Preparation and Characterization of Beads of Sodium Alginate/Carboxymethyl Chitosan/Cellulose Nanofiber Containing Porous Starch Embedded with Gallic Acid: An In Vitro Simulation Delivery Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Loading PS with GA
2.3. Preparation of Beads
2.4. Zeta-Potential
2.5. Rheology
2.6. Characterization of PG and Beads
2.6.1. Texture Analysis
2.6.2. Scanning Electron Microscopy (SEM)
2.6.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Analysis
2.6.4. Thermogravimetric Analysis (TGA)
2.7. Swelling Rate
2.8. Antioxidant Activity of PG and Beads
2.9. In Vitro Release Study
2.10. Statistical Analysis
3. Results and Discussions
3.1. Optimization of Encapsulation Efficiency
3.1.1. Optimization of Loading PS with GA
3.1.2. Optimization of Wall Material Composition
3.2. Characterization of PG, PIPNs and Beads
3.2.1. Zeta-Potential
3.2.2. Rheology
3.2.3. Morphology of Beads
3.2.4. FT-IR
3.2.5. Thermostability
3.3. Texture Analysis
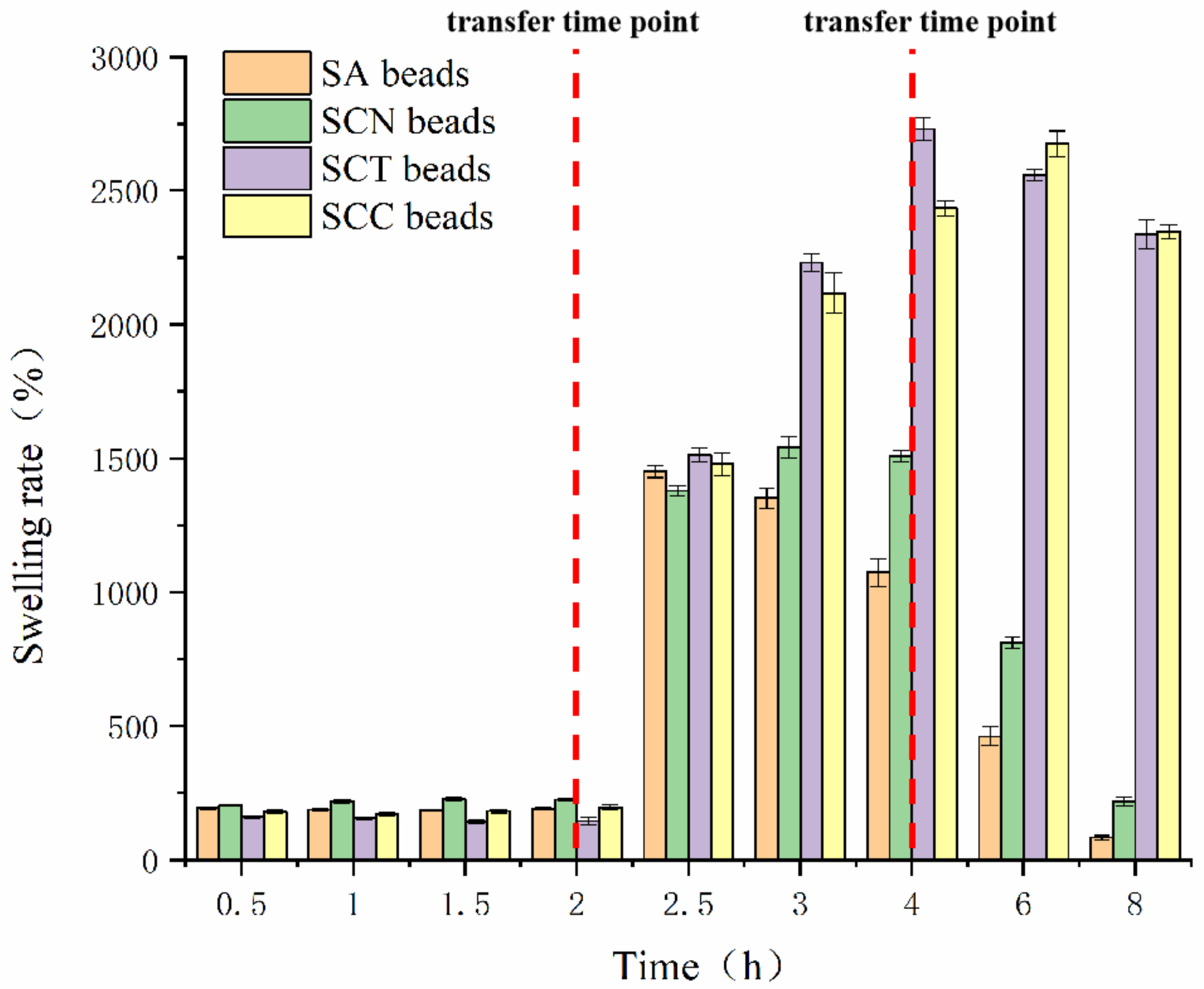
3.4. Swelling Rate
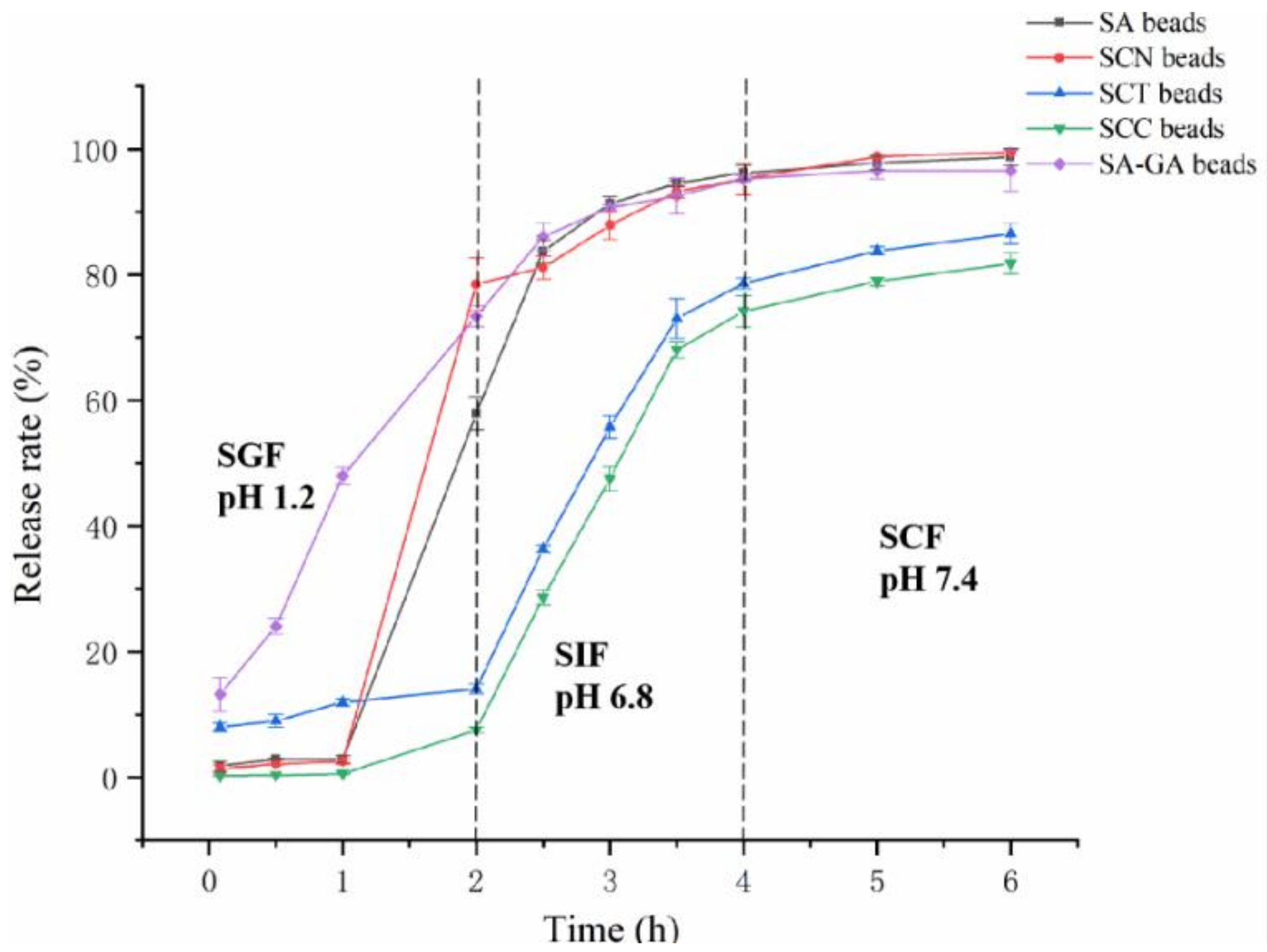
3.5. Release Analysis
3.6. Antioxidant Activity of PG and Beads
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Chen, X.; Huang, X.; Song, B.; Wang, Y.; Wang, Y. Regulation of glucose and lipid metabolism in health and disease. Sci. China Life Sci. 2019, 62, 1420–1458. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Lagarón, J.M.; López-Rubio, A. Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocoll. 2015, 49, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Castellon-Chicas, M.; Arbizu, S.; Talcott, S.; Drury, N.; Smith, S.; Mertens-Talcott, S. Mango (Mangifera indica L.) Polyphenols: Anti-Inflammatory Intestinal Microbial Health Benefits, and Associated Mechanisms of Actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Jothi, G.; Antony, S.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic acid attenuates high-fat diet fed-streptozotocin-induced insulin resistance via partial agonism of PPARγ in experimental type 2 diabetic rats and enhances glucose uptake through translocation and activation of GLUT4 in PI3K/p-Akt signaling pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sheng, Z.; Li, X.; Fan, X.; Jiang, W. In vitro studies on the interactions of blood lipid level-related biological molecules with gallic acid and tannic acid. J. Sci. Food Agric. 2019, 99, 6882–6892. [Google Scholar] [CrossRef] [PubMed]
- Tahereh, S.; Armen, N.; Miroslav, M.; Rahil, N.; Elisabeth, H.; Tahereh, J.; Elisabeth, L.; Michael, G.; Wolfgang, H.; Alexander, H.; et al. Gallic acid, a common dietary phenolic protects against high fat diet induced DNA damage. Eur. J. Nutr. 2019, 58, 2315–2326. [Google Scholar]
- Li, J.; Kim, S.Y.; Chen, X.; Park, H.J. Calcium-alginate beads loaded with gallic acid: Preparation and characterization. LWT 2016, 68, 667–673. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.P.; Gruppen, H.; Hollman, P.C. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Chang, P.R.; Yu, J.; Ma, X. Preparation of porous starch and its use as a structure-directing agent for production of porous zinc oxide. Carbohydr. Polym. 2011, 83, 1016–1019. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Khunkitti, W.; Pongjanyakul, T. Alginate-poloxamer beads for clotrimazole delivery: Molecular interactions, mechanical properties, and anticandidal activity. Int. J. Biol. Macromol. 2020, 148, 1061–1071. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef] [PubMed]
- Faruq, O.; Sayed, S.; Kim, B.; Im, S.; Lee, B. A biphasic calcium phosphate ceramic scaffold loaded with oxidized cellulose nanofiber–gelatin hydrogel with immobilized simvastatin drug for osteogenic differentiation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, X.G.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Sarkar, K.; Soam, S.; Kundu, P.P. Formulation of pH-responsive carboxymethyl chitosan and alginate beads for the oral delivery of insulin. J. Appl. Polym. Sci. 2013, 129, 835–845. [Google Scholar] [CrossRef]
- Yang, J.; Han, S.; Zheng, H.; Dong, H.; Liu, J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr. Polym. 2015, 123, 53–66. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Chang, P.R.; Feng, L.; Yu, J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Colloids Surf. B Biointerfaces 2011, 85, 270–279. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, L.; Chen, W.; Song, Y.; Qian, Z.; Cao, X.; Huang, Q.; Zhang, B.; Chen, H.; Chen, W. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: A novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019, 95, 562–570. [Google Scholar] [CrossRef]
- Zhou, Y.; Mei, Y.; Luo, T.; Chen, W.; Zhong, Q.; Chen, H.; Chen, W. Study on the Relationship between Emulsion Properties and Interfacial Rheology of Sugar Beet Pectin Modified by Different Enzymes. Molecules 2021, 26, 2829. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Hao, W.; Xie, Y.; Chen, L.; Li, Z.; Zhu, B.; Feng, X. Nano-bacterial cellulose/soy protein isolate complex gel as fat substitutes in ice cream model. Carbohydr. Polym. 2018, 198, 620–630. [Google Scholar] [CrossRef]
- Ko, W.; Chang, C.; Wang, H.; Wang, S.; Hsieh, C. Process optimization of microencapsulation of curcumin in gamma-polyglutamic acid using response surface methodology. Food Chem. 2015, 172, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.; Ouyang, X. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, G.; Liu, J.; Meng, Y.; Tang, Y. Adsorptive decolorization of methylene blue by crosslinked porous starch. Carbohydr. Polym. 2013, 93, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yu, Z.; Hu, H.; Lv, J.; Wang, H.; Jiang, S. Adsorption of procyanidins onto chitosan-modified porous rice starch. LWT 2017, 84, 10–17. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Shim, Y.Y.; Wang, Y.; Reaney, M.J. Intermolecular interaction and complex coacervation between bovine serum albumin and gum from whole flaxseed (Linum usitatissimum L.). Food Hydrocoll. 2015, 49, 95–103. [Google Scholar] [CrossRef]
- Li, Q.; Lan, H.; Zhao, Z. Protection effect of sodium alginate against heat-induced structural changes of lactoferrin molecules at neutral pH. LWT 2019, 99, 513–518. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Al-Sabah, A.; Gao, N.; Kyle, S.; Thomas, B.; Badiei, N.; Hawkins, K.; Whitaker, I.S. Printability of pulp derived crystal, fibril and blend nanocellulose-alginate bioinks for extrusion 3D bioprinting. Biofabrication 2019, 11, 045006. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, A.; Lü, Q.; Hui, M. The effects of rheological properties of wall materials on morphology and particle size distribution of microcapsule. Czech J. Food Sci. 2010, 28, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Paulraj, T.; Riazanova, A.V.; Yao, K.; Andersson, R.L.; Müllertz, A.; Svagan, A.J. Bioinspired Layer-by-Layer Microcapsules Based on Cellulose Nanofibers with Switchable Permeability. Biomacromolecules 2017, 18, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, C.; Zhou, W.; Luan, Q.; Li, W.; Deng, Q.; Dong, X.; Tang, H.; Huang, F. A pH-Responsive gel macrosphere based on sodium alginate and cellulose nanofiber for potential intestinal delivery of probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Shi, X.-W.; Du, Y.-M.; Sun, L.-P.; Yang, J.-H.; Wang, X.-H.; Su, X.-L. Ionically Crosslinked Alginate/Carboxymethyl Chitin Beads for Oral Delivery of Protein Drugs. Macromol. Biosci. 2005, 5, 881–889. [Google Scholar] [CrossRef]
- Hu, X.; Du, X. Adsorption of Tea Polyphenols using Microporous Starch: A Study of Kinetics, Equilibrium and Thermodynamics. Molecules 2019, 24, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khurana, R.; Singh, K.; Sapra, B.; Tiwary, A.; Rana, V. Tamarindus indica pectin blend film composition for coating tablets with enhanced adhesive force strength. Carbohydr. Polym. 2014, 102, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jiang, F.-C.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.-H.; Wang, H.-B.; He, J.-R.; Xiong, X.-G. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef]
- Funaki, J.; Minami, M.; Abe, S.; Ueda, R.; Eto, W.; Kugino, K.; Kugino, M.; Abe, K.; Toko, K.; Asakura, T. Effect of Proteolytic Modification on Texture and Mastication of Heat-Treated Egg White Gels. J. Food Process. Preserv. 2016, 41, e128571. [Google Scholar] [CrossRef] [Green Version]
- Hurler, J.; Engesland, A.; Kermany, B.P.; Škalko-Basnet, N. Improved texture analysis for hydrogel characterization: Gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 2012, 125, 180–188. [Google Scholar] [CrossRef]
- Han, W.; Meng, Y.; Hu, C.Y.; Dong, G.; Qu, Y.; Deng, H.; Guo, Y. Mathematical model of Ca 2+ concentration, pH, pectin concentration and soluble solids (sucrose) on the gelation of low methoxyl pectin. Food Hydrocoll. 2017, 66, 37–48. [Google Scholar] [CrossRef]
- Hezaveh, H.; Muhamad, I.I.; Noshadi, I.; Shu, F.L.; Ngadi, N. Swelling behaviour and controlled drug release from cross-linked kappa-carrageenan/NaCMC hydrogel by diffusion mechanism. J. Microencapsul. 2012, 29, 368–379. [Google Scholar] [CrossRef]
- George, M.; Abraham, T. pH sensitive alginate–guar gum hydrogel for the controlled delivery of protein drugs. Int. J. Pharm. 2007, 335, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.A.; Pinilla, C.M.B.; Brandelli, A. Pectin and polygalacturonic acid-coated liposomes as novel delivery system for nisin: Preparation, characterization and release behavior. Food Hydrocoll. 2017, 70, 1–7. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Nunes, M.R.; Benvenutti, E.V.; da Luz, S.R.; D’Avila, R.F.; Rutz, J.K. Microencapsulation of gallic acid in chitosan, β-cyclodextrin and xanthan. Ind. Crops Prod. 2013, 46, 138–146. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Bai, J. Microencapsulation and antimicrobial activity of carvacrol in a pectin-alginate matrix. Food Hydrocoll. 2019, 92, 69–73. [Google Scholar] [CrossRef]
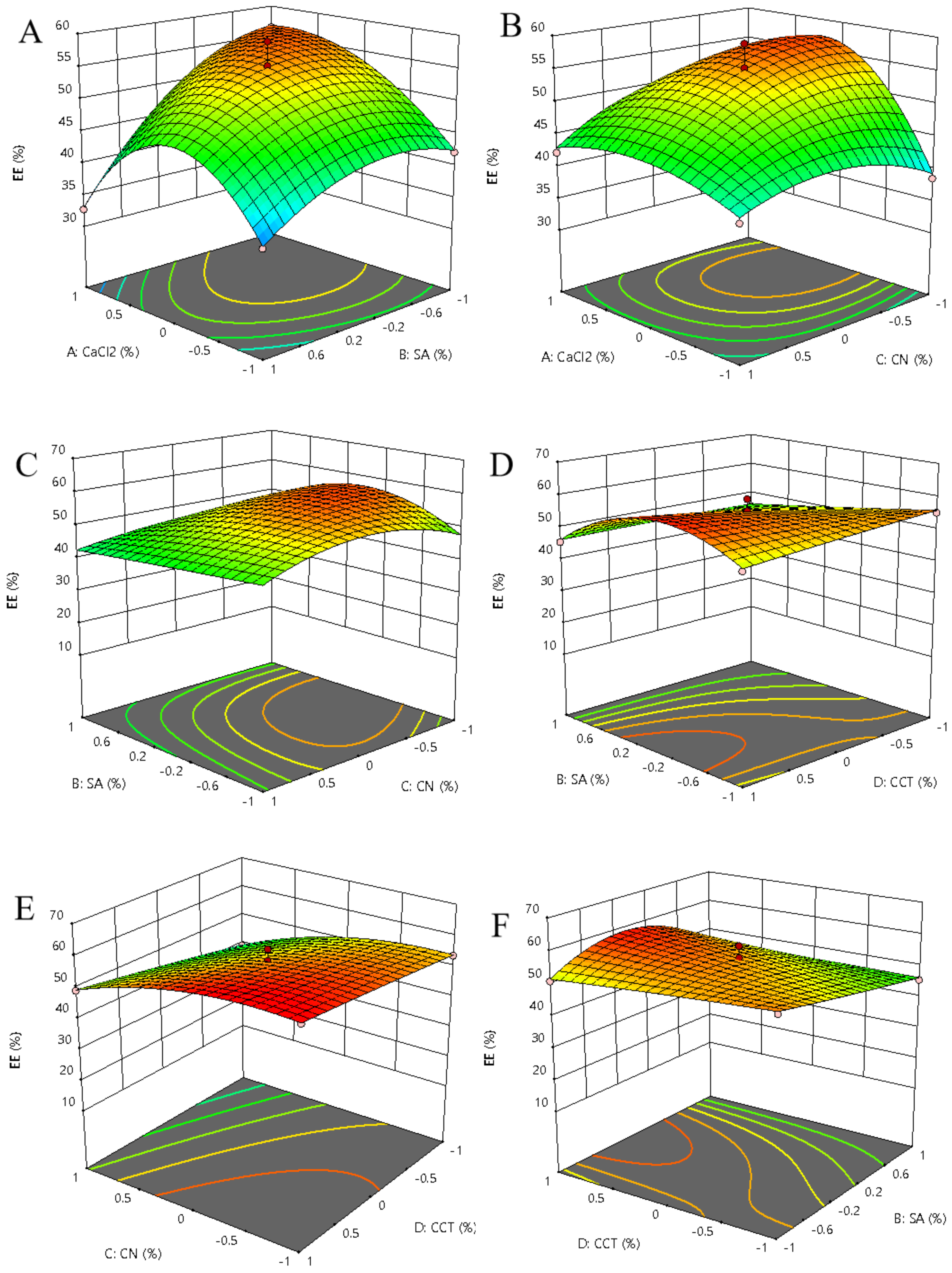

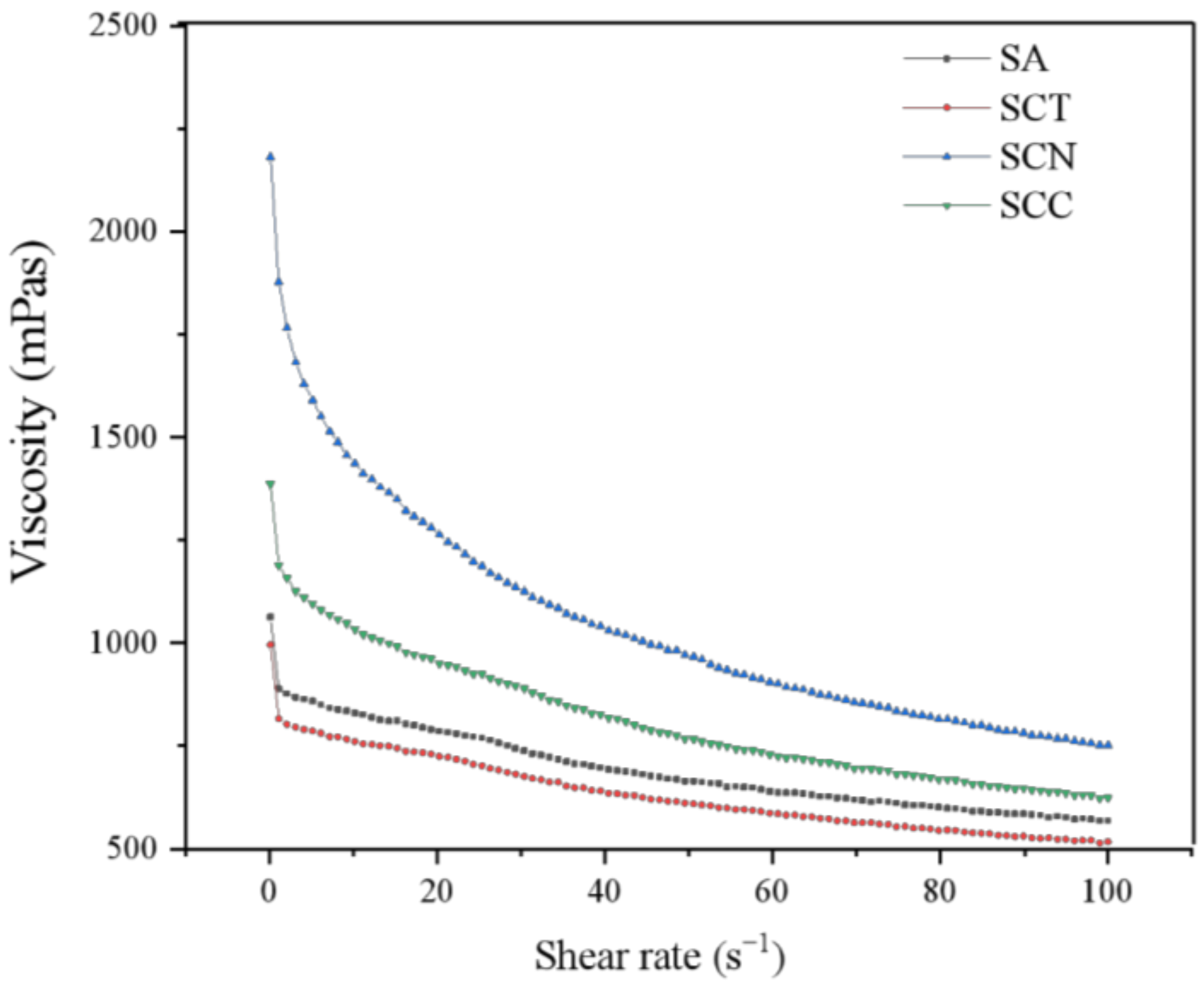
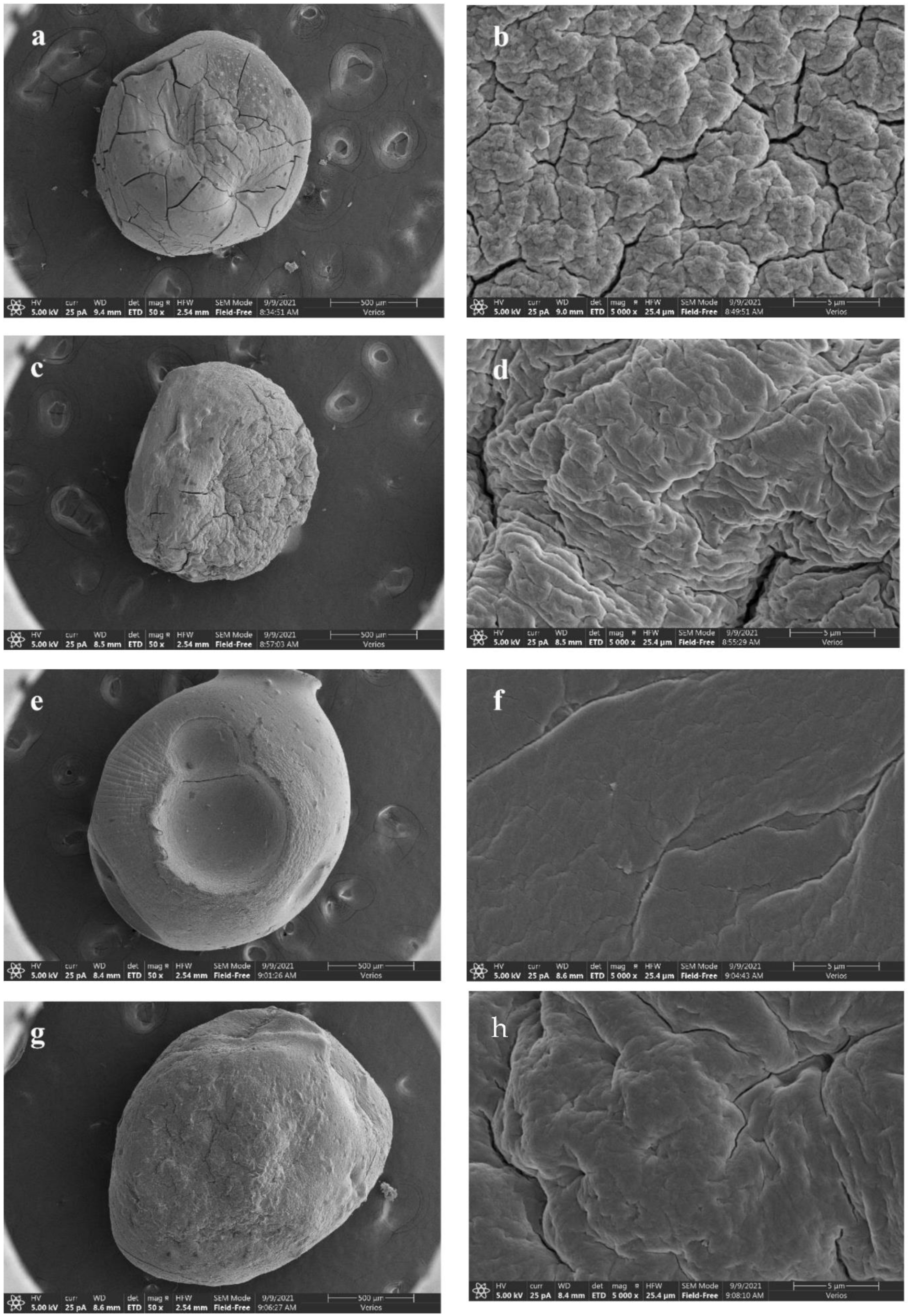
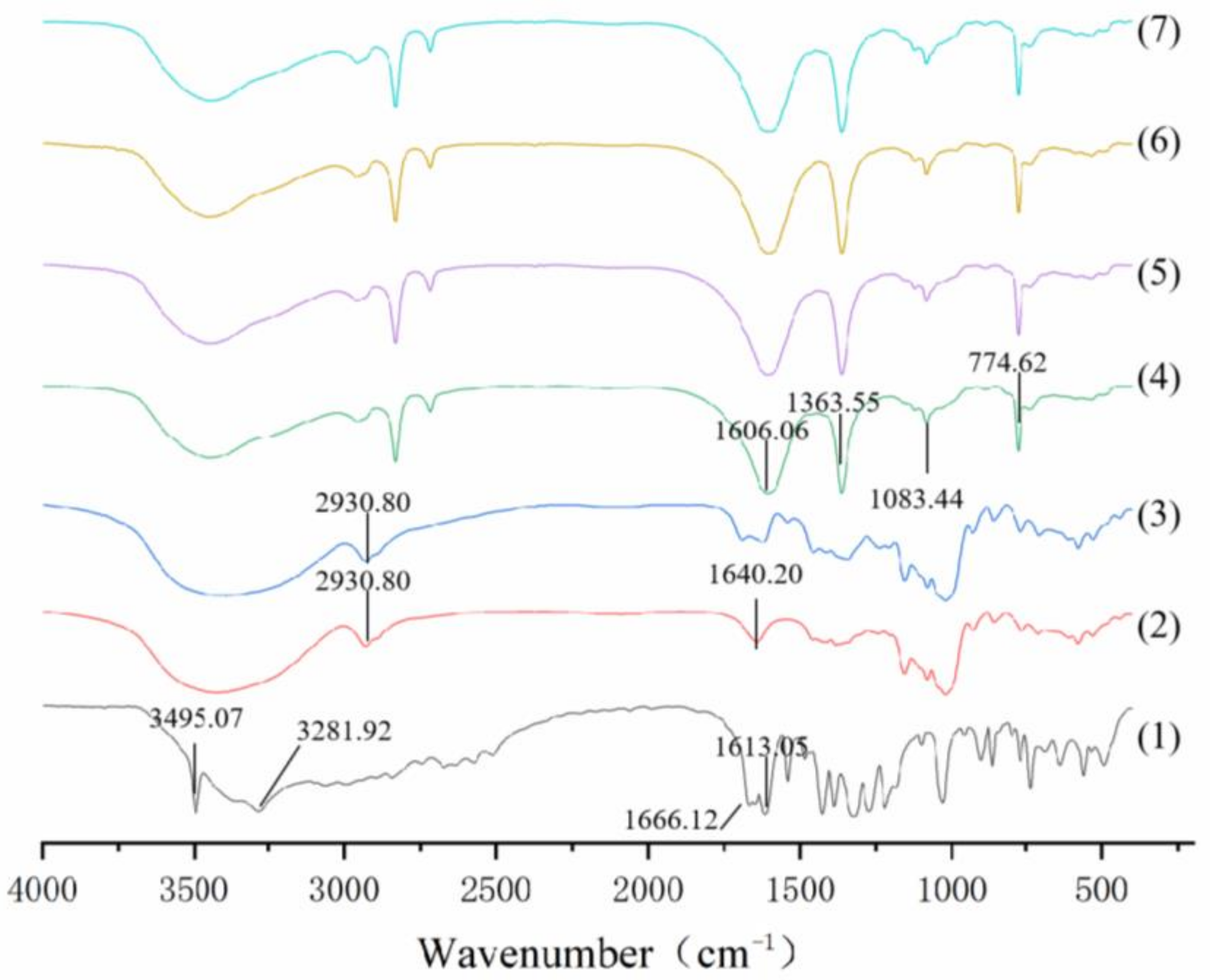

| Level | Factors | |||
|---|---|---|---|---|
| A-CaCl2 (%) | B-SA (%) | C-CN (%) | D-CCT (%) | |
| −1 | 1.0 | 1.5 | 0.4 | 0.5 |
| 0 | 2.0 | 2.0 | 0.5 | 1.0 |
| 1 | 3.0 | 2.5 | 0.6 | 1.5 |
| Source | Sum of Square | df | Mean Square | F-Value | p-Value | Significance a |
|---|---|---|---|---|---|---|
| Model | 1381.7 | 22 | 62.8 | 9.05 | 0.0057 | *** |
| A-CaCl2 | 41.22 | 1 | 41.22 | 5.94 | 0.0507 | * |
| B-SA | 55.8 | 1 | 55.8 | 8.04 | 0.0297 | ** |
| C-CN | 149.82 | 1 | 149.82 | 21.59 | 0.0035 | *** |
| D-CCT | 62.25 | 1 | 62.25 | 8.97 | 0.0241 | ** |
| AB | 77.35 | 1 | 77.35 | 11.15 | 0.0156 | ** |
| AC | 10.63 | 1 | 10.63 | 1.53 | 0.2621 | * |
| AD | 102.62 | 1 | 102.62 | 14.79 | 0.0085 | *** |
| BC | 2.81 | 1 | 2.81 | 0.4044 | 0.5483 | * |
| BD | 4.45 | 1 | 4.45 | 0.6417 | 0.4536 | * |
| CD | 4.49 | 1 | 4.49 | 0.6478 | 0.4516 | * |
| A2 | 427.04 | 1 | 427.04 | 61.55 | 0.0002 | *** |
| B2 | 151.8 | 1 | 151.8 | 21.88 | 0.0034 | *** |
| C2 | 112.6 | 1 | 112.6 | 16.23 | 0.0069 | *** |
| D2 | 0.3073 | 1 | 0.3073 | 0.0443 | 0.8403 | * |
| A2B | 27.86 | 1 | 27.86 | 4.02 | 0.0919 | * |
| A2C | 54.81 | 1 | 54.81 | 7.9 | 0.0307 | ** |
| A2D | 44.18 | 1 | 44.18 | 6.37 | 0.0451 | ** |
| AB2 | 0.3741 | 1 | 0.3741 | 0.0539 | 0.8241 | * |
| AC2 | 0.4608 | 1 | 0.4608 | 0.0664 | 0.8052 | * |
| B2C | 47.09 | 1 | 47.09 | 6.79 | 0.0404 | ** |
| B2D | 50.1 | 1 | 50.1 | 7.22 | 0.0362 | ** |
| BC2 | 11.45 | 1 | 11.45 | 1.65 | 0.2463 | * |
| Residual | 41.63 | 6 | 6.94 | |||
| Lack of Fit | 16.79 | 2 | 8.4 | 1.35 | 0.3559 | * |
| Pure Error | 24.83 | 4 | 6.21 | |||
| Cor Total | 1423.33 | 28 | ||||
| R2 | 0.9708 | |||||
| Adj R2 | 0.8635 | |||||
| Pred R2 | −0.7264 |
| Samples | Hardness (g) | Adhesiveness (g·s) | Resilience | Cohesiveness | Springiness | Gumminess (N) |
|---|---|---|---|---|---|---|
| SA beads | 84.28 ± 1.01 c | −0.35 ± 0.02 a | 25.59 ± 1.23 a | 0.58 ± 0.03 a | 70.94 ± 0.30 a | 48.81 ± 3.17 c |
| SCN beads | 72.90 ± 5.29 c | −0.40 ± 0.13 a | 25.32 ± 0.49 a | 0.58 ± 0.02 a | 74.16 ± 5.58 a | 41.97 ± 3.08 c |
| SCT beads | 102.32 ± 6.24 b | −0.11 ± 0.36 a | 22.24 ± 1.21 b | 0.54 ± 0.01 a | 57.47 ± 4.85 b | 55.26 ± 3.33 b |
| SCC beads | 147.15 ± 5.47 a | −0.63 ± 0.65 a | 25.01 ± 0.82 a | 0.59 ± 0.02 a | 62.49 ± 2.70 b | 86.11 ± 3.10 a |
| Encapsulation Efficiency (%) | Percentage of Inhibition (PI) (%) | |
|---|---|---|
| GA | 94.90 ± 0.86 | |
| PG | 91.47 ± 0.70 | |
| SA beads | 40.61 ± 0.68 | 87.16 ± 0.33 |
| SCN beads | 42.36 ± 0.73 | 82.86 ± 1.09 |
| SCT beads | 48.65 ± 0.64 | 72.48 ± 1.19 |
| SCC beads | 56.46 ± 0.48 | 61.00 ± 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, W.; Wang, Z.; Chen, W.; Zhang, M.; Zhong, Q.; Pei, J.; Chen, H. Preparation and Characterization of Beads of Sodium Alginate/Carboxymethyl Chitosan/Cellulose Nanofiber Containing Porous Starch Embedded with Gallic Acid: An In Vitro Simulation Delivery Study. Foods 2022, 11, 1394. https://doi.org/10.3390/foods11101394
Li W, Chen W, Wang Z, Chen W, Zhang M, Zhong Q, Pei J, Chen H. Preparation and Characterization of Beads of Sodium Alginate/Carboxymethyl Chitosan/Cellulose Nanofiber Containing Porous Starch Embedded with Gallic Acid: An In Vitro Simulation Delivery Study. Foods. 2022; 11(10):1394. https://doi.org/10.3390/foods11101394
Chicago/Turabian StyleLi, Wei, Wenxue Chen, Zhiyang Wang, Weijun Chen, Ming Zhang, Qiuping Zhong, Jianfei Pei, and Haiming Chen. 2022. "Preparation and Characterization of Beads of Sodium Alginate/Carboxymethyl Chitosan/Cellulose Nanofiber Containing Porous Starch Embedded with Gallic Acid: An In Vitro Simulation Delivery Study" Foods 11, no. 10: 1394. https://doi.org/10.3390/foods11101394
APA StyleLi, W., Chen, W., Wang, Z., Chen, W., Zhang, M., Zhong, Q., Pei, J., & Chen, H. (2022). Preparation and Characterization of Beads of Sodium Alginate/Carboxymethyl Chitosan/Cellulose Nanofiber Containing Porous Starch Embedded with Gallic Acid: An In Vitro Simulation Delivery Study. Foods, 11(10), 1394. https://doi.org/10.3390/foods11101394







