Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder
Abstract
:1. Introduction
1.1. Definition
1.2. Epidemiology
1.3. Treatment
2. Monoaminergic Neuromodulation, HPA, Inflammation and MDD
3. Diet and MDD
4. 5-Hydroxy-l-tryptophan and MDD
| Mushroom | Form | Bioactive Indole Derivatives Compound Concentration [mg/100 g] | References | ||||
|---|---|---|---|---|---|---|---|
| Serotonin | l-Tryptophane | 5-HTP | Tryptamine | Melatonin | |||
| Agaricus bisporus (White bottom mushroom) | Fruiting bodies | 5.21 | 0.39 | <0.001 | 0.06 | 0.11 | [63] |
| Armillaria mellea (Honey mushroom) | Fruiting bodies | 2.21 | 4.47 | <0.001 | 2.74 | <0.001 | [64] |
| Boletus badius (Bay bolete) | Fruiting bodies | 0.52 | 0.68 | <0.001 | 0.47 | <0.001 | [64] |
| Boletus edulis (King bolete) | Fruiting bodies | 10.14 | 0.39 | 0.18 | 1.17 | 0.68 | [64] |
| Cantharellus cibarius (Chanterelle) | Fruiting bodies | 29.61 | 0.01 | 0.02 | 0.01 | 0.14 | [63] |
| Ganoderma applanatum (Bracket fungus) | Mycelium | NA | 1.76 | <0.001 | 1.12 | 0.02 | [65] |
| Ganoderma lucidum (Reishi) | Mycelium | 10.58 | NA | NA | NA | 0.98 | [65] |
| Hericium erinaceus (Lion’s mane) | Mycelium | NA | NA | 152.72 | 11.88 | 1.04 | [66] |
| Fruiting bodies | NA | NA | 92.19 | 1.19 | <0.001 | [66] | |
| Lactarius deliciosus (Saffron milk cap) | Fruiting bodies | 18.42 | <0.001 | 0.25 | <0.001 | 1.29 | [63] |
| Laetiporus sulphureus (Chicken of the wood) | Mycelium | NA | 14.08 | 1.5 | 1.16 | <0.001 | [65] |
| Leccinum rufum (Birch bolete) | Fruiting bodies | 31.71 | <0.001 | 0.02 | 1.05 | 0.08 | [63] |
| Leccinum scabrum (Rough-stemmed bolet) | Fruiting bodies | 13.99 | 9.56 | <0.001 | <0.001 | <0.001 | [67] |
| Lentinula edodes (Shitake) | Fruiting bodies | 1.03 | 0.58 | 24.83 | 0.04 | 0.13 | [67] |
| Macrolepiota procera (Parasol mushroom) | Fruiting bodies | <0.001 | 3.47 | 22.94 | 0.92 | 0.07 | [67] |
| Pleurotus citrinopileatus (Golden oyster mushroom) | Mycelium | <0.001 | 7.82 | 368.67 | 3.71 | <0.001 | [68] |
| Fruiting bodies | <0.001 | 13.84 | 128.89 | 1.29 | <0.001 | [68] | |
| Pleurotus djamor (Pink oyster mushroom) | Mycelium | <0.001 | 24.34 | 703.56 | <0.001 | <0.001 | [68] |
| Fruiting bodies | 7.68 | 24.84 | 193.95 | 3.54 | <0.001 | [68] | |
| Pleurotus eryngii (King trumpet mushroom) | Mycelium | 8.54 | 7.60 | 221.51 | 2.67 | 0.08 | [68] |
| Fruiting bodies | 13.18 | 35.28 | 149.73 | 17.84 | 0.13 | [68] | |
| Pleurotus florida (Pearl oyster mushroom) | Mycelium | <0.001 | <0.001 | 215.53 | <0.001 | 0.09 | [68] |
| Fruiting bodies | 3.31 | 10.84 | 95.21 | 1.52 | <0.001 | [68] | |
| Pleurotus ostreatus (Oyster mushroom) | Mycelium | <0.001 | 1.89 | 120.11 | 1.03 | 4.45 | [68] |
| Fruiting bodies | 6.52 NA | <0.001 5.79 | 2.08 67.45 | 0.91 1.04 | <0.001 0.33 | [68] | |
| Pleurotus pulmonarius (Indian oyster) | Mycelium | <0.001 | 17.29 | 553.87 | <0.001 | <0.001 | [68] |
| Fruiting bodies | <0.001 | 11.85 | 117.02 | <0.001 | <0.001 | [68] | |
| Suillus bovinus (Jersey cow mushroom) | Fruiting bodies | <0.001 | 25.90 | 15.83 | 3.15 | <0.001 | [67] |
| Suillus luteus (Slippery Jack) | Fruiting bodies | 34.11 | 2.61 | 1.63 | <0.001 | 0.71 | [69] |
| Trametes versicolor (Turkey tail) | Mycelium | NA | 3.91 | 0.9 | 1.69 | 0.01 | [65] |
| Tricholoma equestre (Man on horseback) | Mycelium | 0.59 | 1.03 | 0.34 | 0.59 | 0.32 | [70] |
| Fruiting bodies | 0.18 | 2.85 | 0.58 | 2.01 | <0.001 | [70] | |
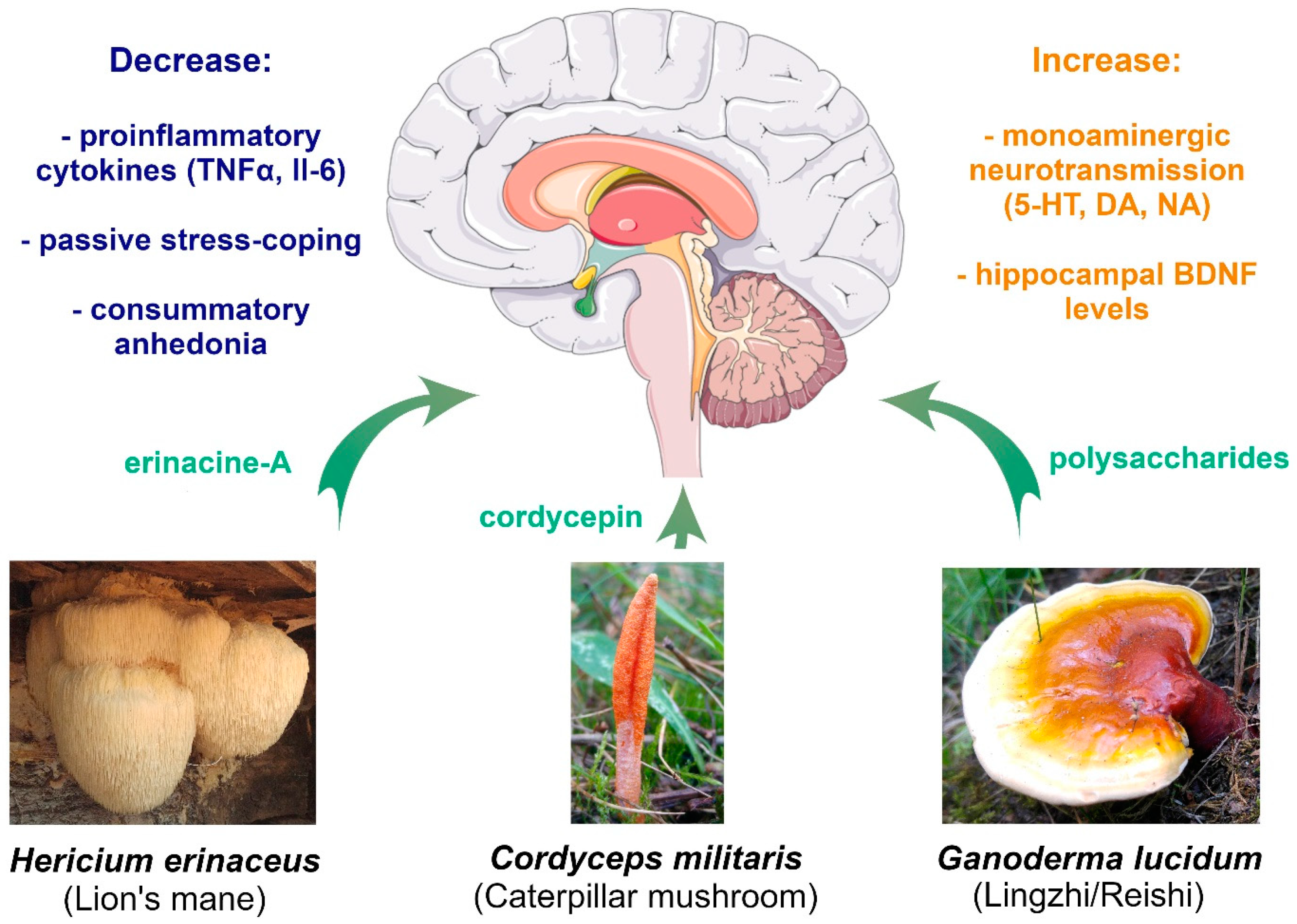
5. Medicinal Mushroom Species and MDD
5.1. Hericium erinaceus
| Herricium erinaceus | |||
|---|---|---|---|
| Biological effect | Neuroprotective effect | Effects in preclinical in vivo studies | Reported effects in clinical studies |
| Anti-inflammatory | Erinacine-A promotes neuronal survival in mouse hippocampus via BDNF and NFκB increase in response to LPS [78]. | Reduction in passive stress-coping induced by LPS [78]. Decrease in plasma proinflammatory cytokines: TNFα [77,78], and Il-6 [78]. Increase of plasma anti-inflammatory cytokine Il-10 [77]. | Reduction in self-reported depression [80,81] and anxiety symptoms [80]. Higher noradrenaline turnover [82]. Modulation of gut microbiota [75]. |
| Antioxidative | Neuroprotective against DEHP [72] and high corticosterone levels [73] via antioxidative and antiapoptotic activity in vitro. | no data | |
| Gut microbiota | no data | Polysaccharides regulate inflammation in the gut via microbiota [74]. | |
| HPA axis | Neuroprotective against high corticosterone levels in vitro [73]. | Reversal of passive stress-coping induced by repeated restraint stress in mice [78]. Prevents a decrease in noradrenaline, serotonin and dopamine in hippocampi of stressed mice [78]. | |
| Mitochondria protection | Neuroprotective against DEHP-induced mitochondrial dysfunction in vitro [72]. | no data | |
| Neurogenesis and BDNF | Erinacine-A increases proliferation of hippocampal progenitors in the subgranular zone of the dentate gyrus [79] and increases via BDNF and NFκB signaling [78]. | Reduction in passive stress-coping compared to non-supplemented mice [79]. | |
| Cordyceps militaris | |||
| Biological effect | Neuroprotective effect | Effects in preclinical in vivo studies | Reported effects in clinical studies |
| Anti-inflammatory | no data | Cordycepin normalized hippocampal IL-6 and TNFα levels increased by chronic stress in mice [93], and serum IL-1β in chronically stressed rats [94] | no data |
| Antioxidative | no data | Increase in brain antioxidant levels in rats [94]. | |
| HPA axis | no data | Reversal of passive stress-coping and consummatory anhedonia induced by chronic unpredictable mild stress in mice [93] and rats [94,95]. Recovery of noradrenalin, dopamine, serotonin and glucocorticoid receptor levels in the hypothalamus of chronically stressed rats [95]. | |
| Neurogenesis and BDNF | no data | Cordycepin slightly upregulated hippocampal BDNF levels decreased by chronic stress in mice [93]. | |
| Ganoderma lucidum | |||
| Biological effect | Neuroprotective effect | Effects in preclinical in vivo studies | Reported effects in clinical studies |
| Anti-inflammatory | no data | Polysaccharides normalized hippocampal proinflammatory (Il-6, TNFα) and anti-inflammatory (Il-10) cytokine levels increased by chronic stress in mice [96]. | Improvement [97] or worsening [98] of self-reported fatigue and improvement of well-being [96,98]. Reduction [99] or no change [98] in depression and anxiety symptoms. |
| HPA axis | Polysaccharides are neuroprotective against high corticosterone levels in vitro [100]. | Polysaccharides reverse passive stress-coping and consummatory anhedonia induced by chronic unpredictable mild stress in mice [97,100]. | |
| Neurogenesis and BDNF | Triterpenes promote neuronal survival via NGF and BDNF signaling in vitro [101]. | Polysaccharides restore hippocampal [97] and frontal cortex [100] BDNF levels decreased by chronic stress in mice. | |
5.2. Cordyceps spp.
5.3. Ganoderma lucidum
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Horwitz, A.V.; Wakefield, J.C.; Lorenzo-Luaces, L. History of Depression. In The Oxford Handbook of Mood Disorders; Oxford University Press: New York, NY, USA, 2015; pp. 11–23. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Bachmann, S. Epidemiology of Suicide and the Psychiatric Perspective. Int. J. Environ. Res. Public Health 2018, 15, 1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Aglio, L.; Lewis, C.M.; Pain, O. Delineating the Genetic Component of Gene Expression in Major Depression. Biol. Psychiatry 2020, 89, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J.; Akil, H.; Gordon, J.; et al. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder—Translating Findings From Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar]
- Schmaal, L.; For the ENIGMA-Major Depressive Disorder Working Group; Hibar, D.; Sämann, P.; Hall, G.; Baune, B.; Jahanshad, N.; Cheung, J.; Van Erp, T.; Bos, D.; et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry 2016, 22, 900–909. [Google Scholar] [CrossRef] [Green Version]
- Schmaal, L.; for the ENIGMA-Major Depressive Disorder Working Group; Veltman, D.J.; Van Erp, T.; Sämann, P.; Frodl, T.; Jahanshad, N.; Loehrer, E.; Tiemeier, H.; Hofman, A.; et al. Subcortical brain alterations in major depressive disorder: Findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry 2015, 21, 806–812. [Google Scholar] [CrossRef] [Green Version]
- Gartlehner, G.; Wagner, G.; Matyas, N.; Titscher, V.; Greimel, J.; Lux, L.; Gaynes, B.N.; Viswanathan, M.; Patel, S.; Lohr, K.N. Pharmacological and non-pharmacological treatments for major depressive disorder: Review of systematic reviews. BMJ Open 2017, 7, e014912. [Google Scholar] [CrossRef]
- Howland, R.H. Sequenced Treatment Alternatives to Relieve Depression (STAR*D)—Part 2: Study Outcomes. J. Psychosoc. Nurs. Ment. Health Serv. 2008, 46, 21–24. [Google Scholar] [CrossRef]
- Cuijpers, P.; Berking, M.; Andersson, G.; Quigley, L.; Kleiboer, A.; Dobson, K. A Meta-Analysis of Cognitive-Behavioural Therapy for Adult Depression, Alone and in Comparison with other Treatments. Can. J. Psychiatry 2013, 58, 376–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Marcantoni, W.S.; Akoumba, B.S.; Wassef, M.; Mayrand, J.; Lai, H.; Richard-Devantoy, S.; Beauchamp, S. A Systematic Review and Meta-Analysis of the Efficacy of Intravenous Ketamine Infusion for Treatment Resistant Depression: January 2009–January 2019. J. Affect. Disord. 2020, 277, 831–841. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S.; Carvalho, I.P.; Lui, L.M.; Majeed, A.; Masand, P.S.; Gill, H.; Rodrigues, N.B.; Lipsitz, O.; Coles, A.C.; Lee, Y.; et al. The effect of intravenous, intranasal, and oral ketamine in mood disorders: A meta-analysis. J. Affect. Disord. 2020, 276, 576–584. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Salloum, N.C.; Hock, R.S.; Jha, M.K.; Murrough, J.W.; Mathew, S.J.; Iosifescu, D.V.; Fava, M. Efficacy of Esketamine Augmentation in Major Depressive Disorder. J. Clin. Psychiatry 2020, 81, 6603. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.A.; Fricker, L.D. Transmitter and Peptide Receptors: Basic Principles. In Neuroscience in the 21st Century; Springer: New York, NY, USA, 2013; pp. 1505–1523. [Google Scholar]
- Booij, L.; Van Der Does, W.; Benkelfat, C.; Bremner, J.D.; Cowen, P.J.; Fava, M.; Gillin, C.; Leyton, M.; Moore, P.; Smith, K.A.; et al. Predictors of Mood Response to Acute Tryptophan Depletion A Reanalysis. Neuropsychopharmacology 2002, 27, 852–861. [Google Scholar] [CrossRef]
- Melhem, N.M.; Zhong, Y.; Miller, J.M.; Zanderigo, F.; Ogden, R.T.; Sublette, M.E.; Newell, M.; Burke, A.; Keilp, J.G.; Lesanpezeshki, M.; et al. Brain 5-HT1A Receptor PET Binding, Cortisol Responses to Stress, and the Familial Transmission of Suicidal Behavior. Int. J. Neuropsychopharmacol. 2022, 25, 36–45. [Google Scholar] [CrossRef]
- Charney, D.S.; Manji, H.K. Life Stress, Genes, and Depression: Multiple Pathways Lead to Increased Risk and New Opportunities for Intervention. Sci. STKE 2004, 2004, re5. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Admon, R.; Mellem, M.S.; Belleau, E.L.; Kaiser, R.H.; Clegg, R.; Beltzer, M.; Goer, F.; Vitaliano, G.; Ahammad, P.; et al. Machine Learning Identifies Large-Scale Reward-Related Activity Modulated by Dopaminergic Enhancement in Major Depression. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 163–172. [Google Scholar] [CrossRef]
- Meyer, J.H.; Krüger, S.; Wilson, A.A.; Christensen, B.K.; Goulding, V.S.; Schaffer, A.; Minifie, C.; Houle, S.; Hussey, D.; Kennedy, S. Lower dopamine transporter binding potential in striatum during depression. NeuroReport 2001, 12, 4121–4125. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, J.; Tolosa, E.; Valles, A. Parkinson’s disease with depression: A possible subgroup of idiopathic parkinsonism. Neurology 1986, 36, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Logrip, M.; Koob, G.F. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front. Neuroendocr. 2014, 35, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeroff, C.B.; Widerlöv, E.; Bissette, G.; Walléus, H.; Karlsson, I.; Eklund, K.; Kilts, C.D.; Loosen, P.T.; Vale, W. Elevated Concentrations of CSF Corticotropin-Releasing Factor-Like Immunoreactivity in Depressed Patients. Science 1984, 226, 1342–1344. [Google Scholar] [CrossRef]
- Chaves, T.; Fazekas, C.L.; Horváth, K.; Correia, P.; Szabó, A.; Török, B.; Bánrévi, K.; Zelena, D. Stress Adaptation and the Brainstem with Focus on Corticotropin-Releasing Hormone. Int. J. Mol. Sci. 2021, 22, 9090. [Google Scholar] [CrossRef]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Gałecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Arnone, D.; Saraykar, S.; Salem, H.; Teixeira, A.L.; Dantzer, R.; Selvaraj, S. Role of Kynurenine pathway and its metabolites in mood disorders: A systematic review and meta-analysis of clinical studies. Neurosci. Biobehav. Rev. 2018, 92, 477–485. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2019, 24, 965–986. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahe, C.; Unrath, M.; Berger, K. Dietary patterns and the risk of depression in adults: A systematic review of observational studies. Eur. J. Nutr. 2014, 53, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Sánchez-Pedreño, F.O.; Van der Does, W.; Martínez-González, M.A. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Butterworth, P. Dietary Patterns and Depressive Symptoms over Time: Examining the Relationships with Socioeconomic Position, Health Behaviours and Cardiovascular Risk. PLoS ONE 2014, 9, e87657. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Villegas, A.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Covas, M.I.; Arós, F.; Romaguera, D.; Gómez-Gracia, E.; Lapetra, J.; et al. Mediterranean dietary pattern and depression: The PREDIMED randomized trial. BMC Med. 2013, 11, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bot, M.; Brouwer, I.; Roca, M.; Kohls, E.; Penninx, B.W.J.H.; Watkins, E.; van Grootheest, G.; Cabout, M.; Hegerl, U.; Gili, M.; et al. Effect of Multinutrient Supplementation and Food-Related Behavioral Activation Therapy on Prevention of Major Depressive Disorder Among Overweight or Obese Adults With Subsyndromal Depressive Symptoms. JAMA 2019, 321, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.; Solmi, M.; Stubbs, B.; Schuch, F.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Stapel, B.; Nösel, P.; Heitland, I.; Mahmoudi, N.; Lanfermann, H.; Kahl, K.; Ding, X. In vivo magnetic resonance spectrometry imaging demonstrates comparable adaptation of brain energy metabolism to metabolic stress induced by 72 h of fasting in depressed patients and healthy volunteers. J. Psychiatr. Res. 2021, 143, 422–428. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Fagundes, C.P.; Andridge, R.; Peng, J.; Malarkey, W.B.; Habash, D.; Belury, M.A. Depression, daily stressors and inflammatory responses to high-fat meals: When stress overrides healthier food choices. Mol. Psychiatry 2017, 22, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornung, J.-P. The Neuronatomy of the Serotonergic System. In Handbook of Behavioral Neuroscience; Elsevier: London, UK, 2010; pp. 51–64. [Google Scholar]
- Linthorst, A.C.; Reul, J.M. The Impact of Stress on Serotonergic Neurotransmission. Handb. Behav. Neurosci. 2010, 21, 475–491. [Google Scholar] [CrossRef]
- Bristow, G.C.; Eisenlohr-Moul, T.; Lotesto, K.; Sodhi, M.S. Sex differences in the transcription of monoamine transporters in major depression. J. Affect. Disord. 2021, 295, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Van der Plas, M.; Braun, V.; Stauch, B.J.; Hanslmayr, S. Stimulation of the left dorsolateral prefrontal cortex with slow rTMS enhances verbal memory formation. PLoS Biol. 2021, 19, e3001363. [Google Scholar] [CrossRef]
- Sambeth, A.; Blokland, A.; Harmer, C.J.; Kilkens, T.O.; Nathan, P.J.; Porter, R.J.; Schmitt, J.A.; Scholtissen, B.; Sobczak, S.; Young, A.H.; et al. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: A pooled analysis of nine studies. Neurosci. Biobehav. Rev. 2007, 31, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Fujii, T.; Koga, N.; Hori, H.; Teraishi, T.; Hattori, K.; Noda, T.; Higuchi, T.; Motohashi, N.; Kunugi, H. PlasmaL-Tryptophan Concentration in Major Depressive Disorder. J. Clin. Psychiatry 2014, 75, e906–e915. [Google Scholar] [CrossRef]
- Javelle, F.; Lampit, A.; Bloch, W.; Häussermann, P.; Johnson, S.L.; Zimmer, P. Effects of 5-hydroxytryptophan on distinct types of depression: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 77–88. [Google Scholar] [CrossRef]
- Shaw, K.; Turner, J.; Del Mar, C. Are Tryptophan and 5-Hydroxytryptophan Effective Treatments for Depression? A Meta-Analysis. Aust. N. Z. J. Psychiatry 2002, 36, 488–491. [Google Scholar] [CrossRef]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Springer: Cham, Switzerland, 2016; Volume 31, pp. 117–138. [Google Scholar] [CrossRef]
- Krystal, J.H.; Abdallah, C.; Sanacora, G.; Charney, D.S.; Duman, R.S. Ketamine: A Paradigm Shift for Depression Research and Treatment. Neuron 2019, 101, 774–778. [Google Scholar] [CrossRef] [Green Version]
- Pompili, M.; Lionetto, L.; Curto, M.; Forte, A.; Erbuto, D.; Montebovi, F.; Seretti, M.E.; Berardelli, I.; Serafini, G.; Innamorati, M.; et al. Tryptophan and Kynurenine Metabolites: Are They Related to Depression? Neuropsychobiology 2018, 77, 23–28. [Google Scholar] [CrossRef]
- Chiu, Y.-C.; Yang, B.-H.; Yang, K.-C.; Liu, M.-N.; Hu, L.-Y.; Liou, Y.-J.; Chan, L.-Y.; Chou, Y.-H. A study of tryptophan, kynurenine and serotonin transporter in first-episode drug-naïve major depressive disorder. Psychiatry Res. Neuroimaging 2021, 312, 111296. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Allers, K.A.; Beekman, A.T.; Giltay, E.J.; Keller, S.; Schoevers, R.A.; Süssmuth, S.D.; Niessen, H.G.; Penninx, B.W. The association between plasma tryptophan catabolites and depression: The role of symptom profiles and inflammation. Brain, Behav. Immun. 2021, 97, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.P.; Krystal, A.D.; Krishnan, K.R.R.; Caron, M.G. Adjunctive 5-Hydroxytryptophan Slow-Release for Treatment-Resistant Depression: Clinical and Preclinical Rationale. Trends Pharmacol. Sci. 2016, 37, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, K.W.; Fuller, R.W. Extracellular 5-hydroxytryptamine concentration in rat hypothalamus after administration of fluoxetine plus l-5-hydroxytryptophan. J. Pharm. Pharmacol. 1993, 45, 759–761. [Google Scholar] [CrossRef]
- Sargent, P.A.; Williamson, D.J.; Cowen, P.J. Brain 5-HT neurotransmission during paroxetine treatment. Br. J. Psychiatry 1998, 172, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Ekiert, H. Indole compounds in fruiting bodies of some edible Basidiomycota species. Food Chem. 2011, 125, 1306–1308. [Google Scholar] [CrossRef]
- Muszyńska, B.; Sułkowska-Ziaja, K. Analysis of indole compounds in edible Basidiomycota species after thermal processing. Food Chem. 2012, 132, 455–459. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Grabowska, K.; Apola, A.; Kryczyk-Poprawa, A.; Muszyńska, B. Mycelial culture extracts of selected wood-decay mushrooms as a source of skin-protecting factors. Biotechnol. Lett. 2021, 43, 1051–1061. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Fijałkowska, A.; Karol, J.; Piotr, Z.; Lazur, J.; Katarzyna, S.-Z.; Bożena, M. Edible and Medicinal Mushroom Hericium Erinceus-Potential Natural Material with Influence on Brain Functions. Med. Int. Rev. Int. Med. Rev. 2020, 29, 4–10. [Google Scholar]
- Muszyńska, B.; Sułkowska-Ziaja, K.; Wójcik, A. Levels of Physiologically Active Indole Derivatives in the Fruiting Bodies of Some Edible Mushrooms (Basiciomycota) before and after Thermal Processing. Mycoscience 2013, 54, 321–326. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected edible medicinal mushrooms from Pleurotus genus as an answer for human civilization diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef] [PubMed]
- Muszynska, B.; Sułkowska-Ziaja, K.; Ekiert, H. Indole Compounds in Some Culinary-Medicinal Higher Basidiomycetes from Poland. Int. J. Med. Mushrooms 2011, 13, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B. Jadalne Grzyby Wielkoowocnikowe Jako Źródło Fizjologicznie Aktywnych Niehalucynogennych Związków Indolowych; Wydawnictwo ZOZ Ośrodka UMEA Shinoda-Kuracejo: Kraków Poland, 2012. [Google Scholar]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus(Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Scuto, M.; Zappalà, A.; Ontario, M.L.; Petralia, A.; Abid-Essefi, S.; Maiolino, L.; Signorile, A.; Salinaro, A.T.; Calabrese, V. Hericium Erinaceus Prevents DEHP-Induced Mitochondrial Dysfunction and Apoptosis in PC12 Cells. Int. J. Mol. Sci. 2020, 21, 2138. [Google Scholar] [CrossRef] [Green Version]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef]
- Xie, X.-Q.; Geng, Y.; Guan, Q.; Ren, Y.; Guo, L.; Lv, Q.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients 2021, 13, 1008. [Google Scholar] [CrossRef]
- Huang, H.-T.; Ho, C.-H.; Sung, H.-Y.; Lee, L.-Y.; Chen, W.-P.; Chen, Y.-W.; Chen, C.-C.; Yang, C.-S.; Tzeng, S.-F. Hericium erinaceus mycelium and its small bioactive compounds promote oligodendrocyte maturation with an increase in myelin basic protein. Sci. Rep. 2021, 11, 6551. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.-C.; Dong, C.; Zhuang, C.; Hirota, S.; Inanaga, K.; Hashimoto, K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015, 136, 7–12. [Google Scholar] [CrossRef]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, J.-L.; Lin, W.-H.; Mong, M.-C. Erinacine A-Enriched Hericium erinaceus Mycelium Produces Antidepressant-Like Effects through Modulating BDNF/PI3K/Akt/GSK-3β Signaling in Mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.; Kim, H.G.; Kim, J.Y.; Kim, S.Y.; Cho, K.-O. Hericium erinaceus Extract Reduces Anxiety and Depressive Behaviors by Promoting Hippocampal Neurogenesis in the Adult Mouse Brain. J. Med. Food 2018, 21, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vigna, L.; Morelli, F.; Agnelli, G.M.; Napolitano, F.; Ratto, D.; Occhinegro, A.; Di Iorio, C.; Savino, E.; Girometta, C.; Brandalise, F.; et al. Hericium erinaceus Improves Mood and Sleep Disorders in Patients Affected by Overweight or Obesity: Could Circulating Pro-BDNF and BDNF Be Potential Biomarkers? Evidence-Based Complement. Altern. Med. 2019, 2019, 7861297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Okamura, H.; Anno, N.; Tsuda, A.; Inokuchi, T.; Uchimura, N.; Inanaga, K. The effects of Hericium erinaceus (Amyloban® 3399) on sleep quality and subjective well-being among female undergraduate students: A pilot study. Pers. Med. Universe 2015, 4, 76–78. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Wu, Y.-K.; Hu, J.-H.; Li, I.-C.; Lin, T.-W.; Chen, C.-C.; Kuo, C.-F. Preclinical Bioavailability, Tissue Distribution, and Protein Binding Studies of Erinacine A, a Bioactive Compound from Hericium erinaceus Mycelia Using Validated LC-MS/MS Method. Molecules 2021, 26, 4510. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-H.; Li, I.-C.; Lin, T.-W.; Chen, W.-P.; Lee, L.-Y.; Chen, C.-C.; Kuo, C.-F. Absolute Bioavailability, Tissue Distribution, and Excretion of Erinacine S in Hericium erinaceus Mycelia. Molecules 2019, 24, 1624. [Google Scholar] [CrossRef] [Green Version]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Polzella, M.; Fabrizi, C.; Fornai, F. Potential Antidepressant Effects of Scutellaria baicalensis, Hericium erinaceus and Rhodiola rosea. Antioxidants 2020, 9, 234. [Google Scholar] [CrossRef] [Green Version]
- Inanaga, K. Marked improvement of neurocognitive impairment after treatment with compounds from Hericium erinaceum: A case study of recurrent depressive disorder. Pers. Med. Universe 2014, 3, 46–48. [Google Scholar] [CrossRef]
- Bagby, R.M.; Ryder, A.G.; Schuller, D.R.; Marshall, M.B. The Hamilton Depression Rating Scale: Has the Gold Standard Be-come a Lead Weight? Am. J. Psychiatry 2004, 161, 2163–2177. [Google Scholar] [CrossRef]
- Kriston, L.; Von Wolff, A. Not as golden as standards should be: Interpretation of the Hamilton Rating Scale for Depression. J. Affect. Disord. 2011, 128, 175–177. [Google Scholar] [CrossRef]
- Timmerby, N.; Andersen, J.; Søndergaard, S.; Østergaard, S.; Bech, P. A Systematic Review of the Clinimetric Properties of the 6-Item Version of the Hamilton Depression Rating Scale (HAM-D6). Psychother. Psychosom. 2017, 86, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2020, 21, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commons, K.G.; Cholanians, A.B.; Babb, J.A.; Ehlinger, D.G. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci. 2017, 8, 955–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.; Zhang, J.-C.; Yao, W.; Ren, Q.; Yang, C.; Ma, M.; Han, M.; Saito, R.; Hashimoto, K. Effects of escitalopram, R-citalopram, and reboxetine on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2016, 144, 7–12. [Google Scholar] [CrossRef]
- Tianzhu, Z.; Shihai, Y.; Juan, D. Antidepressant-Like Effects of Cordycepin in a Mice Model of Chronic Unpredictable Mild Stress. Evidence-Based Complement. Altern. Med. 2014, 2014, 438506. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Wang, J.; Liu, Y.; Zhang, J. Vanadium-Enriched Cordyceps sinensis, a Contemporary Treatment Approach to Both Diabetes and Depression in Rats. Evidence-Based Complement. Altern. Med. 2011, 2011, 450316. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.; Li, L.; Qi, Y.; Zhang, Y.; Li, L.; Teng, L.; Wang, D. Dopamine and serotonin contribute to Paecilomyces hepiali against chronic unpredictable mild stress induced depressive behavior in Sprague Dawley rats. Mol. Med. Rep. 2017, 16, 5675–5682. [Google Scholar] [CrossRef]
- Zhao, S.; Rong, C.; Gao, Y.; Wu, L.; Luo, X.; Song, S.; Liu, Y.; Wong, J.H.; Wang, H.; Yi, L.; et al. Antidepressant-like effect of Ganoderma lucidum spore polysaccharide-peptide mediated by upregulation of prefrontal cortex brain-derived neurotrophic factor. Appl. Microbiol. Biotechnol. 2021, 105, 8675–8688. [Google Scholar] [CrossRef]
- Singh, R.; Shri, R.; Singh, A.P.; Dhingra, G.S. Valorization of Ganoderma Species: Chemical Characterization and Antidepressant-Like Activity. Waste Biomass Valorization 2021, 12, 2025–2036. [Google Scholar] [CrossRef]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A Randomized, Double-Blind and Placebo-Controlled Study of a Ganoderma lucidum Polysaccharide Extract in Neurasthenia. J. Med. Food 2005, 8, 53–58. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xiao, Y.; Han, L.; Jia, Y.; Luo, S.; Zhang, D.; Zhang, L.; Wu, P.; Xiao, C.; Kan, W.; et al. Ganoderma lucidum polysaccharides ameliorated depression-like behaviors in the chronic social defeat stress depression model via modulation of Dectin-1 and the innate immune system. Brain Res. Bull. 2021, 171, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Shimizu, Y.; Iwata, N.; Kamiuchi, S.; Suzuki, F.; Iizuka, H.; Hibino, Y.; Okazaki, M. Antidepressant-like effects of a water-soluble extract from the culture medium of Ganoderma lucidum mycelia in rats. BMC Complement. Altern. Med. 2013, 13, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.-J.; Nakamura, N.; Min, B.-S.; Hirakawa, A.; Zuo, F.; Hattori, M. Quantitative Determination of Bitter Principles in Specimens of Ganoderma lucidum Using High-Performance Liquid Chromatography and Its Application to the Evaluation of Ganoderma Products. Chem. Pharm. Bull. 2004, 52, 688–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-E.; Chen, Y.-C.; Lu, K.-H.; Huang, Y.-J.; Panyod, S.; Liu, W.-T.; Yang, S.-H.; Lu, Y.-S.; Chen, M.-H.; Sheen, L.-Y. Antidepressant-like effects of water extract of Cordyceps militaris (Linn.) Link by modulation of ROCK2/PTEN/Akt signaling in an unpredictable chronic mild stress-induced animal model. J. Ethnopharmacol. 2021, 276, 114194. [Google Scholar] [CrossRef]
- Koh, J.-H.; Kim, K.-M.; Kim, J.-M.; Song, J.-C.; Suh, H.-J. Antifatigue and Antistress Effect of the Hot-Water Fraction from Mycelia of Cordyceps sinensis. Biol. Pharm. Bull. 2003, 26, 691–694. [Google Scholar] [CrossRef] [Green Version]
- Nishizawa, K.; Torii, K.; Kawasaki, A.; Katada, M.; Ito, M.; Terashita, K.; Aiso, S.; Matsuoka, M. Antidepressant-Like Effect of Cordyceps sinensis in the Mouse Tail Suspension Test. Biol. Pharm. Bull. 2007, 30, 1758–1762. [Google Scholar] [CrossRef] [Green Version]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef]
- Kumar, R.; Negi, P.; Singh, B.; Ilavazhagan, G.; Bhargava, K.; Sethy, N.K. Cordyceps sinensis promotes exercise endurance capacity of rats by activating skeletal muscle metabolic regulators. J. Ethnopharmacol. 2011, 136, 260–266. [Google Scholar] [CrossRef]
- Xu, Y.-F. Effect of Polysaccharide from Cordyceps militaris (Ascomycetes) on Physical Fatigue Induced by Forced Swimming. Int. J. Med. Mushrooms 2016, 18, 1083–1092. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, L.; Yang, F.; Yang, W.; Sun, Y.; Hu, Q. Evaluation of anti-fatigue property of the extruded product of cereal grains mixed with Cordyceps militaris on mice. J. Int. Soc. Sports Nutr. 2017, 14, 15. [Google Scholar] [CrossRef] [Green Version]
- Thongsawang, S.; Krataithong, T.; ChorCharoenying, S.; Norchai, P.; Nokkaew, N. Applying Cordyceps Sinensis to Boost Endurance Performance in Long-Distance Runners. J. Exerc. Physiol. 2021, 24, 11–25. [Google Scholar]
- Chen, S.; Li, Z.; Krochmal, R.; Abrazado, M.; Kim, W.; Cooper, C.B. Effect of Cs-4® (Cordyceps sinensis) on Exercise Performance in Healthy Older Subjects: A Double-Blind, Placebo-Controlled Trial. J. Altern. Complement. Med. 2010, 16, 585–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-W.; Hong, T.-W.; Wang, Y.-J.; Chen, K.-C.; Pei, J.-C.; Lai, W.-S.; Tsai, S.-H.; Chu, R.; Chen, W.-C.; Sheen, L.-Y.; et al. Ophiocordyceps formosana improves hyperglycemia and depression-like behavior in an STZ-induced diabetic mouse model. BMC Complement. Altern. Med. 2016, 16, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Hou, Y.; Zhu, M.; Bao, H.; Nie, J.; Zhang, G.Y.; Shan, L.; Yao, Y.; Du, K.; Yang, H.; et al. 3′-Deoxyadenosine (Cordycepin) Produces a Rapid and Robust Antidepressant Effect via Enhancing Prefrontal AMPA Receptor Signaling Pathway. Int. J. Neuropsychopharmacol. 2016, 19, pyv112. [Google Scholar] [CrossRef] [Green Version]
- Holliday, J. Cordyceps: A Highly Coveted Medicinal Mushroom. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Singapore, 2017; pp. 59–91. [Google Scholar]
- Song, J.; Liu, Y.; Zhang, H.F.; Niu, Q. The RAS/PI3K Pathway is Involved in the Impairment of Long-term Potentiation Induced by Acute Aluminum Treatment in Rats. Biomed. Environ. Sci. 2016, 29, 782–789. [Google Scholar]
- Boh, B.; Berovic, M.; Zhang, J.; Zhi-Bin, L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007, 13, 265–301. [Google Scholar] [CrossRef]
- Xu, J.-W.; Ji, S.-L.; Li, H.-J.; Zhou, J.-S.; Duan, Y.-Q.; Dang, L.-Z.; Mo, M.-H. Increased polysaccharide production and biosynthetic gene expressions in a submerged culture of Ganoderma lucidum by the overexpression of the homologous α-phosphoglucomutase gene. Bioprocess Biosyst. Eng. 2014, 38, 399–405. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorganic Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Ip, F.C.; Zhang, D.-M.; Chen, L.-X.; Zhang, W.; Li, Y.-L.; Ip, N.Y.; Ye, W.-C. Triterpenoids with neurotrophic activity fromGanoderma lucidum. Nat. Prod. Res. 2011, 25, 1607–1613. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore Powder of Ganoderma lucidum Improves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evidence-Based Complement. Altern. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef] [Green Version]
- Bao, P.-P.; Lu, W.; Cui, Y.; Zheng, Y.; Gu, K.; Chen, Z.; Zheng, W.; Shu, X.O. Ginseng and Ganoderma lucidum Use after Breast Cancer Diagnosis and Quality of Life: A Report from the Shanghai Breast Cancer Survival Study. PLoS ONE 2012, 7, e39343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazzi, F.; Adsuar, J.; Domínguez-Muñoz, F.; García-Gordillo, M.; Gusi, N.; Collado-Mateo, D. Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia. Healthcare 2020, 8, 520. [Google Scholar] [CrossRef] [PubMed]



| Name of the Compound | Chemical Structure | Occurrence in Mushrooms |
|---|---|---|
| L-Tryptophan | 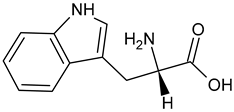 | Commonly found in edible mushrooms. |
| 5–Hydroxy-L-tryptophan | 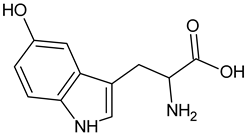 | Commonly found in edible mushrooms. |
| Serotonin | 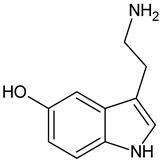 | Commonly found in edible mushrooms. |
| Erinacine S |  | Hericium erinaceus |
| Cordycepin | 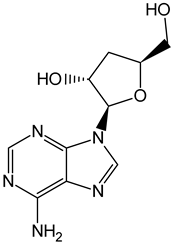 | Cordyceps militaris |
| Methyl ganoderate A acetonid | 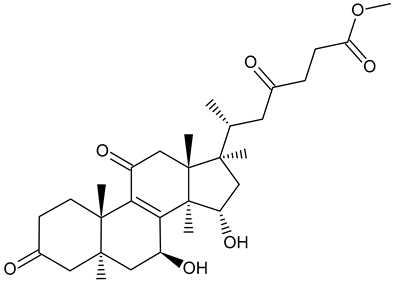 | Ganoderma lucidum |
| n-Butyl ganoderate H | 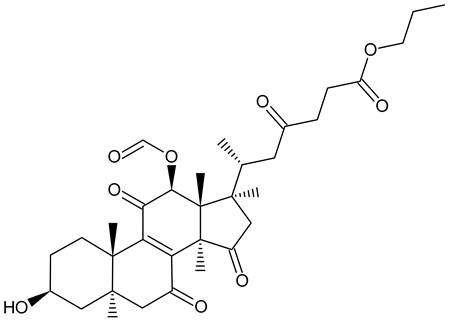 | Ganoderma lucidum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fijałkowska, A.; Jędrejko, K.; Sułkowska-Ziaja, K.; Ziaja, M.; Kała, K.; Muszyńska, B. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods 2022, 11, 1489. https://doi.org/10.3390/foods11101489
Fijałkowska A, Jędrejko K, Sułkowska-Ziaja K, Ziaja M, Kała K, Muszyńska B. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods. 2022; 11(10):1489. https://doi.org/10.3390/foods11101489
Chicago/Turabian StyleFijałkowska, Agata, Karol Jędrejko, Katarzyna Sułkowska-Ziaja, Marek Ziaja, Katarzyna Kała, and Bożena Muszyńska. 2022. "Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder" Foods 11, no. 10: 1489. https://doi.org/10.3390/foods11101489
APA StyleFijałkowska, A., Jędrejko, K., Sułkowska-Ziaja, K., Ziaja, M., Kała, K., & Muszyńska, B. (2022). Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods, 11(10), 1489. https://doi.org/10.3390/foods11101489






