The Underlying Physicochemical Properties and Starch Structures of indica Rice Grains with Translucent Endosperms under Low-Moisture Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rice Varieties and Planting Conditions
2.2. Rice Flour and Starch Preparation and Sorption Properties Determination
2.3. Physical and Chemical Quality Analyses
2.4. Section Scanning and Transparency Analysis of Milled Rice
2.5. Starch Crystalline Structure Analysis
2.5.1. Powder X-ray Diffraction (XRD)
2.5.2. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR)
2.5.3. Small-Angle X-ray Scattering (SAXS)
2.6. Starch Fine Structure Analysis
2.7. Data Analysis
3. Results
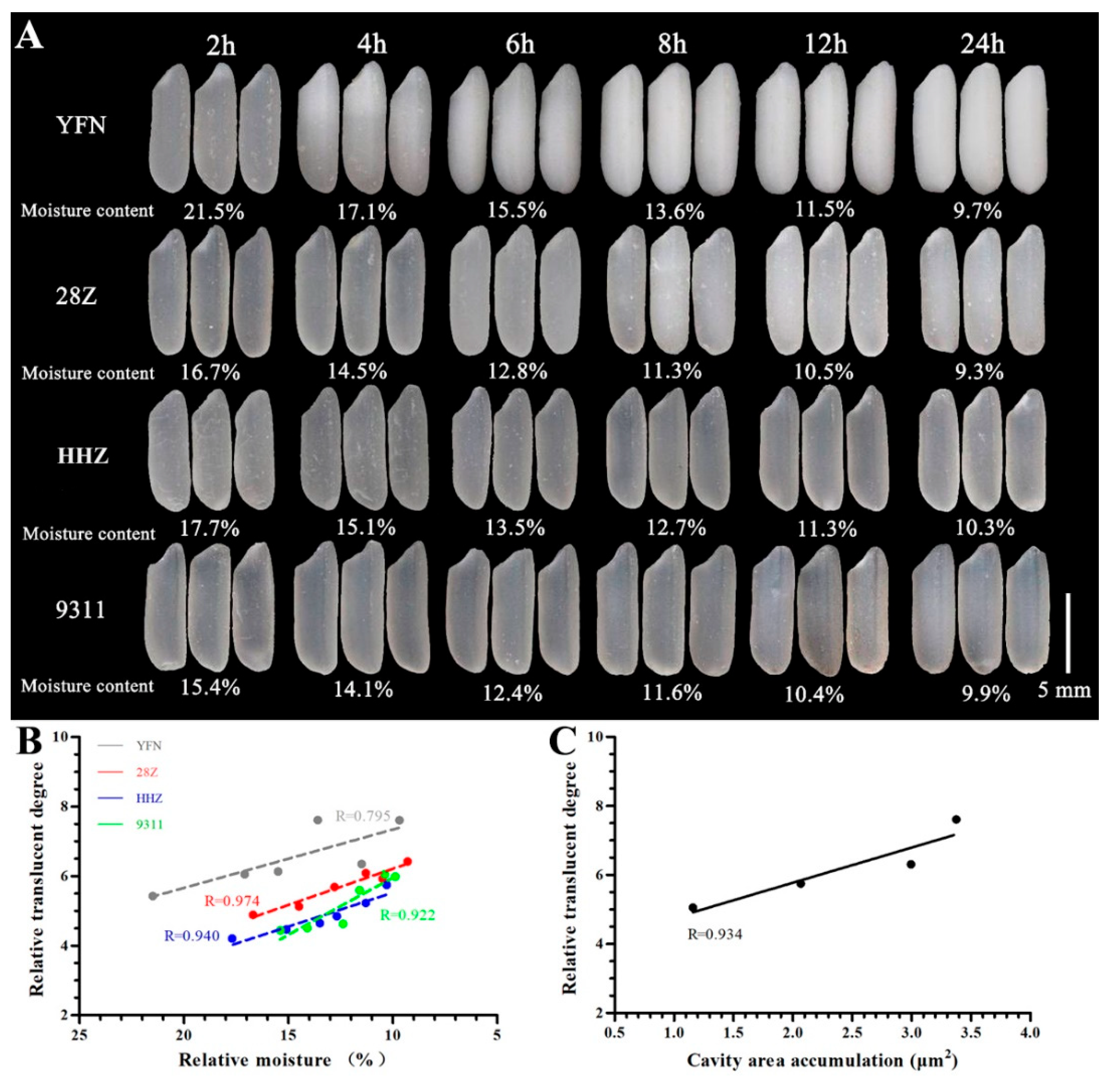
3.1. Analysis of Rice Grain Appearance and Water Content
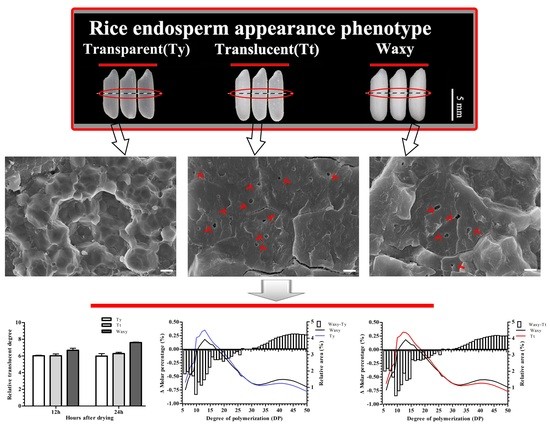
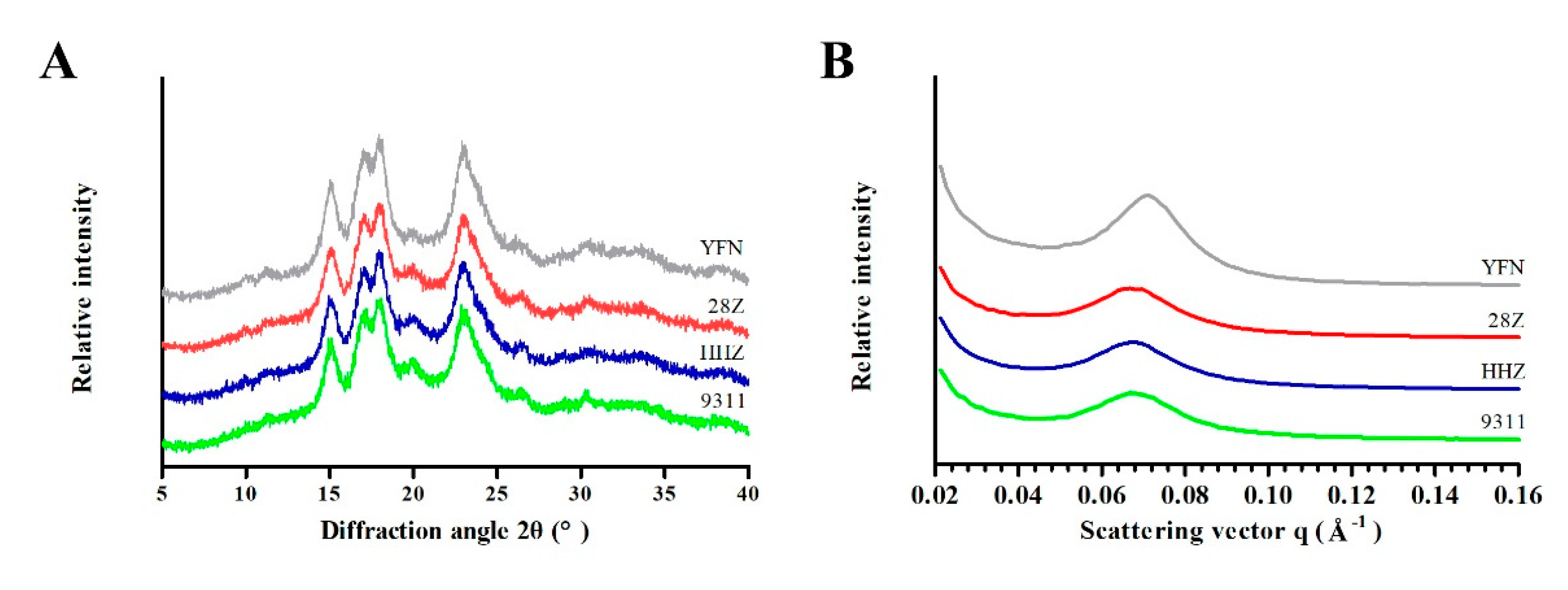
3.2. Analysis of Starch Structure
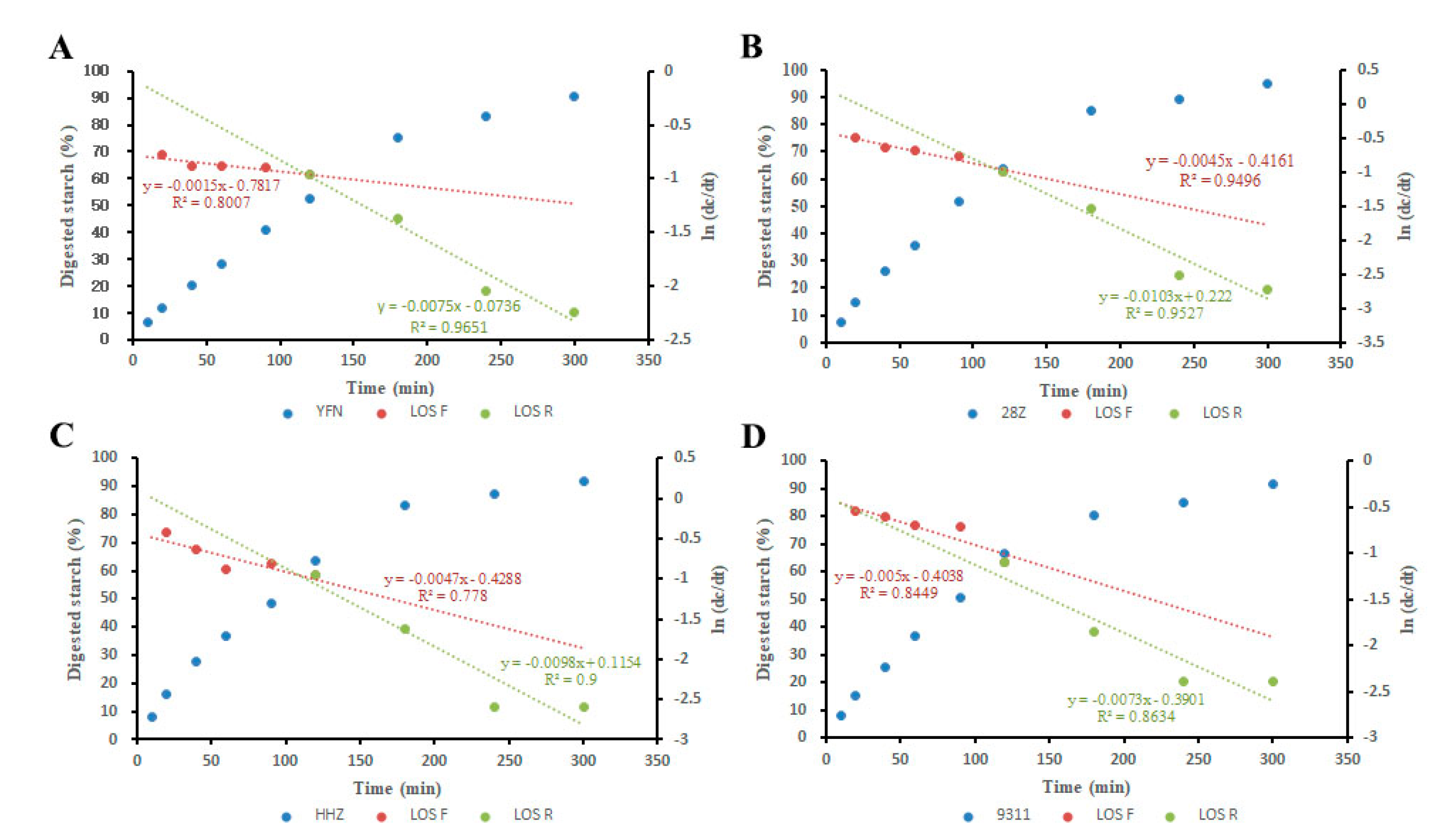
3.3. Eating and Physicochemical Characteristics of Rice
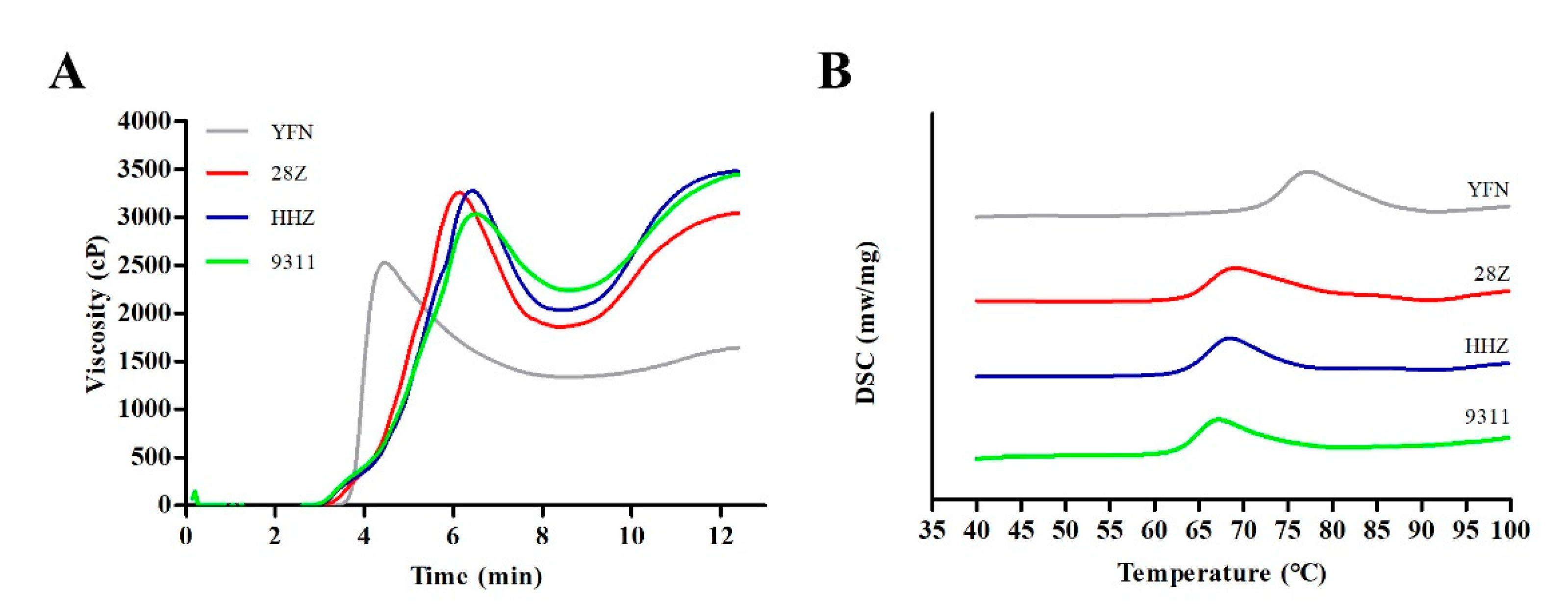
3.4. Analysis of Starch Pasting and Thermal Properties
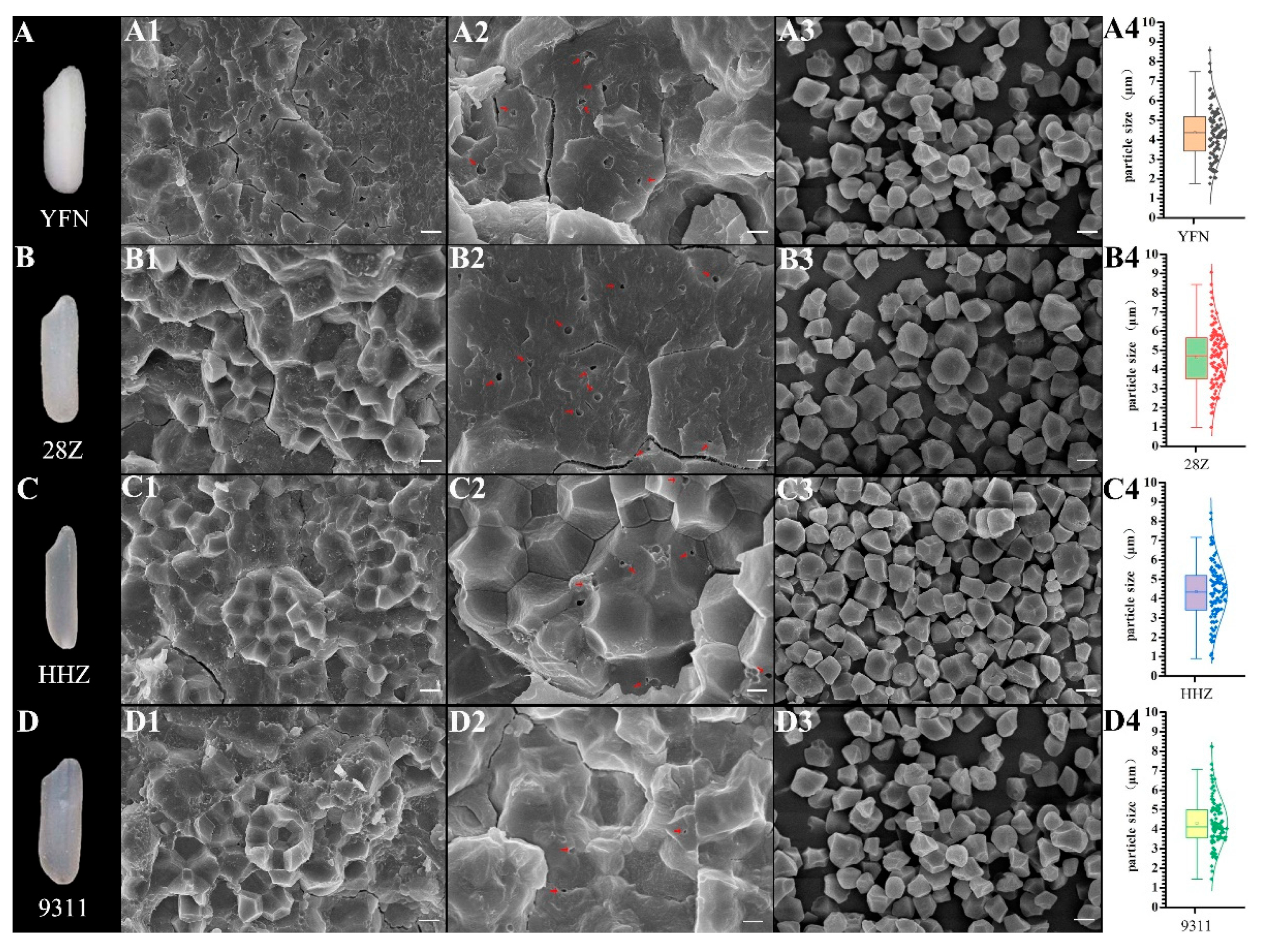
3.5. Microstructures of Starch Granules
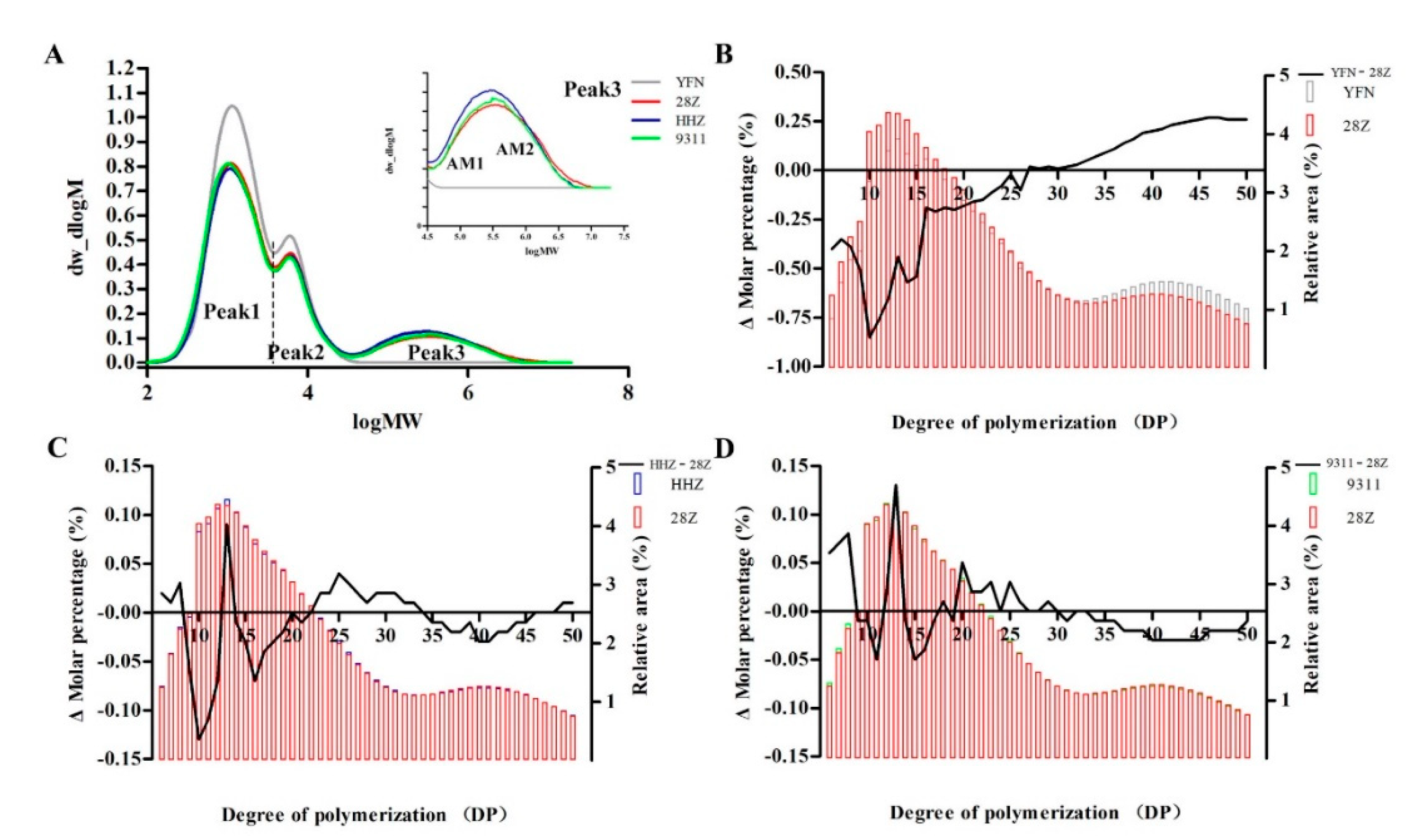
3.6. Starch Fine Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, M.A.; Mccouch, S.R.; Hall, R.D. Not just a grain of rice: The quest for quality. Trends Plant Sci. 2009, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Bal, S. Cooking quality and instrumental textural attributes of cooked rice for different milling fractions. J. Food Eng. 2006, 73, 253–259. [Google Scholar] [CrossRef]
- Tian, Z.; Qian, Q.; Liu, Q.; Yan, M.; Liu, X.; Yan, C.; Liu, G.; Gao, Z.; Tang, S.; Zeng, D.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gilbert, R.G. Starch molecular structure: The basis for an improved understanding of cooked rice texture. Carbohydr. Polym. 2018, 195, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Liu, X.L.; Wan, X.Y.; Weng, J.F.; Liu, S.J.; Liu, X.; Chen, M.J.; Li, J.J.; Su, N.; Wu, F.; et al. Identification of a stable quantitative trait locus for percentage grains with white chalkiness in rice (Oryza sativa). J. Integr. Plant Biol. 2011, 53, 598–607. [Google Scholar]
- Wan, X.Y.; Wan, J.M.; Weng, J.F.; Jiang, L.; Bi, J.F.; Wang, C.M.; Zhai, H.Q. Stability of QTLs for rice grain dimension and endosperm chalkiness characteristics across eight environments. Theor. Appl. Genet. 2005, 110, 1334–1346. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Fang, J.H.; Yan, X.; Liu, J.; Bao, J.S.; Fransson, G.; Andersson, R.; Jansson, C.; Åman, P.; Sun, C.X. Molecular insights into how a deficiency of amylose affects carbon allocation—Carbohydrate and oil analyses and gene expression profiling in the seeds of a rice waxy mutant. BMC Plant Biol. 2012, 12, 230. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Yan, M.; Wang, Y.; Liu, X.; Qian, Q.; Li, J. Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wxb pre-mRNAs in rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 501–509. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zheng, F.Q.; Shen, G.Z.; Gao, J.P.; Snustad, D.P.; Li, M.G.; Zhang, J.L.; Hong, M.M. The amylose content in rice endosperm is related to the post-transcriptional regulation of the waxy gene. Plant J. 2010, 7, 613–622. [Google Scholar] [CrossRef]
- Xi, M.; Zhao, Y.; Lin, Z.; Zhang, X.C.; Ding, C.Q.; Tang, S.; Liu, Z.H.; Wang, S.H.; Ding, Y.F. Comparison of physicochemical characteristics between white-belly and white-core rice grains. J. Cereal Sci. 2016, 69, 392–397. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Yang, Y.; Chen, S.J.; Liu, X.J.; Zhu, J.H.; Zhou, L.H.; Lu, Y.; Li, Q.F.; Fan, X.L.; Tang, S.Z.; et al. A rare Waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integr. Plant Biol. 2021, 63, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.M.; Qi, Y.; Zhang, C.Q.; Liu, Q.Q. Scanning electron microscopic analysis of grain cross-section from rice with different transparency. Chin. J. Rice Sci. 2018, 32, 189–199. [Google Scholar]
- Wang, L.; Gong, Y.; Li, Y.; Tian, Y. Structure and properties of soft rice starch. Int. J. Biol. Macromol. 2020, 157, 10–16. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Liu, S.; Zhu, C.; Jiang, L.; Wang, Y.; Shen, Y.; Ren, Y.; Dong, H.; Chen, L.; et al. Identification and characterization of a novel Waxy allele from a Yunnan rice landrace. Plant Mol. Biol. 2009, 71, 609–626. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S. Effect of composition, granular morphology and crystalline structure on the pasting, textural, thermal and sensory characteristics of Traditional rice cultivars. Food Chem. 2019, 280, 303–309. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Yan, Y.; Hu, Z.; Wang, K.; Zhou, J.; Zhou, Y.; Cao, L.; Wu, S. Influence of starch fine structure and storage proteins on the eating quality of rice varieties with similar amylose contents. J. Sci. Food Agric. 2021, 101, 3811–3818. [Google Scholar] [CrossRef]
- Li, Q.F.; Huang, L.C.; Chu, R.; Li, J.; Jiang, M.Y.; Zhang, C.Q.; Fan, X.L.; Yu, H.X.; Gu, M.H.; Liu, Q.Q. Down-regulation of SSSII-2 gene expression results in novel low-amylose rice with soft, transparent grains. J. Agric. Food Chem. 2018, 66, 9750–9760. [Google Scholar] [CrossRef]
- Tan, Y.F.; Li, J.X.; Yu, S.B.; Xing, Y.Z.; Xu, C.G.; Zhang, Q.F. The three important traits for cooking and eating quality of rice grains are controlled by a single locus in an elite rice hybrid, Shanyou 63. Theor. Appl. Genet. 1999, 99, 642–648. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, Q.Q.; Sang, Y.J.; Gu, M.H.; Shi, Y.C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2010, 120, 94–100. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, S.J.; Ren, X.Y.; Lu, Y.; Liu, D.R.; Cai, X.L.; Li, Q.F.; Gao, J.P.; Liu, Q.Q. Molecular structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.S.; Cai, C.H.; Gilbert, R.G.; Li, E.P.; Wang, J.; Wei, C.X. Relationships between amylopectin molecular structures and functional properties of different-sized fractions of normal and high-amylose maize starches. Food Hydrocoll. 2016, 52, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.X.; Qin, F.L.; Zhou, W.D.; Yu, H.G.; Xu, B.; Chen, C.; Zhu, L.J.; Wang, Y.P.; Gu, M.H.; Liu, Q.Q. Granule structure and distribution of allomorphs in C-Type high-amylose rice starch granule modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010, 58, 11946–11954. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.H.; Lin, L.S.; Man, J.M.; Zhao, L.X.; Wang, Z.F.; Wei, C.X. Different structural properties of high-amylose maize starch fractions varying in granule size. J. Agric. Food Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Zhang, J.; Cai, X.; Liu, Q.; Wei, C. Relationships between transparency, amylose content, starch cavity, and moisture of brown rice kernels. J. Cereal Sci. 2019, 90, 102854. [Google Scholar] [CrossRef]
- Cai, J.W.; Man, J.M.; Huang, J.; Liu, Q.Q.; Wei, W.X.; Wei, C.X. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- Shen, S.Q.; Zhang, J.Y.; Wang, S.Z.; Shu, Q.Y.; Xia, Y.W. Analysis of QTLs with genetic main effect, additive×additive epistatic effect and G×E interaction effect of rice transparency. J. Zhejiang Univ. (Agric. Life Sci.) 2006, 32, 367–371. [Google Scholar]
- Li, Y.B.; Fan, C.C.; Xing, Y.Z.; Yun, P.; Luo, L.J.; Yan, B.; Peng, B.; Xie, W.B.; Wang, G.W.; Li, X.H.; et al. Chalk5 encodes a vacuolar H (+)-translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 2014, 46, 398–404. [Google Scholar] [CrossRef]
- Baldwin, P.M.; Adler, J.; Davies, M.C.; Melia, C.D. Holes in starch granules: Confocal, SEM and light microscopy studies of starch granule structure. Starch 2010, 46, 341–346. [Google Scholar] [CrossRef]
- Glaring, M.A.; Koch, C.B.; Blennow, A. Genotype-specific spatial distribution of starch molecules in the starch granule: A combined CLSM and SEM approach. Biomacromolecules 2006, 7, 2310–2320. [Google Scholar] [CrossRef]
- Liu, T.; Ma, M.; Guo, K.; Hu, G.; Zhang, L.; Wei, C. Structural, thermal, and hydrolysis properties of large and small granules from C-type starches of four Chinese chestnut varieties. Int. J. Biol. Macromol. 2019, 137, 712–720. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Zhang, C.Q.; Rhowell, N.T., Jr.; Liu, Q.Q. Post-genomics revolution in designing premium quality rice in the high yielding background to address the demands of consumers from the 21st Century. Plant Commun. 2022, 3, 100271. [Google Scholar] [CrossRef]
- Han, X.Z.; Hamaker, B.R. Amylopectin fine structure and rice starch paste breakdown. J. Cereal Sci. 2001, 34, 279–284. [Google Scholar] [CrossRef]
- Shi, S.; Wang, E.; Li, C.; Cai, M.; Cheng, B.; Cao, C.; Jiang, Y. Use of protein content, amylose content, and RVA parameters to evaluate the taste quality of rice. Front. Nutr. 2022, 8, 758547. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yasui, T.; Matsuki, J. Effect of amylose content on gelatinization, retrogradation, and pasting properties of starches from Waxy and Nonwaxy wheat and their F1 seeds. Cereal Chem. 2000, 77, 58–63. [Google Scholar] [CrossRef]
- Zeng, M.; Morris, C.F.; Batey, I.L.; Wrigley, C.W. Sources of variation for starch gelatinization, pasting, and gelation properties in wheat. Cereal Chem. 1997, 74, 63–71. [Google Scholar] [CrossRef]
- Cheetham, N.; Tao, L.J.C.P. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Sangeeta, P.; Gilbert, R.G. High-amylose rice: Starch molecular structural features controlling cooked rice texture and preference. Carbohydr. Polym. 2019, 219, 251–260. [Google Scholar] [CrossRef]
- Jane, J.; Chen, Y.Y.; Lee, L.F.; McPherson, A.E.; Wong, K.S.; Radosavljevic, M.; Kasemsuwan, T. Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem. 1999, 76, 629–637. [Google Scholar] [CrossRef]
| Rice Variety | AAC (%) | TV (Points) | MC (%) | PC (%) | TS (%) |
|---|---|---|---|---|---|
| YFN | 4.64 ± 0.74 d | — | 11.87 ± 1.58 a | 9.38 ± 1.22 a | 76.8 ± 2.1 a |
| 28Z | 13.05 ± 0.14 c | 83.34 ± 1.81 b | 10.94 ± 0.41 a | 10.44 ± 1.21 a | 76.1 ± 2.1 a |
| HHZ | 16.16 ± 0.22 a | 78.05 ± 3.41 a | 12.03 ± 0.70 a | 9.50 ± 1.07 a | 77.9 ± 1.7 a |
| 9311 | 15.11 ± 0.18 b | 76.54 ± 3.24 a | 11.04 ± 0.55 a | 9.87 ± 0.76 a | 77.7 ± 2.2 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Lu, Y.; Pan, L.; Fan, X.; Li, Q.; Huang, L.; Zhao, D.; Zhang, C.; Liu, Q. The Underlying Physicochemical Properties and Starch Structures of indica Rice Grains with Translucent Endosperms under Low-Moisture Conditions. Foods 2022, 11, 1378. https://doi.org/10.3390/foods11101378
Chen F, Lu Y, Pan L, Fan X, Li Q, Huang L, Zhao D, Zhang C, Liu Q. The Underlying Physicochemical Properties and Starch Structures of indica Rice Grains with Translucent Endosperms under Low-Moisture Conditions. Foods. 2022; 11(10):1378. https://doi.org/10.3390/foods11101378
Chicago/Turabian StyleChen, Fei, Yan Lu, Lixu Pan, Xiaolei Fan, Qianfeng Li, Lichun Huang, Dongsheng Zhao, Changquan Zhang, and Qiaoquan Liu. 2022. "The Underlying Physicochemical Properties and Starch Structures of indica Rice Grains with Translucent Endosperms under Low-Moisture Conditions" Foods 11, no. 10: 1378. https://doi.org/10.3390/foods11101378
APA StyleChen, F., Lu, Y., Pan, L., Fan, X., Li, Q., Huang, L., Zhao, D., Zhang, C., & Liu, Q. (2022). The Underlying Physicochemical Properties and Starch Structures of indica Rice Grains with Translucent Endosperms under Low-Moisture Conditions. Foods, 11(10), 1378. https://doi.org/10.3390/foods11101378