A dPCR Method for Quantitative Authentication of Wild Lingonberry (Vaccinium vitis-idaea) versus Cultivated American Cranberry (V. macrocarpon)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and DNA Extraction
2.2. Primer Design and Specificity
2.3. Quantitative Chip-Based Digital-PCR (dPCR) with DNA Mixes
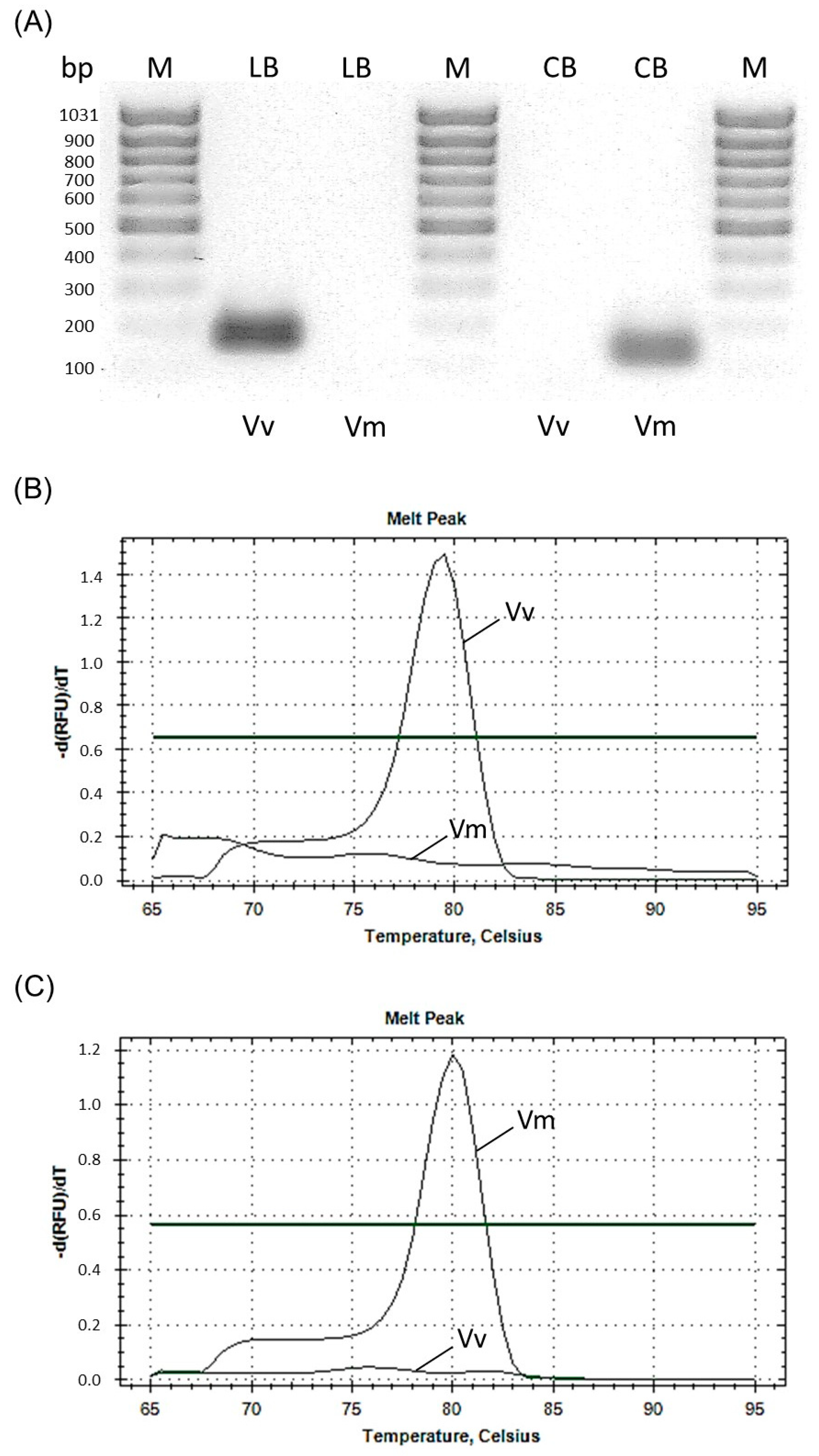
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the developmental and environmental regulation of secondary metabolism in Vaccinium spp. berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kron, K.A.; Powell, E.A.; Luteyn, J.L. Phylogenetic relationships within the blueberry tribe (Vaccinieae, Ericaceae) based on sequence data from matK and nuclear ribosomal ITS regions, with comments on the placement of Satyria. Am. J. Bot. 2002, 89, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Pascual-Díaz, J.P.; Gers, A.; Ilga, K.; Serçe, S.; Vitales, D.; Garcia, S. Contribution to the knowledge of genome size evolution in edible blueberries (genus Vaccinium). J. Berry Res. 2020, 10, 243–257. [Google Scholar] [CrossRef]
- Lee, J. Anthocyanin analyses of Vaccinium fruit dietary supplements. Food Sci. Nutr. 2016, 4, 742–752. [Google Scholar] [CrossRef] [PubMed]
- Hurkova, K.; Uttl, L.; Rupert, J.; Navratilova, K.; Kocourek, V.; Stranska-Zachariasova, M.; Paprstein, F.; Hajslova, J. Cranberries versus lingonberries: A challenging authentication of similar Vaccinium fruit. Food Chem. 2019, 284, 162–170. [Google Scholar] [CrossRef]
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of berries and berry-based food products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Liaudanskas, M.; Raudone, L. Development, validation, and application of UPLC-PDA method for anthocyanins profiling in Vaccinium L. berries. J. Berry Res. 2021, 11, 583–599. [Google Scholar] [CrossRef]
- Raclariu, A.C.; Heinrich, M.; Ichim, M.C.; de Boer, H. Benefits and limitations of DNA barcoding and metabarcoding in herbal product authentication. Phytochem. Anal. 2018, 29, 123–128. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Yang, Y.; Jiang, L.; Liu, M.; Wang, B.; Wang, Y. Authentication of small berry fruit in fruit products by DNA barcoding method. J. Food Sci. 2018, 83, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Jaakola, L.; Suokas, M.; Häggman, H. Novel approaches based on DNA barcoding and high-resolution melting of amplicons for authenticity analyses of berry species. Food Chem. 2010, 123, 494–500. [Google Scholar] [CrossRef]
- Baker, M. Digital PCR hits its stride. Nat. Methods 2012, 9, 541–544. [Google Scholar] [CrossRef]
- Floren, C.; Wiedemann, I.; Brenig, B.; Schütz, E.; Beck, J. Species identification and quantification in meat and meat products using droplet digital PCR (ddPCR). Food Chem. 2015, 173, 1054–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, R.; Dasgupta, K.; Xing, Y.P.; Hernandez, B.T.; Shao, M.; Rohozinski, D.; Kovak, E.; Lin, J.; de Oliveira, M.L.P.; Stover, E.; et al. Accurate measurement of transgene copy number in crop plants using droplet digital PCR. Plant J. 2017, 90, 1014–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Košir, A.B.; Spilsberg, B.; Holst-Jensen, A.; Žel, J.; Dobnik, D. Development and inter-laboratory assessment of droplet digital PCR assays for multiplex quantification of 15 genetically modified soybean lines. Sci. Rep. 2017, 7, 8601. [Google Scholar] [CrossRef] [Green Version]
- Basanisi, M.G.; La Bella, G.; Nobili, G.; Coppola, R.; Damato, A.M.; Cafiero, M.A.; La Salandra, G. Application of the novel droplet digital PCR technology for identification of meat species. Int. J. Food Sci. Technol. 2020, 55, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Scollo, F.; Egea, L.A.; Gentile, A.; La Malfa, S.; Dorado, G.; Hernandez, P. Absolute quantification of olive oil DNA by droplet digital-PCR (ddPCR): Comparison of isolation and amplification methodologies. Food Chem. 2016, 213, 388–394. [Google Scholar] [CrossRef]
- Yu, N.; Han, J.; Deng, T.; Chen, L.; Zhang, J.; Xing, R.; Wang, P.; Zhao, G.; Chen, Y. A novel analytical droplet digital PCR method for identification and quantification of raw health food material powder from Panax notoginseng. Food Anal. Methods 2021, 14, 552–560. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, J.; Oh, D.R.; Kim, A.Y.; Choi, C. Comparative analysis of complete chloroplast genome sequences and insertion-deletion (indel) polymorphisms to distinguish five Vaccinium species. Forests 2020, 11, 927. [Google Scholar] [CrossRef]
- Kim, K.; Lee, S.C.; Lee, J.; Lee, H.O.; Joh, H.J.; Kim, N.H.; Park, H.S.; Yang, T.J. Comprehensive survey of genetic diversity in chloroplast genomes and 45S nrDNAs within Panax ginseng species. PLoS ONE 2015, 10, e0117159. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Cheon, K.S.; Yoo, K.O.; Lee, H.O.; Cho, K.S.; Suh, J.T.; Kim, S.J.; Nam, J.H.; Sohn, H.B.; Kim, Y.H. Complete chloroplast genome sequences and comparative analysis of Chenopodium quinoa and C. album. Front. Plant Sci. 2017, 8, 1696. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Henry, R.J.; Rossetto, M.; Wang, Y.; Chen, S. Plant DNA barcoding: From gene to genome. Biol. Rev. 2015, 90, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kumar, A.; Nagireddy, A.; Mani, D.N.; Shukla, A.K.; Tiwari, R.; Sundaresan, V. DNA barcoding: An efficient tool to overcome authentication challenges in the herbal market. Plant Biotechnol. J. 2016, 14, 8–21. [Google Scholar] [CrossRef]
- An, J.; Moon, J.C.; Kim, J.H.; Kim, G.S.; Jang, C.S. Development of DNA-based species-specific real-time PCR markers for four berry fruits and their application in commercial berry fruit foods. Appl. Biol. Chem. 2019, 62, 10. [Google Scholar] [CrossRef] [Green Version]
- Little, D.P. A DNA mini-barcode for land plants. Mol. Ecol. Resour. 2014, 14, 437–446. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, Y.; Wang, X.; Wei, X.; Han, J. DNA mini-barcoding: A derived barcoding method for herbal molecular identification. Front. Plant Sci. 2019, 10, 987. [Google Scholar] [CrossRef]
- Ortola-Vidal, A.; Schnerr, H.; Rojmyr, M.; Lysholm, F.; Knight, A. Quantitative identification of plant genera in food products using PCR and Pyrosequencing® technology. Food Control 2007, 18, 921–927. [Google Scholar] [CrossRef]
- Song, M.; Dong, G.Q.; Zhang, Y.Q.; Liu, X.; Sun, W. Identification of processed Chinese medicinal materials using DNA mini-barcoding. Chin. J. Nat. Med. 2017, 15, 0481–0486. [Google Scholar] [CrossRef]
- Faller, A.C.; Ragupathy, S.; Shanmughanandhan, D.; Zhang, Y.; Lu, Z.; Chang, P.; Swanson, G.; Newmaster, S.G. DNA quality and quantity analysis of Camellia sinensis through processing from fresh leaves to a green tea extract. J. AOAC Int. 2019, 102, 1798–1807. [Google Scholar] [CrossRef]
- Yu, X.; Tan, W.; Gao, H.; Miao, L.; Tian, X. Development of a specific mini-barcode from plastome and its application for qualitative and quanitative identification of processed herbal products using DNA metabarcoding technique: A case study on Senna. Front. Pharmacol. 2020, 11, 585687. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, L.; Bozza, E.; Giongo, L. Soft fruit traceability in food matrices using real-time PCR. Nutrients 2009, 1, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, M.; Niu, N.; Wang, H.; Wang, B.; Li, M.; Wang, Y.; Wu, Y. Identification of small berry species in food and juice using TaqMan-based real-time PCR. J. AOAC Int. 2019, 102, 1552–1566. [Google Scholar] [CrossRef]
- Morcia, C.; Bergami, R.; Scaramagli, S.; Ghizzoni, R.; Carnevali, P.; Terzi, V. A chip digital PCR assay for quantification of common wheat contamination in pasta production chain. Foods 2020, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Deng, T.; Huang, W.; Chen, Y.; Ge, Y. A digital PCR method for identifying and quantifying adulteration of meat species in raw and processed food. PLoS ONE 2017, 12, e0173567. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Locus | Locus Location | Primer Sequence 5′-3′ | Amplicon Size (bp) | Primer Efficiency (%) |
|---|---|---|---|---|
| trnI-CAU–trnL-CAA | 92554–96620 1 | LB_F-TAGGCCTTGAAAGGAGAAGGAG | 174 | 104.7 |
| (Vaccinium vitis-idaea) | LB_R-GCTCGTAATCCAGCCGATAAAG | |||
| trnI-CAU–trnL-CAA | 95178–98419 2 | CB_F-CGTGCATTAAGACACGAAGG | 136 | 108.6 |
| (Vaccinium macrocarpon) | CB_R-TAAGGCTCCACTGCCTATGG |
| Lingonberry | Cranberry | |||
|---|---|---|---|---|
| DNA Mix (w/w) % | DNA Copies per µL | Measured Value % | DNA Copies per µL | Measured Value % |
| 100/0 | 860 1 | 100 | 1374 | 100 |
| 99/1 | 851 | 98.95 (−0.05) | 1322 | 96.22 (−2.80) |
| 95/5 | 847 | 98.49 (+3.67) | 1257 | 91.48 (−3.71) |
| 90/10 | 828 | 96.28 (+6.98) | 1170 | 85.15 (−5.39) |
| 75/25 | 729 | 84.77 (+13.03) | 1003 | 73.00 (−2.67) |
| 50/50 | 490 | 56.98 (+13.96) | 682 | 49.64 (−0.72) |
| 25/75 | 233 | 27.09 (+8.36) | 347 | 25.25 (+1.00) |
| 10/90 | 84 | 9.77 (−2.30) | 153 | 11.14 (+11.40) |
| 5/95 | 52 | 6.05 (+21.00) | 66 | 4.80 (−4.00) |
| 1/99 | 10 | 1.16 (+16.00) | 12 | 0.87 (−13.00) |
| 0/100 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karppinen, K.; Avetisyan, A.; Hykkerud, A.L.; Jaakola, L. A dPCR Method for Quantitative Authentication of Wild Lingonberry (Vaccinium vitis-idaea) versus Cultivated American Cranberry (V. macrocarpon). Foods 2022, 11, 1476. https://doi.org/10.3390/foods11101476
Karppinen K, Avetisyan A, Hykkerud AL, Jaakola L. A dPCR Method for Quantitative Authentication of Wild Lingonberry (Vaccinium vitis-idaea) versus Cultivated American Cranberry (V. macrocarpon). Foods. 2022; 11(10):1476. https://doi.org/10.3390/foods11101476
Chicago/Turabian StyleKarppinen, Katja, Anna Avetisyan, Anne Linn Hykkerud, and Laura Jaakola. 2022. "A dPCR Method for Quantitative Authentication of Wild Lingonberry (Vaccinium vitis-idaea) versus Cultivated American Cranberry (V. macrocarpon)" Foods 11, no. 10: 1476. https://doi.org/10.3390/foods11101476
APA StyleKarppinen, K., Avetisyan, A., Hykkerud, A. L., & Jaakola, L. (2022). A dPCR Method for Quantitative Authentication of Wild Lingonberry (Vaccinium vitis-idaea) versus Cultivated American Cranberry (V. macrocarpon). Foods, 11(10), 1476. https://doi.org/10.3390/foods11101476







