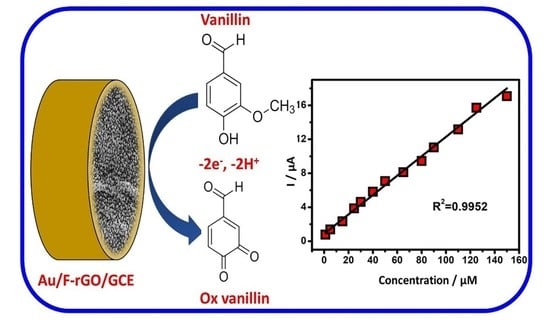
Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Sensor Fabrication
2.3. Physicochemical Characterization and Electrochemical Techniques
3. Results and Discussion
3.1. Surface Characterization
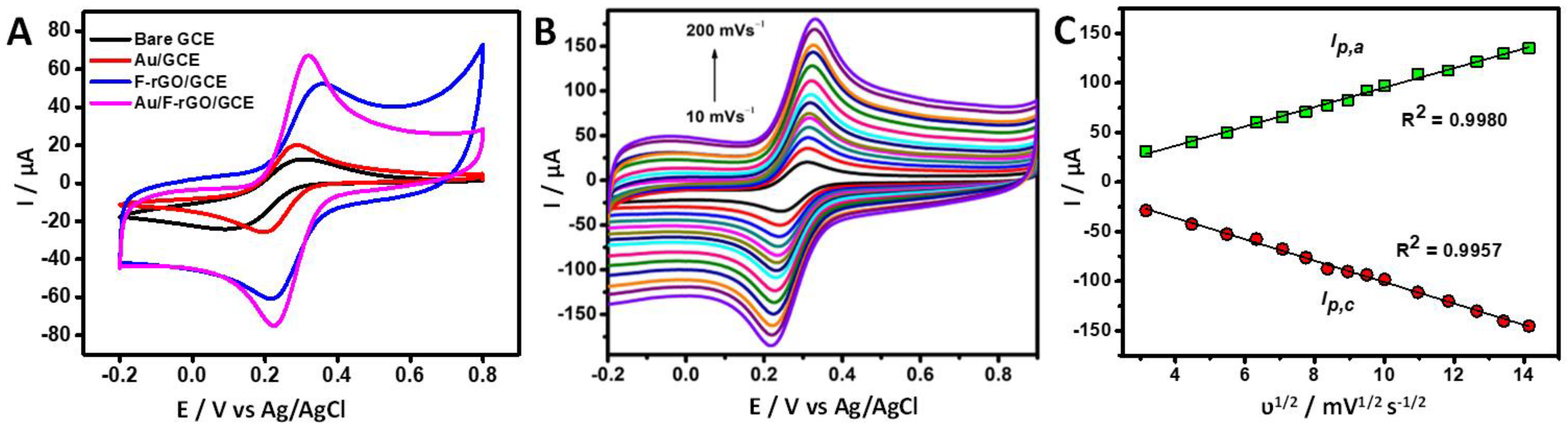
3.2. Electrochemical Characterization
3.3. Electrochemical Behavior of Vanillin Using CV and DPV
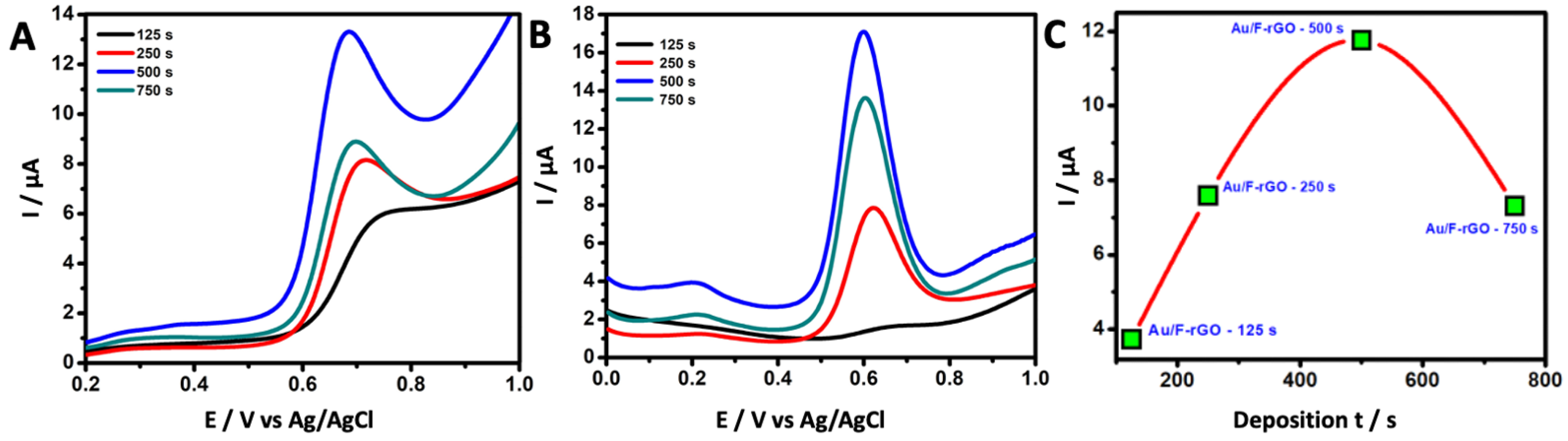
3.4. Optimization of Au-Deposition Time
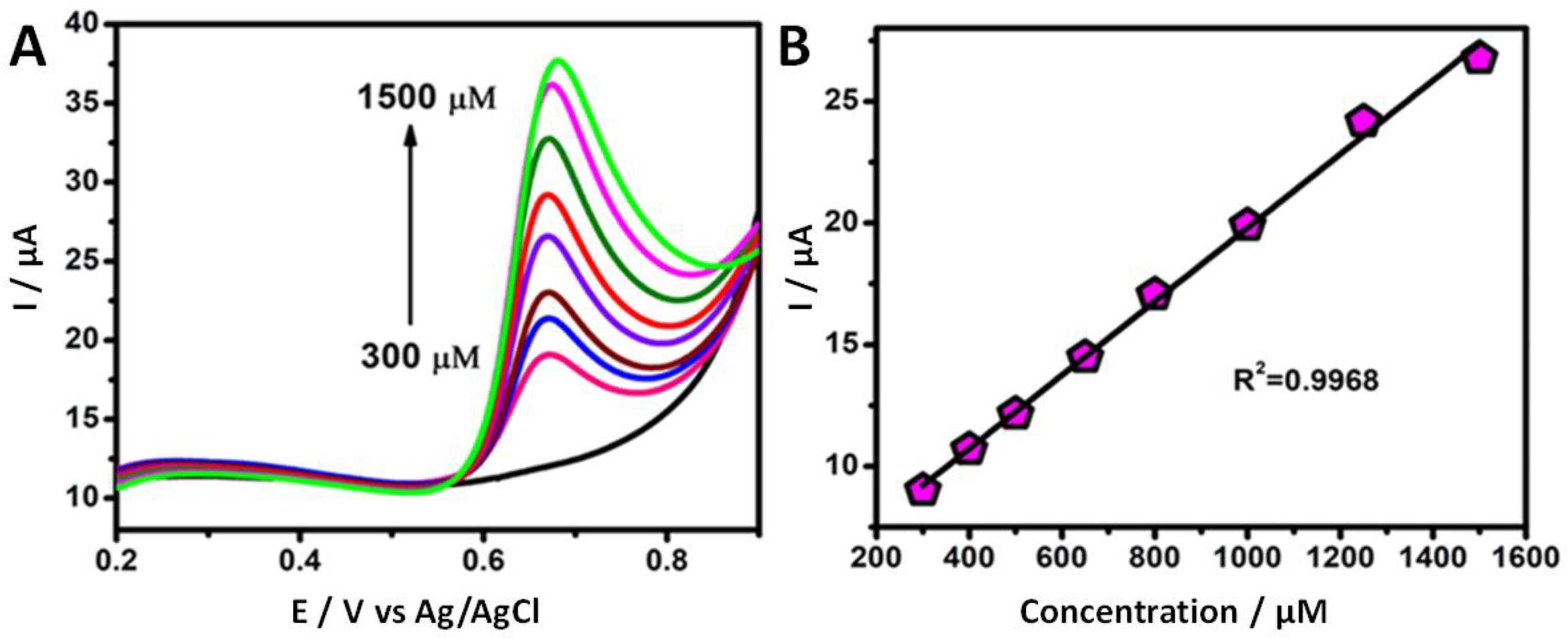
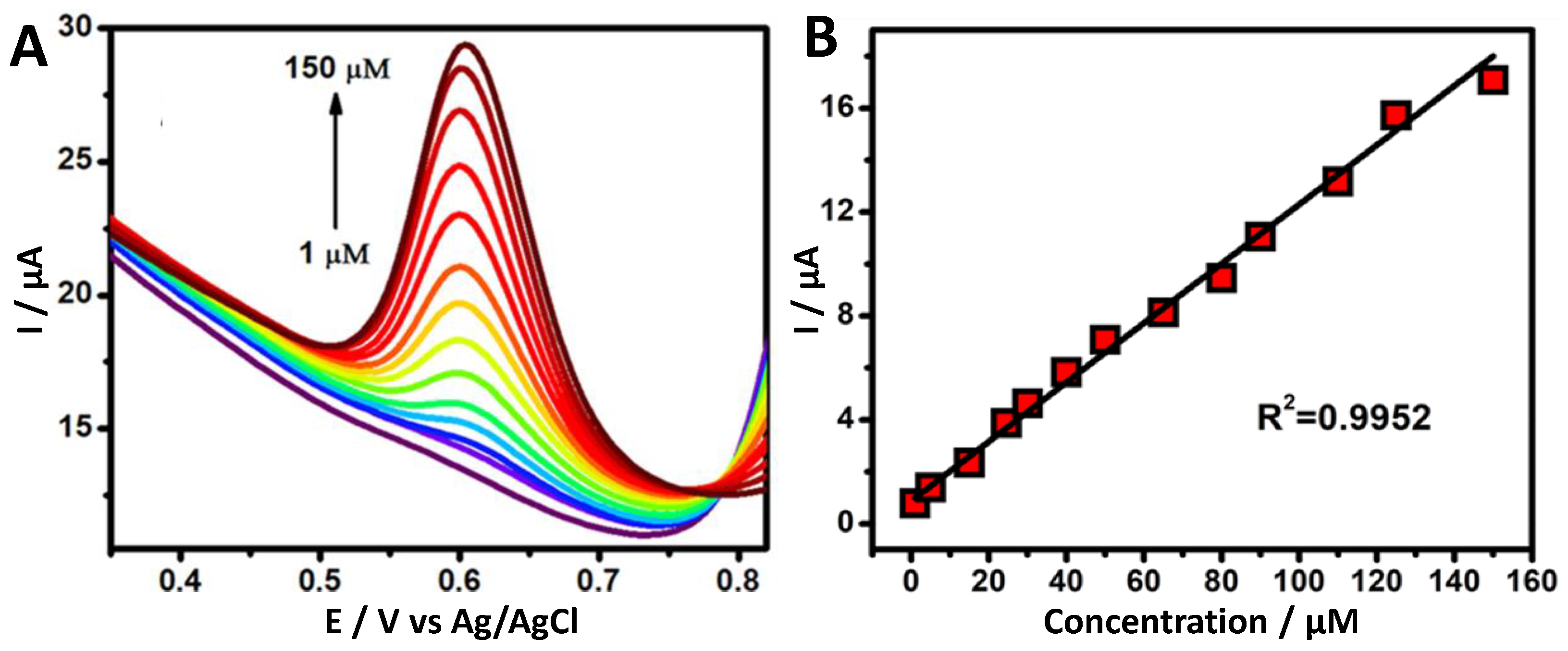
3.5. Effect of the Vanillin Concentration
3.6. Analytical Determination of Vanillin
3.7. Stability, Reproducibility and Interference Tests as Well as Real-Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fort, C.I.; Cobzac, S.C.A.; Turdean, G.L. Second-order derivative of square-wave voltammetry for determination of vanillin at platinum electrode. Food Chem. 2022, 385, 132711. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Motta, O.; Camin, F.; Bontempo, L.; Proto, A. Application of 13C quantitative NMR spectroscopy to isotopic analyses for vanillin authentication source. Foods 2021, 10, 2635. [Google Scholar] [CrossRef] [PubMed]
- Taouri, L.; Bourouina, M.; Bourouina-Bacha, S.; Hauchard, D. Fullerene-MWCNT nanostructured-based electrochemical sensor for the detection of vanillin as food additive. J. Food Compos. Anal. 2021, 100, 103811. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Mathiyarasu, J.; Kim, B.-S. Environmental-assisted shape-controlled synthesis and electrocatalytic performance of CuS nanostructures for vanillin detection in commercial food products. Appl. Mater. Today 2022, 27, 101428. [Google Scholar] [CrossRef]
- Kuznetsov, A.; Komarova, N.; Andrianova, M.; Grudstov, V.; Kuznetsov, E. Aptamer based vanillin sensor using an ion-sensitive field-effect transistor. Microchim. Acta 2018, 185, 3. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Zeng, R.; Ding, C. Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene–polyvinylpyrrolidone composite film. Food Chem. 2015, 180, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhao, J.; Zhao, L. Fabrication and characterization of magnetic molecularly imprinted polymer based on deep eutectic solvent for specific recognition and quantification of vanillin in infant complementary food. Food Chem. 2022, 374, 131720. [Google Scholar] [CrossRef]
- Cañadas, R.; González-Miquel, M.; González, E.J.; de Prado, A.N.; Díaz, I.; Rodríguez, M. Sustainable recovery of high added-value vanilla compounds from wastewater using green solvents. ACS Sustain. Chem. Eng. 2021, 9, 4850–4862. [Google Scholar] [CrossRef]
- Chbani, M.; Matthäus, B.; Charrouf, Z.; Monfalouti, H.E.; Kartah, B.; Gharby, S.; Willenberg, I. Characterization of phenolic compounds extracted from cold pressed cactus (Opuntia ficus-indica L.) seed oil and the effect of roasting on their composition. Foods 2020, 9, 1098. [Google Scholar] [CrossRef]
- Motshakeri, M.; Sharma, M.; Phillips, A.R.J.; Kilmartin, P.A. Electrochemical methods for the analysis of milk. J. Agric. Food Chem. 2022, 70, 2427–2449. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; van der Zalm, J.; Chen, A. Graphene oxide-based nanomaterials for the electrochemical sensing of isoniazid. ACS Appl. Nano Mater. 2021, 4, 3696–3706. [Google Scholar] [CrossRef]
- Cobb, S.J.; Macpherson, J.V. Enhancing square wave voltammetry measurements via electrochemical analysis of the non-faradaic potential window. Anal. Chem. 2019, 91, 7935–7942. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Deng, P.; Wu, Y.; Liu, J.; Li, J.; Li, G. High sensitive voltammetric sensor for nanomolarity vanillin detection in food samples vis manganese dioxide nanowires hybridized electrode. Microchem. J. 2020, 157, 104885. [Google Scholar] [CrossRef]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and sensitive determination of vanillin based on a glassy carbon electrode modified with Cu2O-electrochemically reduced graphene oxide nanocomposite film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Yuan, X.; Zhang, Z.; Zhang, J. Preparation of ZIF-67 derived thin film electrode via electrophoretic deposition for efficient electrocatalytic oxidation of vanillin. Inorg. Chem. 2019, 58, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, L.; Zhao, F.; Zeng, B. A vanillin electrochemical sensor based on molecularly imprinted poly(1-vinyl-3-octylimidazole hexafluoride phosphorus)-multi-walled carbon nanotubes@polydopamine-carboxyl single-walled carbon nanotubes composite. Sens. Actuators B Chem. 2017, 239, 481–487. [Google Scholar] [CrossRef]
- Calam, T.T. Voltammetric determination and electrochemical behavior of vanillin based on 1H-1,2,4-triazole-3-thiol polymer film modified gold electrode. Food Chem. 2020, 328, 127098. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, Y.; Jiang, F.; Li, L.; Mo, F. Electrodeposited nitrogen-doped graphene/carbon nanotubes nanocomposite as enhanced for simultaneous and sensitive voltammetric determination of caffeine and vanillin. Anal. Chim. Acta 2014, 833, 22–28. [Google Scholar] [CrossRef]
- Yardım, Y.; Gülcan, M.; Şentürk, Z. Determination of vanillin in commercial food product by adsorptive stripping voltammetry using a boron-doped diamond electrode. Food Chem. 2013, 141, 1821–1827. [Google Scholar] [CrossRef]
- Zheng, D.; Hu, C.; Gan, T.; Dang, X.; Hu, S. Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy NPs. Sens. Actuators B Chem. 2010, 148, 247–252. [Google Scholar] [CrossRef]
- Silva, T.R.; Brondani, D.; Zapp, E.; Vieira, C.I. Electrochemical sensor based on gold NPs stabilized in poly(Allylamine hydrochloride) for determination of vanillin. Electroanalysis 2015, 27, 465–472. [Google Scholar] [CrossRef]
- Li, J.; Feng, H.; Li, J.; Jiang, J.; Feng, Y.; He, L.; Qian, D. Bimetallic Ag-Pd NPs-decorated graphene oxide: A fascinating three-dimensional nanohybrid as an efficient electrochemical sensing platform for vanillin determination. Electrochim. Acta 2015, 176, 827–835. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, F.; Zeng, B. Sensitive voltammetric determination of vanillin with an AuPd NPs-graphene composite modified electrode. Food Chem. 2014, 151, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wu, S.; Lu, Y.; Wang, Y.; Cao, Q.; Fang, W. Effective control of particle size and electron density of Pd/C and Sn-Pd/C nanocatalysts for vanillin production via base-free oxidation. ACS Catal. 2020, 10, 7699–7709. [Google Scholar] [CrossRef]
- Murtada, K.; Moreno, V. Nanomaterials-based electrochemical sensors for the detection of aroma compounds—Towards analytical approach. J. Electroanal. Chem. 2020, 861, 113988. [Google Scholar] [CrossRef]
- Adhikari, B.-R.; Govindhan, M.; Schraft, H.; Chen, A. Simultaneous and sensitive detection of acetaminophen and valacyclovir based on two dimensional graphene nanosheet. J. Electroanal. Chem. 2016, 780, 241–248. [Google Scholar] [CrossRef]
- Mao, B.; Qian, L.; Govindhan, M.; Liu, Z.; Chen, A. Simultaneous electrochemical detection of guanine and adenine using reduced graphene oxide decorated with AuPt nanoclusters. Microchim. Acta 2021, 188, 276. [Google Scholar] [CrossRef]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-oxide based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, V.S.; O’Driscoll, B.; Bloor, J.M.; Li, B.; Katare, P.; Sethi, J.; Gorthi, S.S.; Jenkins, D. Emerging graphene-based sensors for the detection of food adulterants and toxicants—A review. Food Chem. 2021, 355, 129547. [Google Scholar] [CrossRef]
- Hou, X.; Ding, R.; Yan, S.; Zhao, H.; Yang, Q.; Wu, W. ZrO2 NPs and poly(diallyldimethylammoniumchloride)-doped graphene oxide aerogel-coated stainless-steel mesh for the effective adsorption of organophosphorus pesticides. Foods 2021, 10, 1616. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene materials: From understanding to design and synthesis control. Chem. Rev. 2020, 120, 10336–10453. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Srivastava, S.; Narayanan, T.N.; Mahlotra, B.D.; Vajtai, R.; Ajayan, P.M.; Srivastava, A. Functionalized multilayered graphene platform for urea sensor. ACS Nano 2012, 6, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.S.; Durairaj, S.; Boateng, E.; Sidhureddy, B.; Chen, A. Electrochemical detection of nitrite based on Co3O4-Au nanocomposites for food quality control. J. Electrochem. Soc. 2021, 168, 107505. [Google Scholar] [CrossRef]
- Liu, Z.; Forsyth, H.; Khaper, N.; Chen, A. Sensitive electrochemical detection of nitric oxide based on AuPt and reduced graphene oxide nanocomposites. Analyst 2016, 141, 4074–4083. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Chen, W.; Ge, C.; Li, J.T.; Beckham, J.L.; Yuan, Z.; Wyss, K.M.; Advincula, P.A.; Eddy, L.; Kittrell, C.; Chen, J.; et al. Heteroatom-doped flash graphene. ACS Nano 2022, 16, 6646–6656. [Google Scholar] [CrossRef]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Manna, B.; Raj, C.R. Nanostructured sulfur-doped porous reduced graphene oxide for the ultrasensitive electrochemical detection and efficient removal of Hg (II). ACS Sustain. Chem. Eng. 2018, 6, 6175–6182. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive electrochemical detection of caffeic acid in wine based on fluorine-doped graphene oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [Green Version]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Simple one-step synthesis of fluorine-doped carbon NPs as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar] [CrossRef]
- Wang, T.; Zang, X.; Wang, X.; Gu, X.; Shao, Q.; Cao, N. Recent advances in fluorine-doped/fluorinated carbon-based materials for supercapacitors. Energy Storage Mater. 2020, 30, 367–384. [Google Scholar] [CrossRef]
- Shahzad, F.; Zaidi, S.A.; Koo, C.M. Synthesis of multifunctional electrically tunable fluorine-doped reduced graphene oxide at low temperatures. ACS Appl. Mater. Interfaces 2017, 9, 24179–24189. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, Y.; Feng, Y.; Cao, Y.; Cao, C.; Long, P.; Li, S.; Feng, W. Reduced graphene oxide doped predominantly with CF2 groups as a superior anode material for long-life lithium-ion batteries. Chem. Commun. 2018, 54, 2727–2730. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chaisiwamongkhol, K.; Chen, Y.; Compton, R.G. Rapid electrochemical detection of vanillin in natural vanilla. Electroanalysis 2019, 31, 1067–1074. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G. A simple approach for the electrochemical determination of vanillin at ionic surfactant modified graphene paste electrode. Microchem. J. 2020, 154, 104575. [Google Scholar] [CrossRef]
- Peng, J.; Hou, C.; Hu, X. A graphene-based electrochemical sensor for sensitive detection of vanillin. Int. J. Electrochem. Sci. 2012, 7, 1724–1733. [Google Scholar]
- Calam, T.T.; Uzun, D. Rapid and selective determination of vanillin in the presence of caffeine, its electrochemical behavior on an Au electrode electropolymerized with 3-Amino-1,2,4-triazole-5-thiol. Electroanalysis 2019, 31, 2347–2358. [Google Scholar] [CrossRef]
- Veeramani, V.; Madhu, R.; Chen, S.-M.; Veerakumar, P.; Syu, J.-J.; Liu, S.-B. Cajeput tree bark derived activated carbon for the practical electrochemical detection of vanillin. New J. Chem. 2015, 39, 9109–9119. [Google Scholar] [CrossRef]
- Monnappa, A.B.; Manjunatha, J.G.G.; Bhatt, A.S.; Nagarajappa, H. Sensitive and selective electrochemical detection of vanillin at graphene-based poly (methyl orange) modified electrode. J. Sci. Adv. Mater. Devices 2021, 6, 415–424. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manikandan, V.S.; Boateng, E.; Durairaj, S.; Chen, A. Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles. Foods 2022, 11, 1448. https://doi.org/10.3390/foods11101448
Manikandan VS, Boateng E, Durairaj S, Chen A. Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles. Foods. 2022; 11(10):1448. https://doi.org/10.3390/foods11101448
Chicago/Turabian StyleManikandan, Venkatesh S., Emmanuel Boateng, Sharmila Durairaj, and Aicheng Chen. 2022. "Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles" Foods 11, no. 10: 1448. https://doi.org/10.3390/foods11101448
APA StyleManikandan, V. S., Boateng, E., Durairaj, S., & Chen, A. (2022). Electrochemical Sensing of Vanillin Based on Fluorine-Doped Reduced Graphene Oxide Decorated with Gold Nanoparticles. Foods, 11(10), 1448. https://doi.org/10.3390/foods11101448