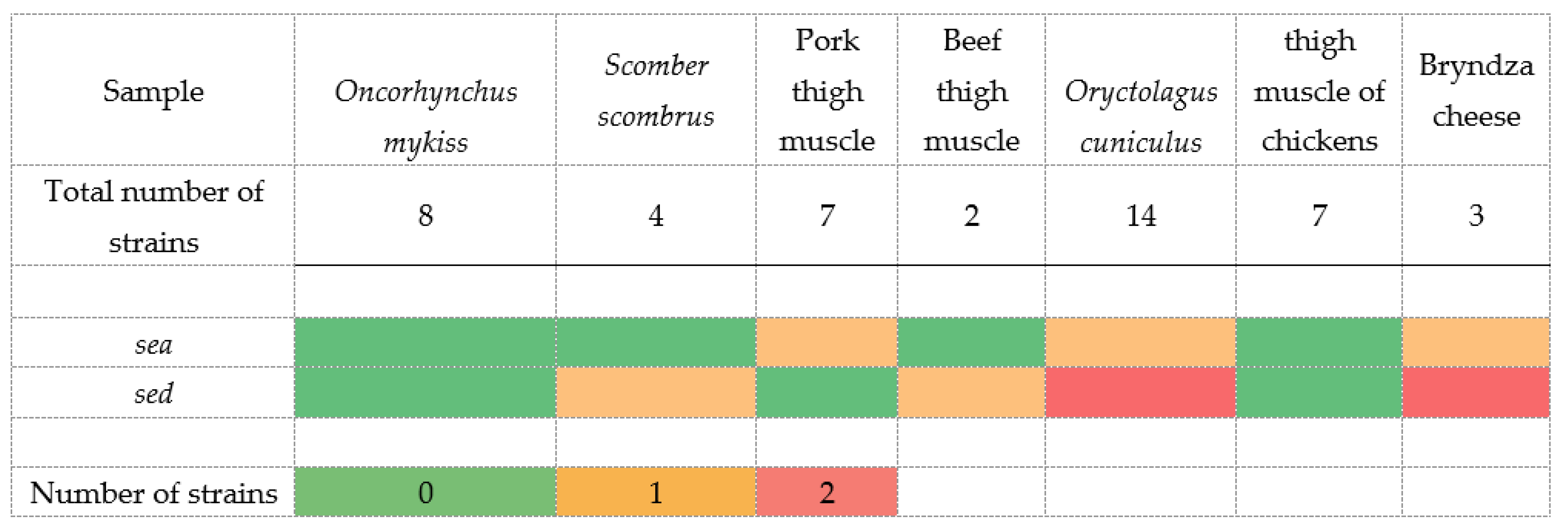
| Oncorhynchus mykiss | 8 | 0 | 2 | 6 | 0 | 0 | 3 | 7 | 0 |
| Scomber scombrus | 4 | 0 | 2 | 1 | 1 | 3 (α) | 2 | 3 | 0 |
| Pork thigh muscle | 7 | 0 | 0 | 6 | 1 | 0 | 3 | 1 | 0 |
| Beef thigh muscle | 2 | 1 | 1 | 0 | 0 | 0 | 1 | 5 | 0 |
| Oryctolagus cuniculus | 14 | 0 | 3 | 7 | 4 | 5 (α) | 4 | 12 | 0 |
| thigh muscle of chickens | 7 | 1 | 0 | 4 | 2 | 1 (α) | 1 | 0 | 0 |
| Bryndza cheese | 3 | 1 | 1 | 1 | 0 | 1 (α) | 3 | 3 | 0 |
| Total | 45 | 9 | 6 | 23 | 7 | 10 | 17 | 31 | 0 |
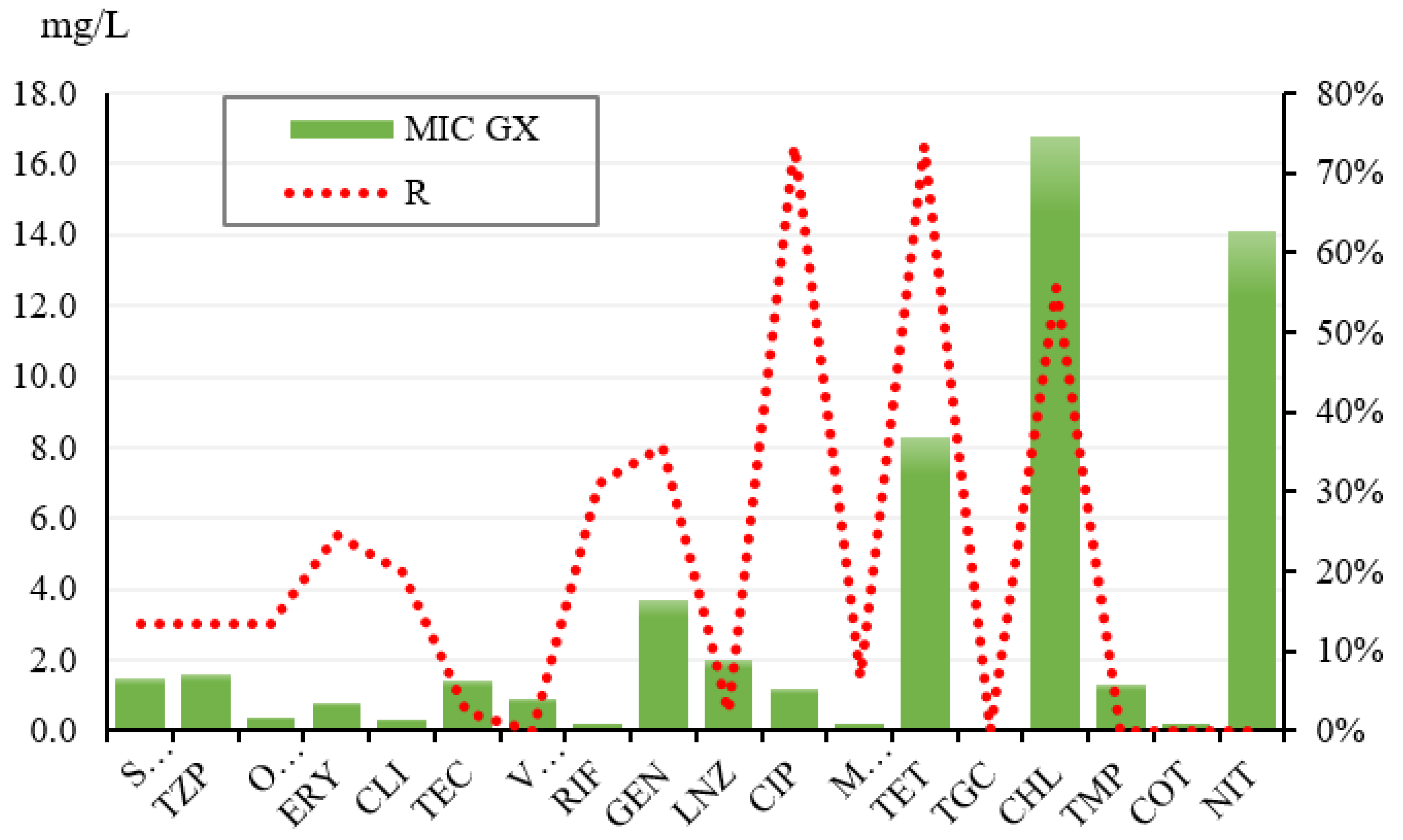
| ATB | S | I | R | GX SI | MIC50 | MIC90 | Total |
|---|---|---|---|---|---|---|---|
| SAM | 86.67% | 0.00% | 13.33% | 0.9 | 1.00 | 64.00 | 45 |
| TZP | 86.67% | 0.00% | 13.33% | 0.8 | 1.00 | 128.00 | 45 |
| OXA | 86.67% | 0.00% | 13.33% | 0.2 | 0.25 | 8.00 | 45 |
| ERY | 75.56% | 0.00% | 24.44% | 0.3 | 0.50 | 16.00 | 45 |
| CLI | 89.00% | 0.00% | 20.00% | 0.1 | 0.25 | 8.00 | 45 |
| TEC | 97.78% | 0.00% | 2.22% | 1.3 | 1.00 | 4.00 | 45 |
| VAN | 100.00% | 0.00% | 0.00% | 0.9 | 1.00 | 2.00 | 45 |
| RIF | 42.22% | 26.67% | 31.11% | 0.1 | 0.50 | 2.00 | 45 |
| GEN | 64.44% | 0.00% | 35.56% | 0.4 | 0.50 | 256.00 | 45 |
| LNZ | 97.78% | 0.00% | 2.22% | 2.0 | 2.00 | 4.00 | 45 |
| CIP | 0.00% | 26.67% | 73.33% | 0.2 | 2.00 | 4.00 | 45 |
| MFX | 93.33% | 0.00% | 6.67% | 0.1 | 0.13 | 0.25 | 45 |
| TET | 26.67% | 0.00% | 73.33% | 0.4 | 16.00 | 32.00 | 45 |
| TGC | 100.00% | 0.00% | 0.00% | 0.1 | 0.06 | 0.13 | 45 |
| CHL | 44.44% | 0.00% | 55.56% | 5.3 | 16.00 | 64.00 | 45 |
| TMP | 0.00% | 100.00% | 0.00% | 1.3 | 2.00 | 2.00 | 45 |
| COT | 100.00% | 0.00% | 0.00% | 0.2 | 0.25 | 0.50 | 45 |
| NIT | 0.00% | 100.00% | 0.00% | 14.1 | 16.00 | 16.00 | 45 |
| Mechanisms of Resistance | Number | % |
|---|---|---|
| MRCNS | 6 | 13.33% |
| Aminogl.PH(2″)-AC(6′) | 15 | 33.33% |
| Fluoroq.incompl.resistance | 32 | 71.11% |
| Constitutive MLSB/c | 1 | 2.22% |
| Inducible MLSB/i | 0 | 0.00% |
| Multi-resistance | 10 | 22.22% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regecová, I.; Výrostková, J.; Zigo, F.; Gregová, G.; Pipová, M.; Jevinová, P.; Becová, J. Detection of Resistant and Enterotoxigenic Strains of Staphylococcus warneri Isolated from Food of Animal Origin. Foods 2022, 11, 1496. https://doi.org/10.3390/foods11101496
Regecová I, Výrostková J, Zigo F, Gregová G, Pipová M, Jevinová P, Becová J. Detection of Resistant and Enterotoxigenic Strains of Staphylococcus warneri Isolated from Food of Animal Origin. Foods. 2022; 11(10):1496. https://doi.org/10.3390/foods11101496
Chicago/Turabian StyleRegecová, Ivana, Jana Výrostková, František Zigo, Gabika Gregová, Monika Pipová, Pavlina Jevinová, and Jana Becová. 2022. "Detection of Resistant and Enterotoxigenic Strains of Staphylococcus warneri Isolated from Food of Animal Origin" Foods 11, no. 10: 1496. https://doi.org/10.3390/foods11101496
APA StyleRegecová, I., Výrostková, J., Zigo, F., Gregová, G., Pipová, M., Jevinová, P., & Becová, J. (2022). Detection of Resistant and Enterotoxigenic Strains of Staphylococcus warneri Isolated from Food of Animal Origin. Foods, 11(10), 1496. https://doi.org/10.3390/foods11101496








