Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of D. maruadsi Surimi
2.3. Preparation of D. maruadsi Surimi Products
2.4. Determination of Moisture Retention
2.4.1. Moisture Content
2.4.2. Low-Field Nuclear Magnetic Resonance (NMR)
2.4.3. Water Loss Rate
2.5. Determination of Gel Properties
2.5.1. Microstructure
2.5.2. Whiteness
2.5.3. Puncture Test
2.6. Protein Conformation and Force Changes
2.6.1. Raman Spectroscopy
2.6.2. Chemical Forces
2.7. Volatile Flavor Substances
2.8. Statistical Analysis
3. Results and Discussion
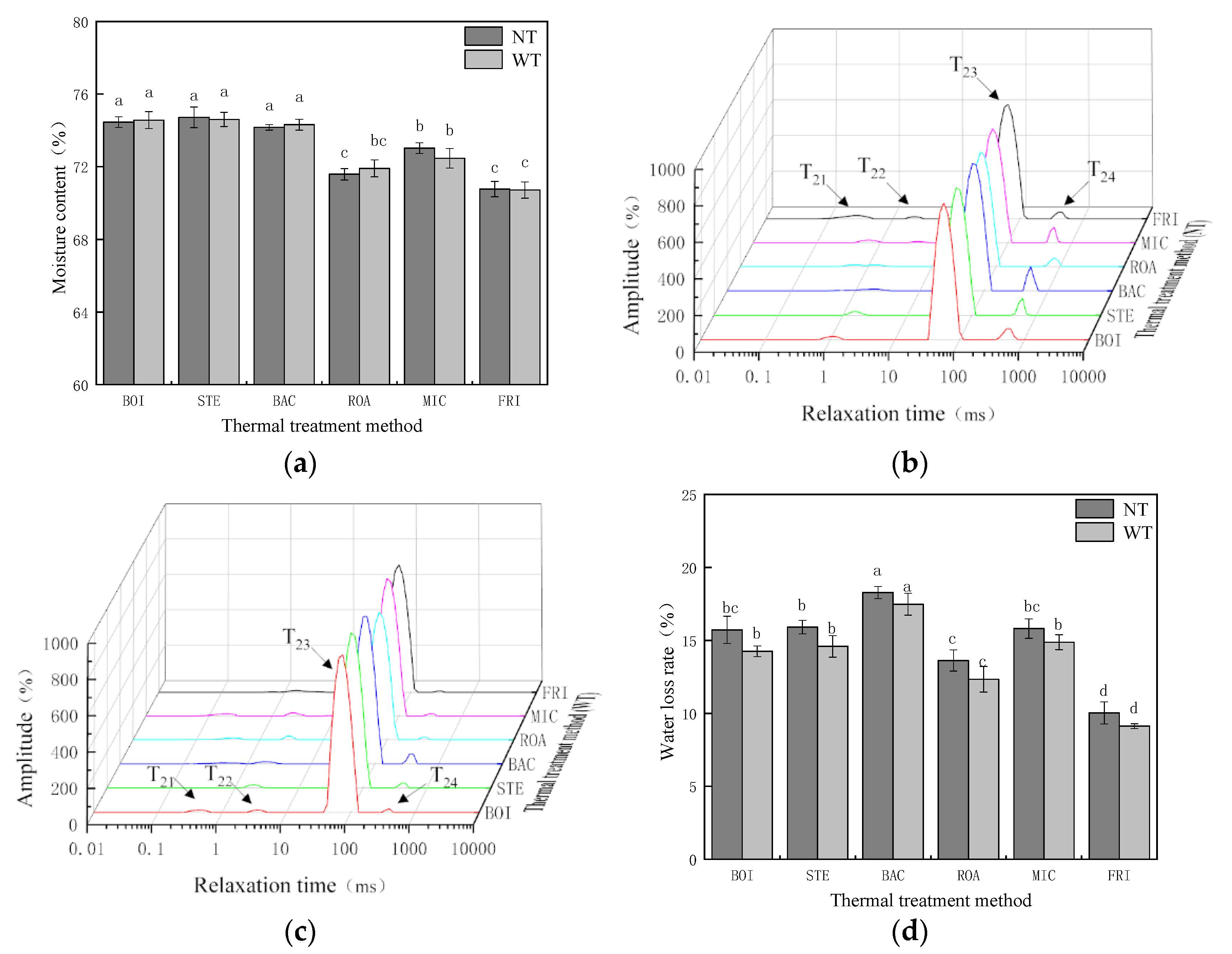
3.1. Analysis of Moisture Retention
3.2. Analysis of Gel Properties
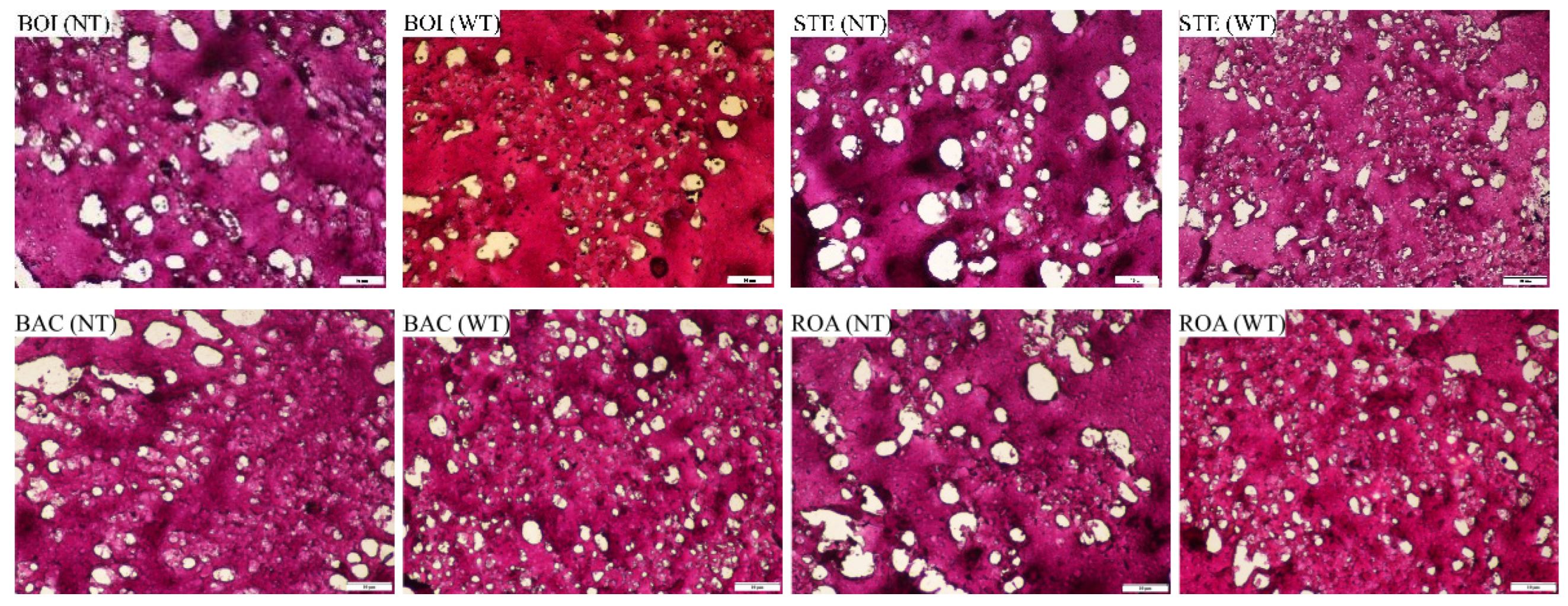
3.2.1. Microstructure
3.2.2. Whiteness
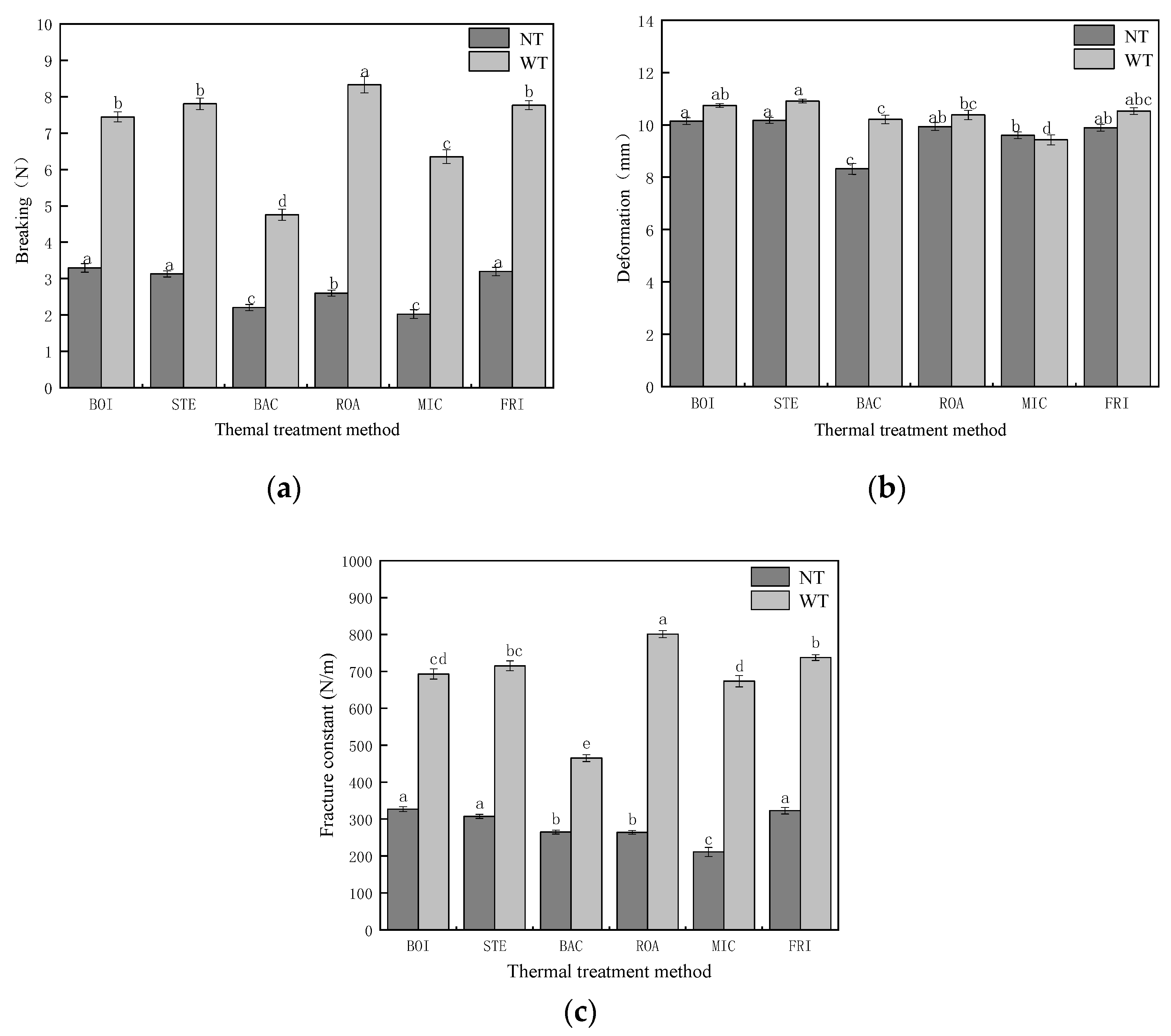
3.2.3. Puncture Tests
3.3. Analysis of Protein Conformation and Force
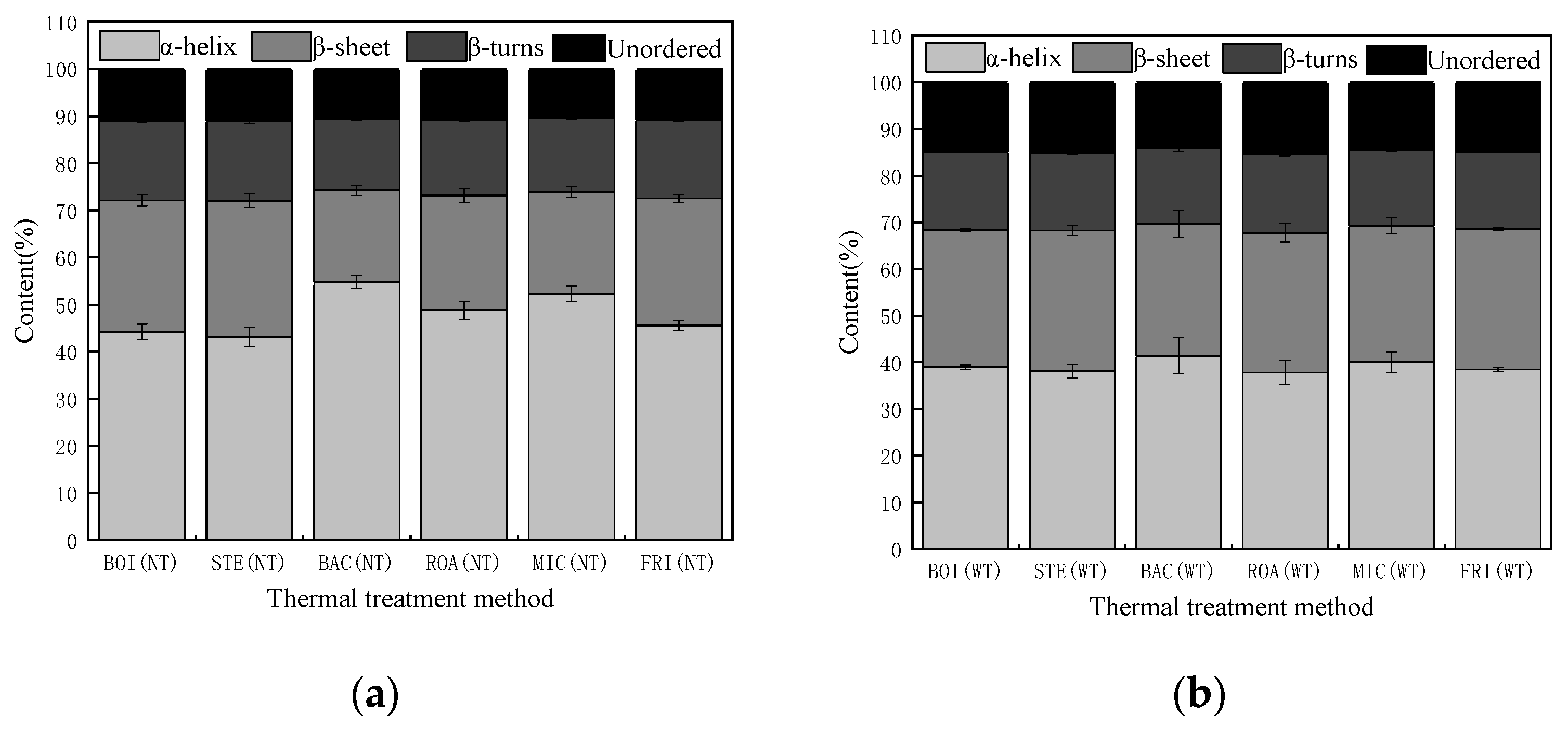
3.3.1. Analysis of Protein Secondary Structure
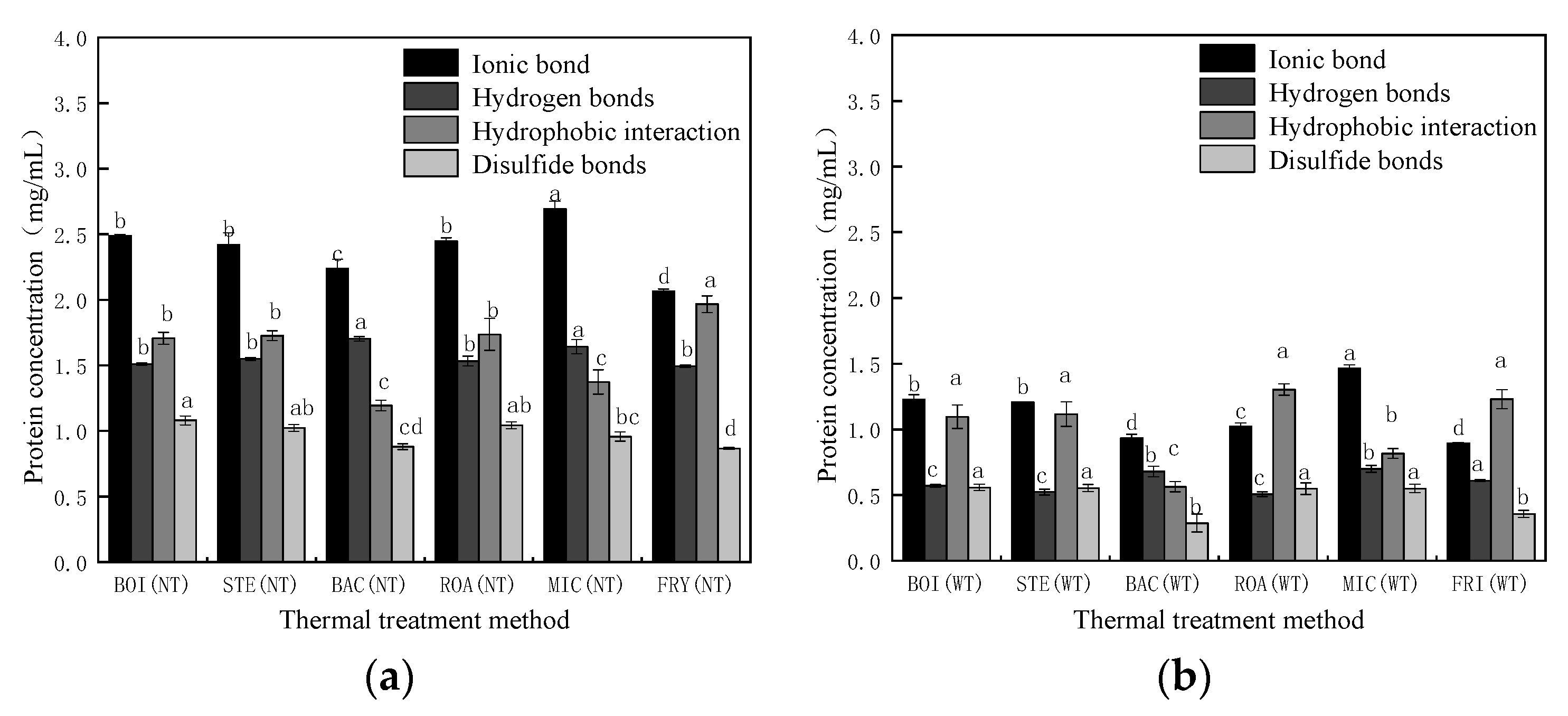
3.3.2. Analysis of Chemical Forces
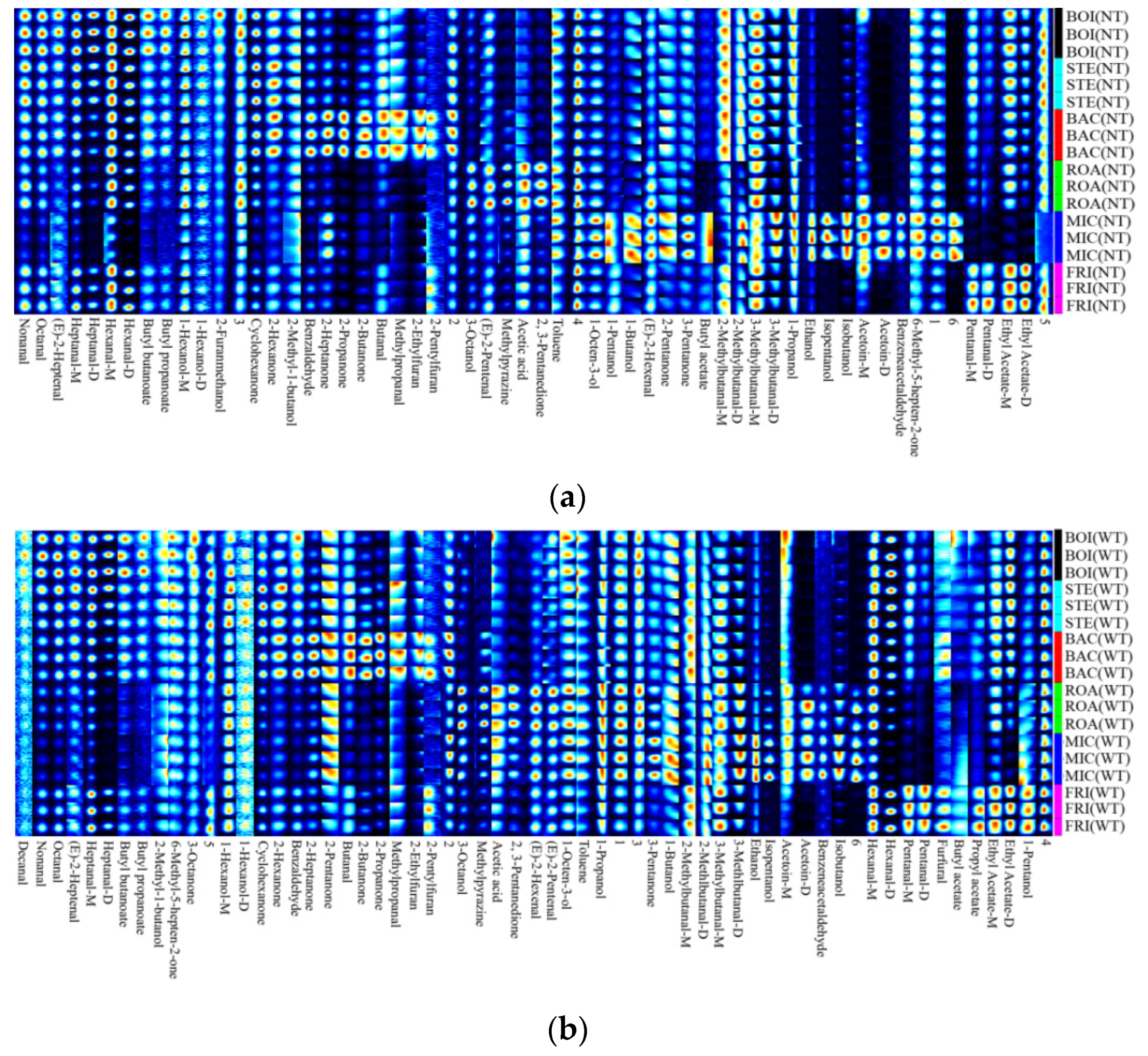
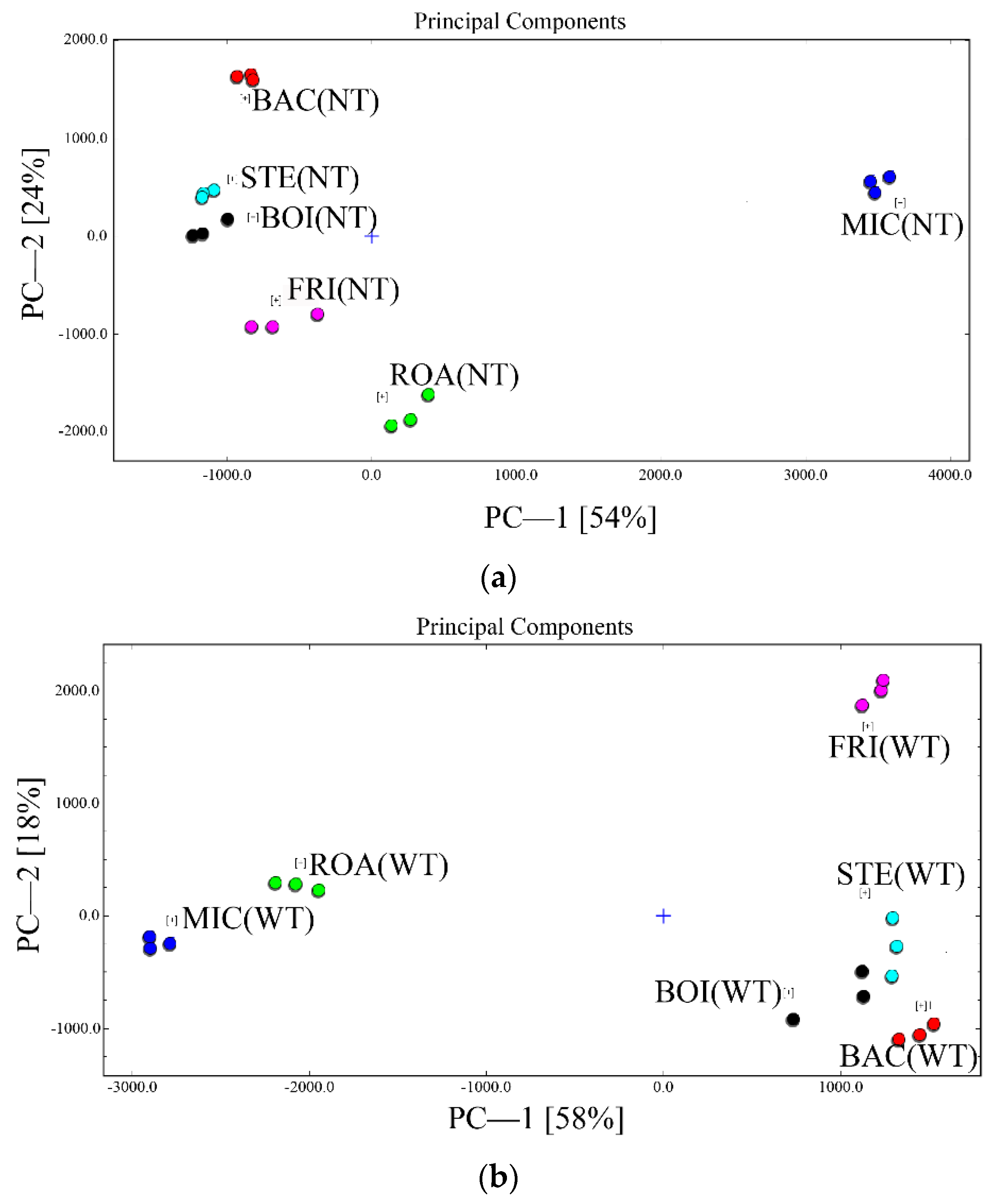
3.4. Qualitative Analysis of Volatile Flavor Substances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohshima, T.; Suzuki, T.; Koizumi, C. New developments in surimi technology. Trends Food Sci. Technol. 1993, 4, 157–163. [Google Scholar] [CrossRef]
- Chen, J.; Deng, T.; Wang, C.; Mi, H.; Yi, S.; Li, X.; Li, J. Effect of hydrocolloids on gel properties and protein secondary structure of silver carp surimi. J. Sci. Food Agric. 2020, 100, 2252–2260. [Google Scholar] [CrossRef]
- Morrissey, M.T.; Tan, S.M. World Resources for Surimi. In Food Science and Technology; Marcel Dekker: New York, NY, USA, 2000; pp. 1–22. [Google Scholar]
- Chen, H.; Wang, S.; Zhou, A.; Miao, J.; Liu, J.; Benjakul, S. A novel antioxidant peptide purified from defatted round scad (Decapterus maruadsi) protein hydrolysate extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2020, 68, 103907. [Google Scholar] [CrossRef]
- Sun, L.; Lin, Y.; Liu, W.; Qiu, X.; Cao, K.; Liu, G.; Cao, M. Effect of pH shifting on conformation and gelation properties of myosin from skeletal muscle of blue round scads (Decapterus maruadsi). Food Hydrocoll. 2019, 93, 137–145. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Shahidi, F. Compositions, functional properties and antioxidative activity of protein hydrolysates prepared from round scad (Decapterus maruadsi). Food Chem. 2007, 103, 1385–1394. [Google Scholar] [CrossRef]
- Jiang, H.; Tong, T.; Sun, J.; Xu, Y.; Zhao, Z.; Liao, D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014, 154, 158–163. [Google Scholar] [CrossRef]
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of cryoprotectants in surimi and factors affecting surimi gel properties: A review. Food Rev. Int. 2020, 1–20. [Google Scholar] [CrossRef]
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Wang, X. Effects of hydrolyzed wheat gluten on the properties of high-temperature (≥100 °C) treated surimi gels. Food Hydrocoll. 2015, 45, 196–202. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Gavahian, M.; Tiwari, B.K.; Chu, Y.H.; Ting, Y.; Farahnaky, A. Food texture as affected by ohmic heating: Mechanisms involved, recent findings, benefits, and limitations. Trends Food Sci. Technol. 2019, 86, 328–339. [Google Scholar] [CrossRef]
- Cao, H.; Jiao, X.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, H.; Wang, M. Microwave irradiation promotes aggregation behavior of myosin through conformation changes. Food Hydrocoll. 2019, 96, 11–19. [Google Scholar] [CrossRef]
- Yu, X.; Li, L.; Xue, J.; Wang, J.; Song, G.; Zhang, Y.; Shen, Q. Effect of air-frying conditions on the quality attributes and lipidomic characteristics of surimi during processing. Innov. Food Sci. Emerg. Technol. 2020, 60, 102305. [Google Scholar] [CrossRef]
- He, C.; Sun, Z.; Qu, X.; Cao, J.; Shen, X.; Li, C. A comprehensive study of lipid profiles of round scad (Decapterus maruadsi) based on lipidomic with UPLC-Q-Exactive Orbitrap-MS. Food Res. Int. 2020, 133, 109138. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Hurtado, J.L.; Perez-Mateos, M. Microstructural behaviour and gelling characteristics of myosystem protein gels interacting with hydrocolloids. Food Hydrocoll. 2000, 14, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Seighalani, F.Z.B.; Bakar, J.; Saari, N.; Khoddami, A. Thermal and physicochemical properties of red tilapia (Oreochromis niloticus) surimi gel as affected by microbial transglutaminase. Anim. Prod. Sci. 2017, 57, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- An, Y.; You, J.; Xiong, S.; Yin, T. Short-term frozen storage enhances cross-linking that was induced by transglutaminase in surimi gels from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 257, 216–222. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemistry: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Lv, Y.; Xie, J. Effects of freeze-thaw cycles on water migration, microstructure and protein oxidation in cuttlefish. Foods 2021, 10, 2576. [Google Scholar] [CrossRef]
- Aksoy, A.; Karasu, S.; Akcicek, A.; Kayacan, S. Effects of different drying methods on drying kinetics, microstructure, color, and the rehydration ratio of minced meat. Foods 2019, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Wijayanti, I.; Singh, A.; Benjakul, S.; Sookchoo, P. Textural, sensory, and chemical characteristic of threadfin bream (Nemipterus sp.) Surimi Gel Fortified with Bio-Calcium from bone of asian sea bass (Lates calcarifer). Foods 2021, 10, 976. [Google Scholar] [CrossRef]
- Herranz, B.; Borderias, A.J.; Solo-de-Zaldivar, B.; Solas, M.T.; Tovar, C.A. Thermostability analyses of glucomannan gels. Concentration influence. Food Hydrocoll. 2012, 29, 85–92. [Google Scholar] [CrossRef]
- Poowakanjana, S.; Mayer, S.G.; Park, J.W. Optimum chopping conditions for Alaska pollock, Pacific whiting, and threadfin bream surimi paste and gel based on rheological and Raman spectroscopic analysis. J. Food Sci. 2012, 77, E88–E97. [Google Scholar] [CrossRef] [PubMed]
- Alix, A.J.P.; Pedanou, G.; Berjot, M. Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman amide I band. J. Mol. Struct. 1988, 174, 159–164. [Google Scholar] [CrossRef]
- Tan, F.J.; Lai, K.M.; Hsu, K.C. A comparative study on physical properties and chemical interactions of gels from tilapia meat pastes induced by heat and pressure. J. Texture Stud. 2010, 41, 153–170. [Google Scholar] [CrossRef]
- Li, M.; Du, H.; Lin, S. Flavor Changes of Tricholoma matsutake Singer under Different Processing Conditions by Using HS-GC-IMS. Foods 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Turp, G.Y. Effects of four different cooking methods on some quality characteristics of low fat Inegol meatball enriched with flaxseed flour. Meat Sci. 2016, 121, 40–46. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Heating surimi products using microwave combined with steam methods: Study on energy saving and quality. Innov. Food Sci. Emerg. Technol. 2018, 47, 231–240. [Google Scholar] [CrossRef]
- Luo, H.; Guo, C.; Lin, L.; Si, Y.; Gao, X.; Xu, D.; Jia, R.; Yang, W. Combined use of rheology, LF-NMR, and MRI for characterizing the gel properties of hairtail surimi with potato starch. Food Bioprocess Tech. 2020, 13, 637–647. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of yeast β-glucan on gel properties, spatial structure and sensory characteristics of silver carp surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- Ji, L.; Xue, Y.; Zhang, T.; Li, Z.; Xue, C. The effects of microwave processing on the structure and various quality parameters of Alaska pollock surimi protein-polysaccharide gels. Food Hydrocoll. 2017, 63, 77–84. [Google Scholar] [CrossRef]
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Biosci. 2013, 1, 39–47. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z.; Xue, C. Effects of high-temperature treatment (≥100 °C) on Alaska Pollock (Theragra chalcogramma) surimi gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Huang, J.; Ye, B.; Wang, W.; Li, J.; Yi, S.; Li, X.; Xu, Y.; Mi, H. Incorporation effect of inulin and microbial transglutaminase on the gel properties of silver carp (Hypophthalmichthys molitrix) surimi. J. Food Meas. Charact. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- Chanarat, S.; Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852. [Google Scholar] [CrossRef]
- Luo, Y.K.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Comparison of gel properties of surimi from Alaska Pollock and three freshwater fish species: Effects of thermal processing and protein concentration. J. Food Sci. 2001, 66, 548–554. [Google Scholar] [CrossRef]
- Feng, D.; Xue, Y.; Li, Z.; Wang, Y.; Yang, W.; Xue, C. Dielectric properties of myofibrillar protein dispersions from Alaska Pollock (Theragra chalcogramma) as a function of concentration, temperature, and NaCl concentration. J. Food Eng. 2015, 166, 342–348. [Google Scholar] [CrossRef]
- Park, J.W. Surimi gel colors as affected by moisture content and physical conditions. J. Food Sci. 1995, 60, 15–18. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, T.; Feng, D.; Xue, Y.; Wang, Y.; Li, Z.; Yang, W.; Xue, C. Effects of modified starches on the gel properties of Alaska Pollock surimi subjected to different temperature treatments. Food Hydrocoll. 2016, 56, 20–28. [Google Scholar] [CrossRef]
- Liang, F.; Lin, L.; He, T.; Zhou, X.; Jiang, S.; Lu, J. Effect of transglutaminase on gel properties of surimi and precocious Chinese mitten crab (Eriocheir sinensis) meat. Food Hydrocoll. 2020, 98, 105261. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Chen, S.; Xu, C.; Yu, Y.; Wang, X.; Shi, X. Determination of the effects of different high-temperature treatments on texture and aroma characteristics in Alaska pollock surimi. Food Sci. Nutr. 2018, 6, 2079–2091. [Google Scholar] [CrossRef]
- Das, S.; Mukhopadhyay, A.K.; Datta, S.; Basu, D. Prospects of microwave processing: An overview. Bull. Mater. Sci. 2009, 32, 1–13. [Google Scholar] [CrossRef]
- Chen, H.; Zou, Y.; Zhou, A.; Xiao, J.; Benjakul, S. Insight into the effect of ice addition on the gel properties of Nemipterus virgatus surimi gel combined with water migration. Foods 2021, 10, 1815. [Google Scholar] [CrossRef]
- Camou, J.P.; Sebranek, J.G.; Olson, D.G. Effect of heating rate and protein concentration on gel strength and water loss of muscle protein gels. J. Food Sci. 1989, 54, 850–854. [Google Scholar] [CrossRef]
- Panthi, R.R.; Kelly, A.L.; Sheehan, J.J.; Bulbul, K.; Vollmer, A.H.; McMahon, D.J. Influence of protein concentration and coagulation temperature on rennet-induced gelation characteristics and curd microstructure. J. Dairy Sci. 2019, 102, 177–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinterits, A.; Arntfield, S.D. Improvement of canola protein gelation properties through enzymatic modification with transglutaminase. LWT Food Sci. Technol. 2008, 41, 128–138. [Google Scholar] [CrossRef]
- Kutemeyer, C.; Froeck, M.; Werlein, H.D.; Watkinson, B.M. The influence of salts and temperature on enzymatic activity of microbial transglutaminase. Food Control 2005, 16, 735–737. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar protein conformation enhance gel properties of mixed surimi gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Bouraoui, M.; Nakai, S.; Li, C. In situ investigation of protein structure in Pacific whiting surimi and gels using Raman spectroscopy. Food Res. Int. 1997, 30, 65–72. [Google Scholar] [CrossRef]
- Nunez-Flores, R.; Cando, D.; Borderias, A.J.; Moreno, H.M. Importance of salt and temperature in myosin polymerization during surimi gelation. Food Chem. 2018, 239, 1226–1234. [Google Scholar] [CrossRef]
- Fang, M.; Xiong, S.; Hu, Y.; Yin, T.; You, J. In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by Microbial Transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160. [Google Scholar] [CrossRef]
- Lauber, S.; Noack, I.; Klostermeyer, H.; Henle, T. Stability of microbial transglutaminase to high pressure treatment. Eur. Food Res. Technol. 2001, 213, 273–276. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Mu, J.; Shi, T.; Yuan, L. Effect of l-histidine on the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosin in low/high ionic strength solution. Food Hydrocoll. 2018, 75, 174–181. [Google Scholar] [CrossRef]
- Yuan, L.; Kong, Y.; Leng, W.; Wang, Y.; Jin, W.; Gao, R. L-glutamic acid affects myosin aggregation and the physical properties of bighead carp (Aristichthys nobilis) surimi gels. Food Biosci. 2021, 40, 100886. [Google Scholar] [CrossRef]
- Reed, Z.H.; Park, J.W. Thermophysical characterization of tilapia myosin and its subfragments. J. Food Sci. 2011, 76, C1050–C1055. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, S.; Ruan, Q.; Gao, Q.; An, Y.; Hu, Y.; Xiong, S. Differences in flavor characteristics of frozen surimi products reheated by microwave, water boiling, steaming, and frying. Food Chem. 2022, 372, 131260. [Google Scholar] [CrossRef]
- An, Y.; Qian, Y.; Magana, A.; Xiong, S.; Qian, M. Comparative characterization of aroma compounds in silver carp (Hypophthalmichthys molitrix), pacific whiting (Merluccius productus), and alaska pollock (Theragra chalcogramma) surimi by aroma extract dilution analysis, odor activity value, and aroma recombination studies. J. Agric. Food Chem. 2020, 68, 10403–10413. [Google Scholar]
- Lorenzo, J.; Franco, D.; Carballo, J. Effect of the inclusion of chestnut in the finishing diet on volatile compounds during the manufacture of dry-cured “Lacón” from Celta pig breed. Meat Sci. 2014, 96, 211–223. [Google Scholar] [CrossRef]
- Fu, C.; Wu, D.; Jin, Z.; Xie, G.; Lu, J. Development of a novel cooking wine with high-efficiency deodorizing capability via a rapid fermentation strategy. LWT Food Sci. Technol. 2022, 153, 112431. [Google Scholar] [CrossRef]
- Song, N.; Tan, C.; Huang, M.; Liu, P.; Eric, K.; Zhang, X.; Xia, S.; Jia, C. Transglutaminase cross-linking effect on sensory characteristics and antioxidant activities of Maillard reaction products from soybean protein hydrolysates. Food Chem. 2013, 136, 144–151. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Fu, X.; Su, G. Reducing the influence of the thermally induced reactions on the determination of aroma-active compounds in soy sauce using SDE and GC-MS/O. Food Anal. Methods 2017, 10, 931–942. [Google Scholar] [CrossRef]
- Yi, S.; Ji, Y.; Guo, Z.; Zhu, J.; Xu, Y.; Li, X.; Li, J. Gel properties and flavor characteristics of blended anchovy (Engraulis japonicus) mince and silver carp (Hypophthalmichthys molitrix) surimi. RSC Adv. 2020, 10, 6563–6570. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Liu, C.; Sun, Y.; Wen, X.; Xia, W. Modification of volatile profiles of silver carp surimi gel by immersion treatment with hydrogen peroxide (H2O2). Int. J. Food Sci. Technol. 2021, 56, 5726–5737. [Google Scholar] [CrossRef]
- Liu, C.; Li, W.; Lin, B.; Yi, S.; Ye, B.; Mi, H.; Li, J.; Wang, J.; Li, X. Effects of ozone water rinsing on protein oxidation, color, and aroma characteristics of grass carp (Ctenopharyngodon idellus) surimi. J. Food Process. Preserv. 2021, 45, e15811. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.; Cheng, J.; Sun, D. Effects of microwave and water bath heating on the interactions between myofibrillar protein from beef and ketone flavour compounds. Int. J. Food Sci. Technol. 2019, 54, 1787–1793. [Google Scholar] [CrossRef]
- Ning, Y.; Niu, S. Preparation and catalytic performance in esterification of a bamboo-based heterogeneous acid catalyst with microwave assistance. Energy Convers. Manag. 2017, 153, 446–454. [Google Scholar] [CrossRef]
- Xiang, J.; Liu, F.; Wang, B.; Chen, L.; Liu, W.; Tan, S. A literature review on maillard reaction based on milk proteins and carbohydrates in food and pharmaceutical products: Advantages, disadvantages, and avoidance strategies. Foods 2021, 10, 1998. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Laye, I.; Kim, Y.; Morr, C. Formation of volatile compounds in whey protein concentrate during elevated temperature storage as a function of water activity. Int. Dairy J. 1996, 6, 485–496. [Google Scholar] [CrossRef]

| Abbreviation | Group | Thermal Treatment Method |
|---|---|---|
| BOI | Boiled D. maruadsi surimi products | Firstly, in a constant temperature water bath at 40 °C for 30 min, and then at 90 °C for 20 min. |
| STE | Steamed D. maruadsi surimi products | Firstly, in a constant temperature water bath at 40 °C for 30 min, and then steam in a steamer with boiling water for 17 min. |
| BAC | Back-pressure sterilized D. maruadsi surimi products | Heated in a back pressure sterilizer at 110 °C and 0.05 MPa for 10 min. |
| ROA | Roasted D. maruadsi surimi products | Firstly, in a constant temperature water bath at 40 °C for 30 min, then removed the nylon casing, and finally roasted in an oven at 100 °C for 18 min. |
| MIC | Microwaved D. maruadsi surimi products | Firstly, in a constant temperature water bath at 40 °C for 30 min, then removed the casing, and finally heated it in a 2450 MHz microwave oven with a power of 3 W/g for 1 min. (The surface temperature was 72.4 ℃) |
| FRI | Fried D. maruadsi surimi products | Firstly, in a constant temperature water bath at 40 °C for 30 min, then removed the casing, and finally fried in a fryer at 150 °C for 3 min. |
| Thermal Treatment Method | T21 + T22 | T23 | T24 |
|---|---|---|---|
| Samples without TGase (NT) | |||
| BOI | 19.76 ± 2.80 a | 954.77 ± 1.23 a | 52.04 ± 3.92 b |
| STE | 25.11 ± 4.23 a | 944.69 ± 4.03 a | 57.59 ± 1.85 ab |
| BAC | 28.84 ± 2.77 a | 915.71 ± 15.39 ab | 65.24 ± 1.96 a |
| ROA | 25.07 ± 5.31 a | 885.56 ± 8.02 bc | 34.87 ± 0.97 cd |
| MIC | 19.74 ± 0.44 a | 881.10 ± 24.57 bc | 37.31 ± 3.62 c |
| FRI | 29.69 ± 5.73 a | 851.85 ± 18.64 c | 27.66 ± 1.17 d |
| Samples with TGase (WT) | |||
| BOI | 23.44 ± 2.55 a | 1113.34 ± 28.79 a | 11.90 ± 1.93 bc |
| STE | 30.71 ± 2.34 a | 1057.69 ± 40.04 ab | 15.78 ± 2.56 b |
| BAC | 21.85 ± 6.87 a | 1018.18 ± 15.94 bc | 34.45 ± 4.10 a |
| ROA | 30.56 ± 5.57 a | 979.97 ± 8.36 c | 7.33 ± 0.38 cd |
| MIC | 27.34 ± 3.19 a | 962.03 ± 5.92 c | 9.31 ± 0.37 bcd |
| FRI | 25.17 ± 2.08 a | 954.91 ± 15.84 c | 3.79 ± 0.08 d |
| Moisture Content | T21 + T22 | T23 | T24 | |
|---|---|---|---|---|
| Samples without TGase (NT) | ||||
| Water loss rate | 0.856 * | −0.316 | 0.678 | 0.862 * |
| Moisture content | 1 | −0.384 | 0.916 * | 0.900 * |
| Samples with TGase (WT) | ||||
| Water loss rate | 0.805 | −0.270 | 0.420 | 0.834 * |
| Moisture content | 1 | −0.200 | 0.851 * | 0.668 |
| Thermal Treatment Method | a* | b* | L* | Whiteness |
|---|---|---|---|---|
| Samples without TGase (NT) | ||||
| BOI | −5.07 ± 0.01 c | 12.54 ± 0.08 b | 74.40 ± 0.23 a | 71.05 ± 0.23 a |
| STE | −5.03 ± 0.03 c | 12.58 ± 0.04 b | 74.5 ± 0.15 a | 71.12 ± 0.12 a |
| BAC | −3.82 ± 0.03 a | 16.57 ± 0.04 a | 74.10 ± 0.08 a | 69.01 ± 0.24 c |
| ROA | −5.07 ± 0.06 c | 11.91 ± 0.06 c | 73.21 ± 0.16 b | 70.25 ± 0.16 b |
| MIC | −4.67 ± 0.02 b | 11.72 ± 0.15 c | 71.49 ± 0.24 c | 68.81 ± 0.21 c |
| FRI | −5.26 ± 0.02 c | 12.23 ± 0.13 c | 73.23 ± 0.07 b | 70.10 ± 0.07 b |
| Samples with TGase (WT) | ||||
| BOI | −5.12 ± 0.01 c | 12.42 ± 0.07 b | 76.42 ± 0.17 a | 72.86 ± 0.17 a |
| STE | −5.09 ± 0.01 c | 12.51 ± 0.02 b | 76.34 ± 0.26 a | 72.75 ± 0.23 a |
| BAC | −3.91 ± 0.08 a | 16.68 ± 0.17 a | 74.81 ± 0.24 b | 69.53 ± 0.20 c |
| ROA | −5.03 ± 0.05 c | 11.95 ± 0.05 c | 74.71 ± 0.25 b | 71.58 ± 0.21 b |
| MIC | −4.42 ± 0.04 b | 11.45 ± 0.03 d | 72.77 ± 0.69 c | 70.13 ± 0.62 c |
| FRI | −5.26 ± 0.02 d | 12.45 ± 0.05 b | 75.10 ± 0.17 b | 71.67 ± 0.15 b |
| Group of without TGase (NT) | Group of with TGase (WT) | ||
|---|---|---|---|
| Gel strength | α-helix | −0.985 ** | −0.992 ** |
| β-sheet | 0.982 ** | 0.957 ** | |
| β-turns | 0.980 ** | 0.866 * | |
| Unordered | 0.907 * | 0.939 ** | |
| Ionic bond | −0.252 | −0.086 | |
| Hydrogen bonds | −0.900 * | −0.892 * | |
| Hydrophobic interaction | 0.867 * | 0.972 ** | |
| Disulfide bonds | 0.432 | 0.539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Yi, S.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products. Foods 2022, 11, 66. https://doi.org/10.3390/foods11010066
Li Q, Yi S, Wang W, Xu Y, Mi H, Li X, Li J. Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products. Foods. 2022; 11(1):66. https://doi.org/10.3390/foods11010066
Chicago/Turabian StyleLi, Qiang, Shumin Yi, Wei Wang, Yongxia Xu, Hongbo Mi, Xuepeng Li, and Jianrong Li. 2022. "Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products" Foods 11, no. 1: 66. https://doi.org/10.3390/foods11010066
APA StyleLi, Q., Yi, S., Wang, W., Xu, Y., Mi, H., Li, X., & Li, J. (2022). Different Thermal Treatment Methods and TGase Addition Affect Gel Quality and Flavour Characteristics of Decapterus maruadsi Surimi Products. Foods, 11(1), 66. https://doi.org/10.3390/foods11010066






