The Beneficial Effects of Two Polysaccharide Fractions from Sargassum fusiform against Diabetes Mellitus Accompanied by Dyslipidemia in Rats and Their Underlying Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Preparation of SFPs
2.3. Animal Experiment
2.3.1. Experiment I
2.3.2. Experiment II
2.3.3. Experiment III
2.4. Oral Glucose Tolerance Test
2.5. Biochemical Index Determination
2.6. Short Chain Fatty Acid Detection
2.7. 16S rRNA Gene Sequencing Analysis
2.8. Quantitative Reverse Transcription PCR
2.9. Statistical Analysis
3. Results
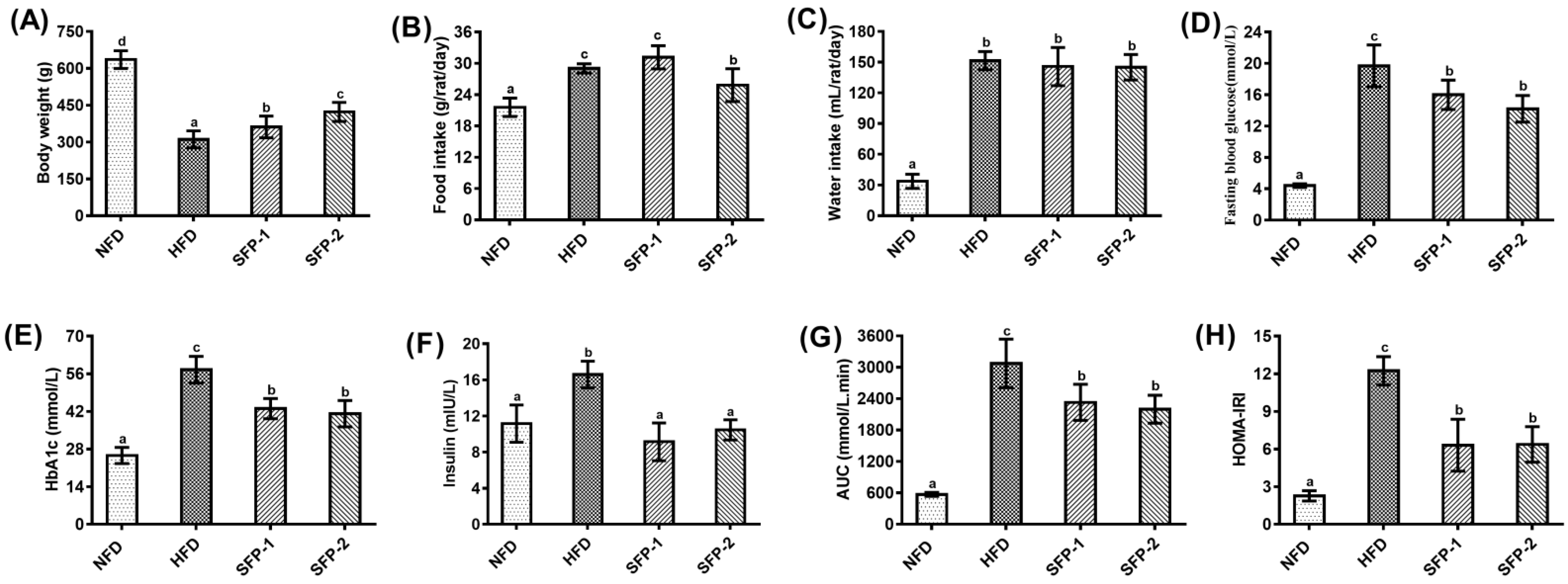
3.1. Effects of SFPs on Carbohydrate Loading Capacity in Rats
3.2. Hypoglycemic Activity of SFPs in Diabetic Rats
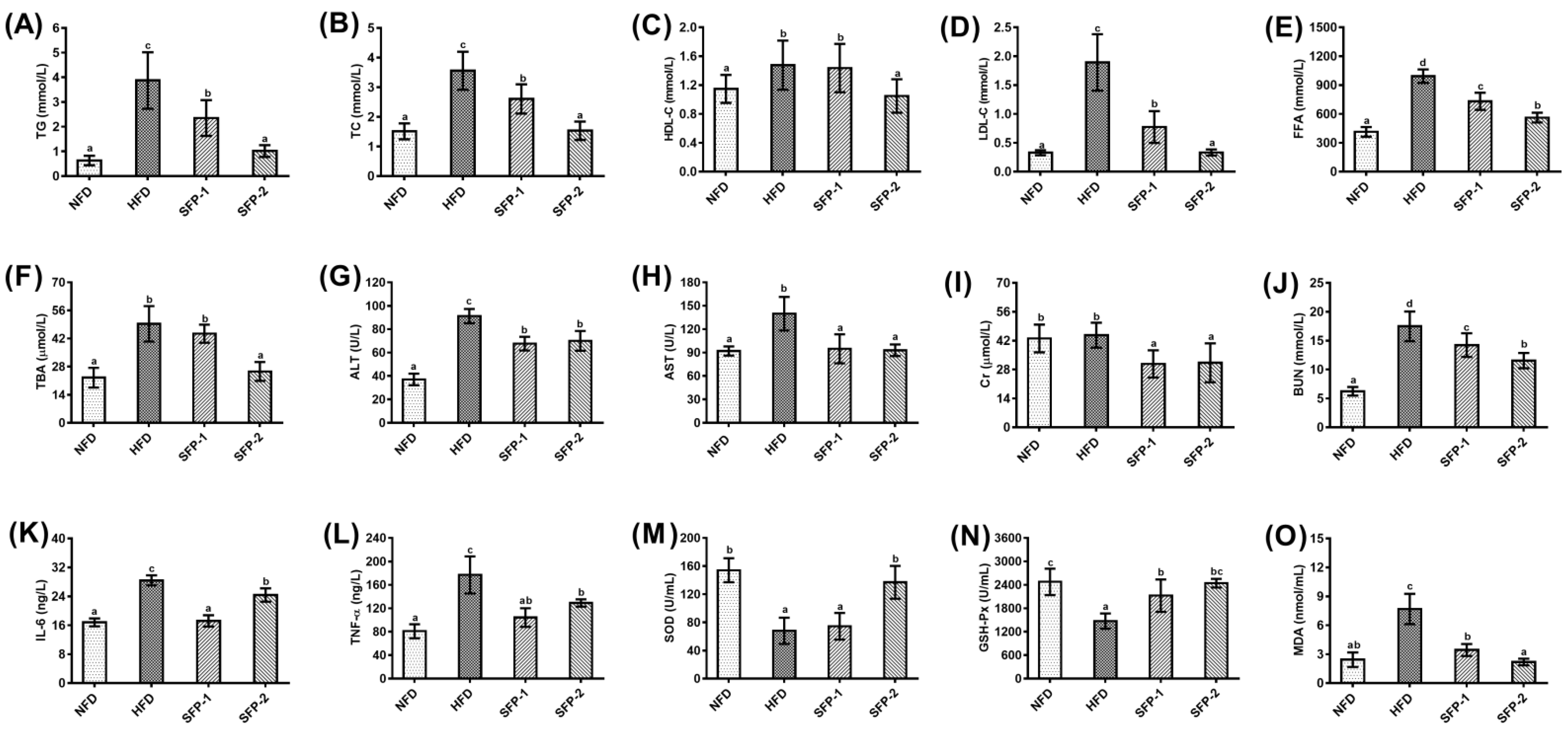
3.3. Effects of SFPs on Serum Profiles in Diabetic Rats
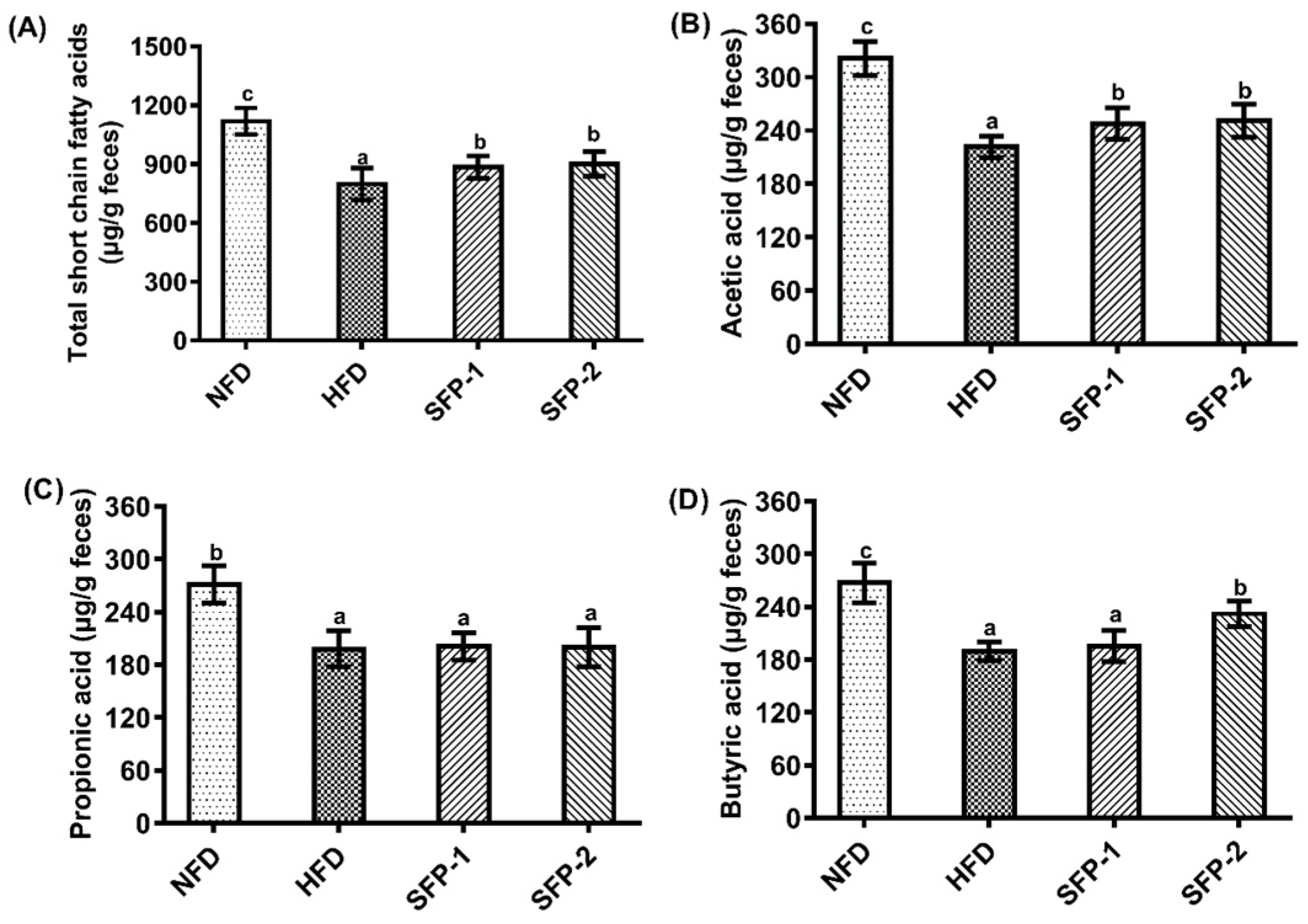
3.4. Effects of SFPs on SCFA Production Capacity in Diabetic Rats
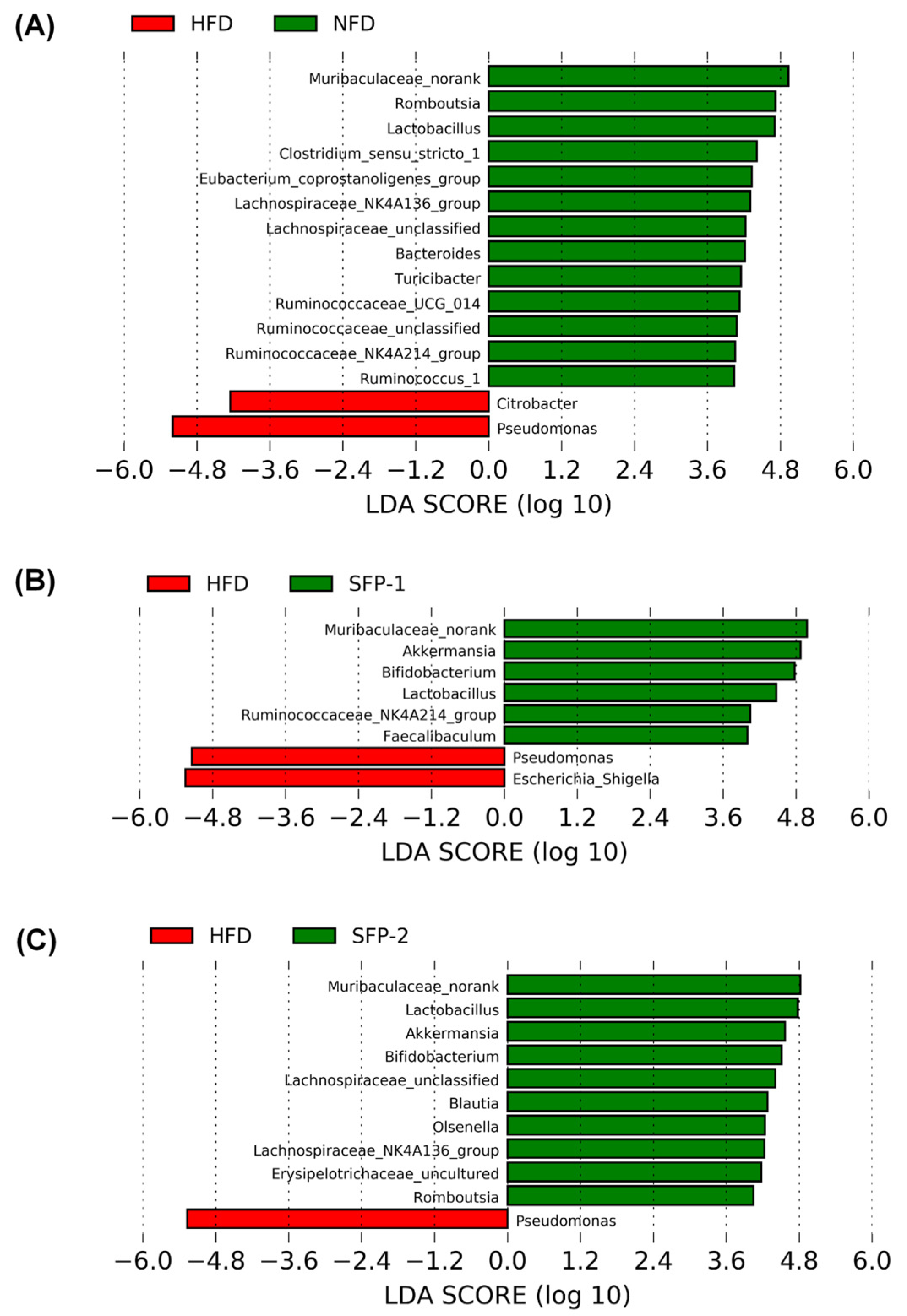
3.5. Effects of SFPs on Gut Flora in Diabetic Rats
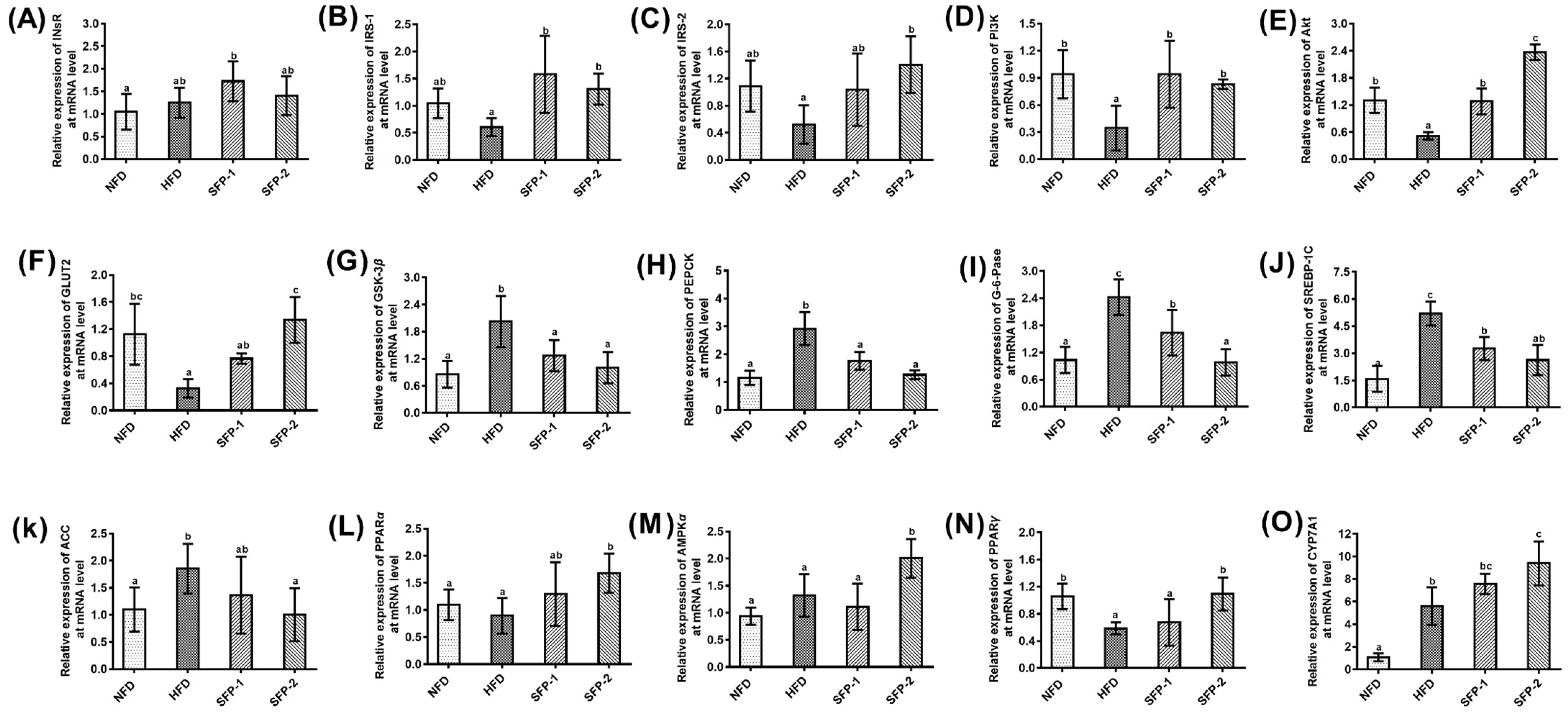
3.6. Effects of SFPs on Gene Expression at the mRNA Level in Diabetic Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campus, G.; Saelem, A.; Maida, C.; Tonolo, G.; Baldoni, E. Clinical aspect of periodontitis in T2DM (non-insulin-dependent-diabetes) patients. J. Dent. Res. 2003, 82, 545. [Google Scholar]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic Complications of Diabetes Mellitus: A. Mini Review. Curr. Diab. Rep. 2017, 13, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 16 February 2022).
- Wu, G.J.; Bai, Z.Y.; Wan, Y.J.; Shi, H.F.; Huang, X.J.; Nie, S.P. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocoll. 2020, 101, 105456. [Google Scholar] [CrossRef]
- De Climens, A.R.; Pain, E.; Boss, A.; Shaunik, A. Understanding reasons for treatment discontinuation, attitudes and education needs among people who discontinue type 2 diabetes treatment: Results from an online patient survey in the USA and UK. Diabetes Ther. 2020, 11, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wu, Q.; Zhang, J.; Xie, Y.; Cai, W.; Tan, J. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 down-regulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol 2017, 196, 47–57. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, Q.M.; Yu, N.J.; Chen, W.D.; Zha, X.Q.; Wu, D.L.; Pan, L.H.; Duan, J.; Luo, J.P. Dendrobium huoshanense polysaccharide regulates hepatic glucose homeostasis and pancreatic β-cell function in type 2 diabetic mice. Carbohydr. Polym. 2019, 211, 39–48. [Google Scholar] [CrossRef]
- Zhu, X.M.; Xu, R.; Wang, H.; Chen, J.Y.; Tu, Z.C. Structural properties, bioactivities, and applications of polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: A review. J. Agr. Food Chem. 2020, 68, 14091–14103. [Google Scholar] [CrossRef]
- Zhu, Y.; Cong, W.; Shen, L.; Wei, H.; Wang, Y.; Wang, L.; Ruan, K.; Wu, F.; Feng, Y. Fecal metabonomic study of a polysaccharide, MDG-1 from Ophiopogon japonicus on diabetic mice based on gas chromatography/time-of-flight mass spectrometry (GC TOF/MS). Mol. Biosyst. 2014, 10, 304–312. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-diabetic effects and mechanisms of dietary polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Chen, H.P.; He, Y.Y.; Chen, W.L.; Chen, J.W.; Gao, L.; Hu, H.Y.; Wang, J. Effects of rich-polyphenols extract of Dendrobium loddigesii on anti-diabetic, anti-inflammatory, anti-oxidant, and gut microbiota modulation in db/db mice. Molecules 2018, 23, 3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, Y.; Wan, X.; Zeng, F.; Zhong, R.; Guo, W.; Lv, X.C.; Zhao, C.; Liu, B. Regulatory effect of Grifola frondosa extract rich in polysaccharides and organic acids on glycolipid metabolism and gut microbiota in rats. Int. J. Biol. Macromol. 2019, 155, 1030–1039. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Xie, L.; Zheng, X.; Chen, W. Dietary fibers as emerging nutritional factors against diabetes: Focus on the involvement of gut microbiota. Crit. Rev. Biomed. Eng. 2019, 39, 524–540. [Google Scholar] [CrossRef]
- Chen, G.; Chen, R.; Chen, D.; Ye, H.; Hu, B.; Zeng, X.; Liu, Z. Tea Polysaccharides as potential therapeutic options for metabolic diseases. J. Agr. Food Chem. 2019, 67, 5350–5360. [Google Scholar] [CrossRef]
- Feng, Y.; Weng, H.; Ling, L.; Zeng, T.; Zhang, Y.; Chen, D.; Li, H. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. Int. J. Biol. Macromol. 2019, 132, 1001–1011. [Google Scholar] [CrossRef]
- Praveen, M.A.; Parvathy, K.R.K.; Balasubramanian, P.; Jayabalan, R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Liu, J.; Song, J.; Yu, P.; Chen, P.; Liao, Z.; Wu, M.; Tong, H. Physicochemical characterization of Sargassum fusiforme fucoidan fractions and their antagonistic effect against P-selectin-mediated cell adhesion, International Journal of Biological. Macromolecules 2019, 133, 656–662. [Google Scholar] [CrossRef]
- Jia, R.B.; Li, Z.R.; Wu, J.; Ou, Z.R.; Zhu, Q.; Sun, B.; Lin, L.; Zhao, M. Physicochemical properties of polysaccharide fractions from Sargassum fusiforme and their hypoglycemic and hypolipidemic activities in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 147, 428–438. [Google Scholar] [CrossRef]
- Staob, A.M. Removal of proteins from polysaccharides methods. Methods Carbohydr. Chem. 1965, 5, 5–9. [Google Scholar]
- Zhang, C.; Liu, A.; Zhang, T.; Li, Y.; Zhao, H. Gas chromatography detection protocol of short-chain fatty acids in mice feces. Bio-Protocol 2020, 10, e3672. [Google Scholar] [CrossRef] [PubMed]
- Raghu, C.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-D-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar] [CrossRef]
- Piliponiene, L.; Velickiene, D.; Kregzdyte, R. Microvascular complications, peripheral artery disease and mortality in patients with type 2 diabetes mellitus, in two counties of southern Lithuania over 13 years: Analysis using a cohort database of the national health insurance. Medicina 2021, 57, 1380. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, X.; Liu, S.; Yu, H.; Li, R.; Wang, X.; Qin, Y.; Li, P. Preparation of low molecular weight Sargassum fusiforme polysaccharide and its anticoagulant activity. J. Oceanol. Limnol. 2018, 36, 882–891. [Google Scholar] [CrossRef]
- Chen, P.; He, D.; Zhang, Y.; Yang, S.; Chen, L.; Wang, S.; Zou, H.; Liao, Z.; Zhang, X.; Wu, M. Sargassum fusiforme polysaccharides activate antioxidant defense by promoting Nrf2-dependent cytoprotection and ameliorate stress insult during aging. Food Funct. 2016, 7, 4576–4588. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Graneri, L.T.; Mamo, J.C.L.; D’Alonzo, Z.; Lam, V.; Takechi, R. Chronic intake of energy drinks and their sugar free substitution similarly promotes metabolic syndrome. Nutrients 2021, 13, 1202. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Jurgonski, A.; Kołodziejczyk, K.; Kosmala, M.; Milala, J.; Zduńczyk, Z.; Fotschki, B.; Żary-Sikorska, E. Blood glucose lowering efficacy of strawberry extracts rich in ellagitannins with different degree of polymerization in rats. Pol. J. Food Nutr. Sci. 2016, 66, 109–117. [Google Scholar] [CrossRef]
- Zhang, B.; Yue, R.; Chen, Y.; Yang, M.; Huang, X.; Shui, J.; Peng, Y.; Chin, J. Gut microbiota, a potential new arget for chinese herbal medicines in treating diabetes mellitus. Evid. Based Complementary Altern. Med. 2019, 2019, 2634898. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Perez-Jimenez, F.; Camargo, A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J. Nutr. Biochem. 2019, 70, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Khondkaryan, L.; Margaryan, S.; Poghosyan, D.M. Impaired inflammatory response to LPS in type 2 diabetes mellitus. Int. J. Inflamm. 2018, 2018, 2157434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballan, R.; Saad, S.M.I. Characteristics of the gut microbiota and potential effects of probiotic supplements in individuals with type 2 diabetes mellitus. Foods 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, M.; Yang, J.; Xu, Q.; Liang, C.; Chen, B.; Zhang, J.; Yang, Y.; Wang, H.; Shang, Y.; et al. Response of gut microbiota in type 2 diabetes to hypoglycemic agents. Endocrine 2019, 66, 485–493. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Liang, J.; Wang, T.; Sun, M.; Zhang, Z. Gymnemic acid ameliorates hyperglycemia through PI3K/AKT- and AMPK-mediated signaling pathways in type 2 diabetes mellitus rats. J. Agric. Food Chem. 2019, 67, 13051–13060. [Google Scholar] [CrossRef]
- Shah, A.M.; Wondisford, F.E. Tracking the carbons supplying gluconeogenesis. J. Biol. Chem. 2020, 295, 14419–14429. [Google Scholar] [CrossRef]
- Qu, X.; Guan, P.; Xu, L.; Liu, B.; Li, M.; Xu, Z.; Huang, X.; Han, L. Riligustilide alleviates hepatic insulin resistance and gluconeogenesis in T2DM mice through multitarget actions. Phytother. Res. 2021, 36, 462–474. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Chung, S.S.M.; Xu, B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 103, 1012–1017. [Google Scholar] [CrossRef]
- Vlavcheski, F.; den Hartogh, D.J.; Giacca, A.; Tsiani, E. Amelioration of high-insulin-induced skeletal muscle cell insulin resistance by resveratrol is linked to activation of AMPK and restoration of GLUT4 translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.X.; Liu, J.; Li, P.Q.; Xie, X.-D.; Guo, Q.; Tian, L.M.; Ma, X.Q.; Zhang, J.P.; Liu, J.; Gao, J.Y. Association of sterol regulatory element-binding protein-1c gene polymorphism with type 2 diabetes mellitus, insulin resistance and blood lipid levels in Chinese population. Diabetes Res. Clin. Pract. 2008, 82, 42–47. [Google Scholar] [CrossRef]
- Dludla, P.V.; Muller, C.J.F.; Louw, J.; Mazibuko-Mbeje, S.E.; Tiano, L.; Silvestri, S.; Orlando, P.; Marcheggiani, F.; Cirilli, I.; Chellan, N.; et al. The Combination effect of aspalathin and phenylpyruvic acid-2-O-β-D-glucoside from Rooibos against hyperglycemia-induced cardiac damage: An in vitro study. Nutrients 2020, 12, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Ins R | CAATGGTGCTGAGGACACTAGG | GTGCTCTTCGTGGCTTGTGG |
| PI3K | GGTGAGGAACGAAGAATGGC | TCCGAGGCAAGACAGGGATA |
| Akt | TGAGACCGACACCAGGTATTTTG | GCTGAGTAGGAGAACTGGGGAAA |
| IRS-1 | ATGTGGAAATGGCTCGGA | TAAGGCAGCAAAGGGTAGGC |
| IRS-2 | TGACCAGTCCCACATCAGGC | CTGCACGGATGACCTTAGCG |
| GLUT2 | CTCTGTGCTGCTTGTGGAGA | CGGCACAGAAAAACATGCC |
| GSK-3β | TCGTCCATCGATGTGTGGTC | TTGTCCAGGGGTGAGCTTTG |
| PEPCK | TGCCCATCGAAGGCATCA | TCTCATGGCAGCTCCTACAAACAC |
| G-6Pase | TCGTCCATCGATGTGTGGTC | TTGTCCAGGGGTGAGCTTTG |
| AMPKα | ACCTGAGAACGTCCTGCTTG | TAAGGCAGCAAAGGGTAGGC |
| PPARα | TCACACAATGCAATCCGTTT | GGCCTTGACCTTGTTCATGT |
| PPARγ | TGTCGGTTTCAGAAGTGCCTTG | TTCAGCTGGTCGATATCACTGGAG |
| ACC | GAGAGGGGTCAAGTCCTTC | ACATCCACTTCCACACACGA |
| SREBP-1C | TACTTCTTGTGGCCCGTACC | TCAGGTCATGTTGGAAACCA |
| CYP7A1 | GCTTTACAGAGTGCTGGCCAA | CTGTCTAGTACCGGCAGGTCATT |
| β-actin | TGCTATGTTGCCCTAGACTTCG | GTTGGCATAGAGGTCTTTACGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, R.-B.; Wu, J.; Luo, D.; Lin, L.; Chen, C.; Xiao, C.; Zhao, M. The Beneficial Effects of Two Polysaccharide Fractions from Sargassum fusiform against Diabetes Mellitus Accompanied by Dyslipidemia in Rats and Their Underlying Mechanisms. Foods 2022, 11, 1416. https://doi.org/10.3390/foods11101416
Jia R-B, Wu J, Luo D, Lin L, Chen C, Xiao C, Zhao M. The Beneficial Effects of Two Polysaccharide Fractions from Sargassum fusiform against Diabetes Mellitus Accompanied by Dyslipidemia in Rats and Their Underlying Mechanisms. Foods. 2022; 11(10):1416. https://doi.org/10.3390/foods11101416
Chicago/Turabian StyleJia, Rui-Bo, Juan Wu, Donghui Luo, Lianzhu Lin, Chong Chen, Chuqiao Xiao, and Mouming Zhao. 2022. "The Beneficial Effects of Two Polysaccharide Fractions from Sargassum fusiform against Diabetes Mellitus Accompanied by Dyslipidemia in Rats and Their Underlying Mechanisms" Foods 11, no. 10: 1416. https://doi.org/10.3390/foods11101416
APA StyleJia, R.-B., Wu, J., Luo, D., Lin, L., Chen, C., Xiao, C., & Zhao, M. (2022). The Beneficial Effects of Two Polysaccharide Fractions from Sargassum fusiform against Diabetes Mellitus Accompanied by Dyslipidemia in Rats and Their Underlying Mechanisms. Foods, 11(10), 1416. https://doi.org/10.3390/foods11101416







