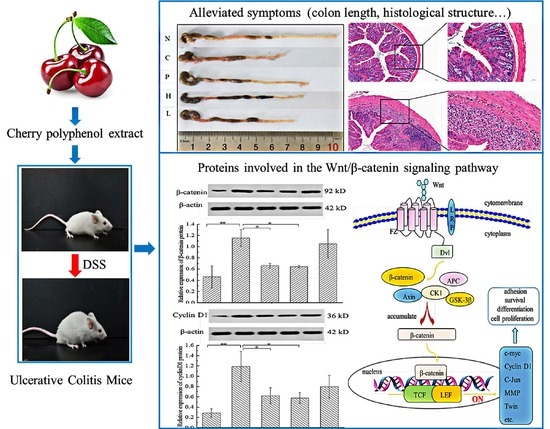
Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/β-Catenin Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction, Purification and Content Determination of the Cherry Polyphenol Extracts
2.3. The Antioxidant Capacities In Vitro of the Free and Bound Polyphenol Extracts
2.4. Analysis of Cherry Polyphenol Extracts (Free and Bound Polyphenols) by HPLC and UPLC-ESI-TOF-MS/MS
2.5. Experimental Animals
2.6. Developing of Ulcerative Colitis and Cherry Polyphenol Extract Treatment
2.7. Histological Assessment
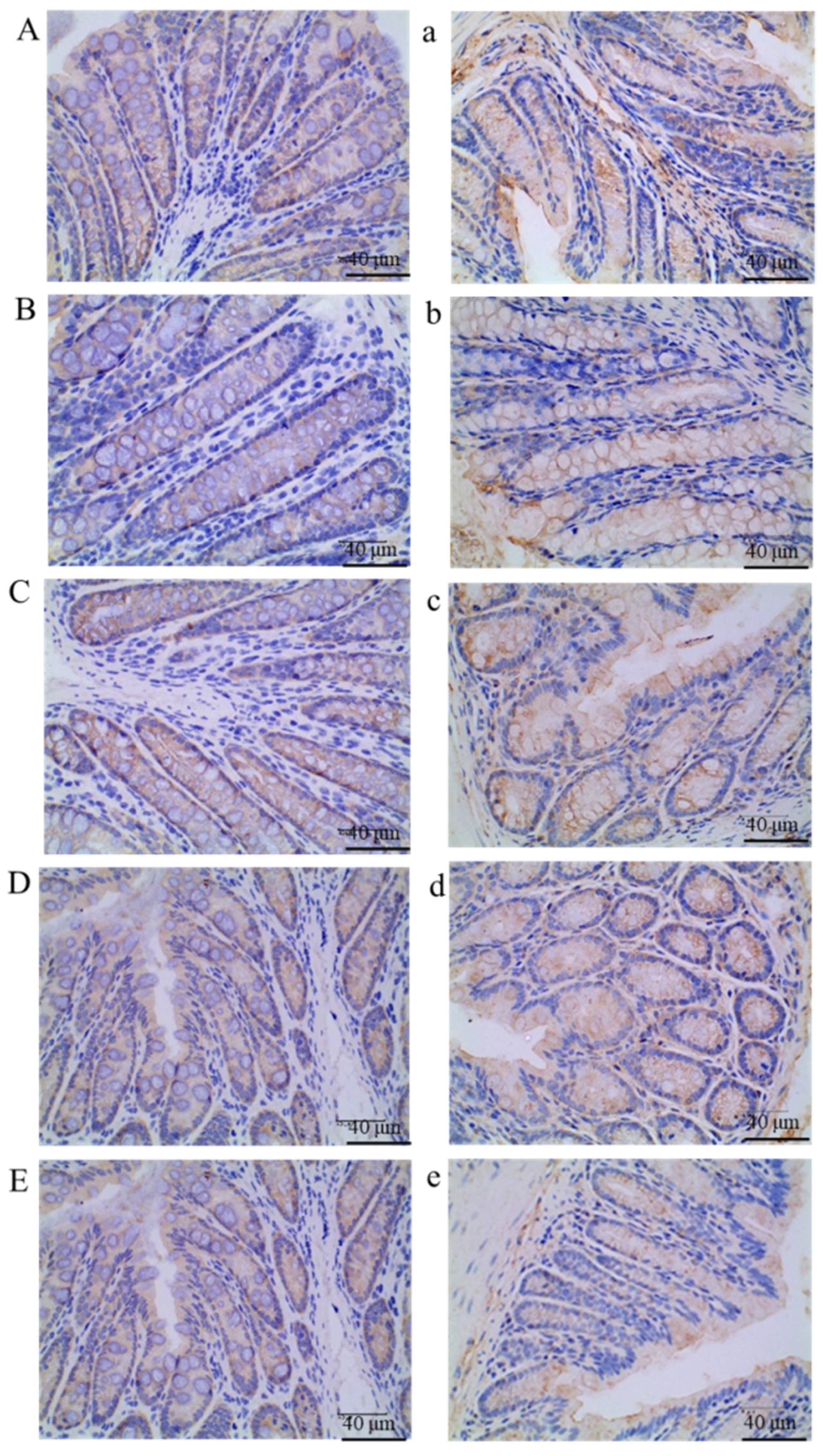
2.8. Immunohistochemistry Analysis
2.9. Determination of Enzymes in Serum
2.10. Determination of Inflammatory Cytokines in the Colon
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. The Polyphenols Content and Antioxidant Capacities In Vitro of the Free and Bound Polyphenol Extract
3.2. The Quantitative Analysis of Phenolic Compounds in the Cherry Extract by HPLC
3.3. Identification of Cherry Polyphenol Extract by UPLC-ESI-TOF-MS/MS
3.4. Effects of the Free Polyphenol Extract of Cherry on DSS-Induced Ulcerative Colitis in Mice
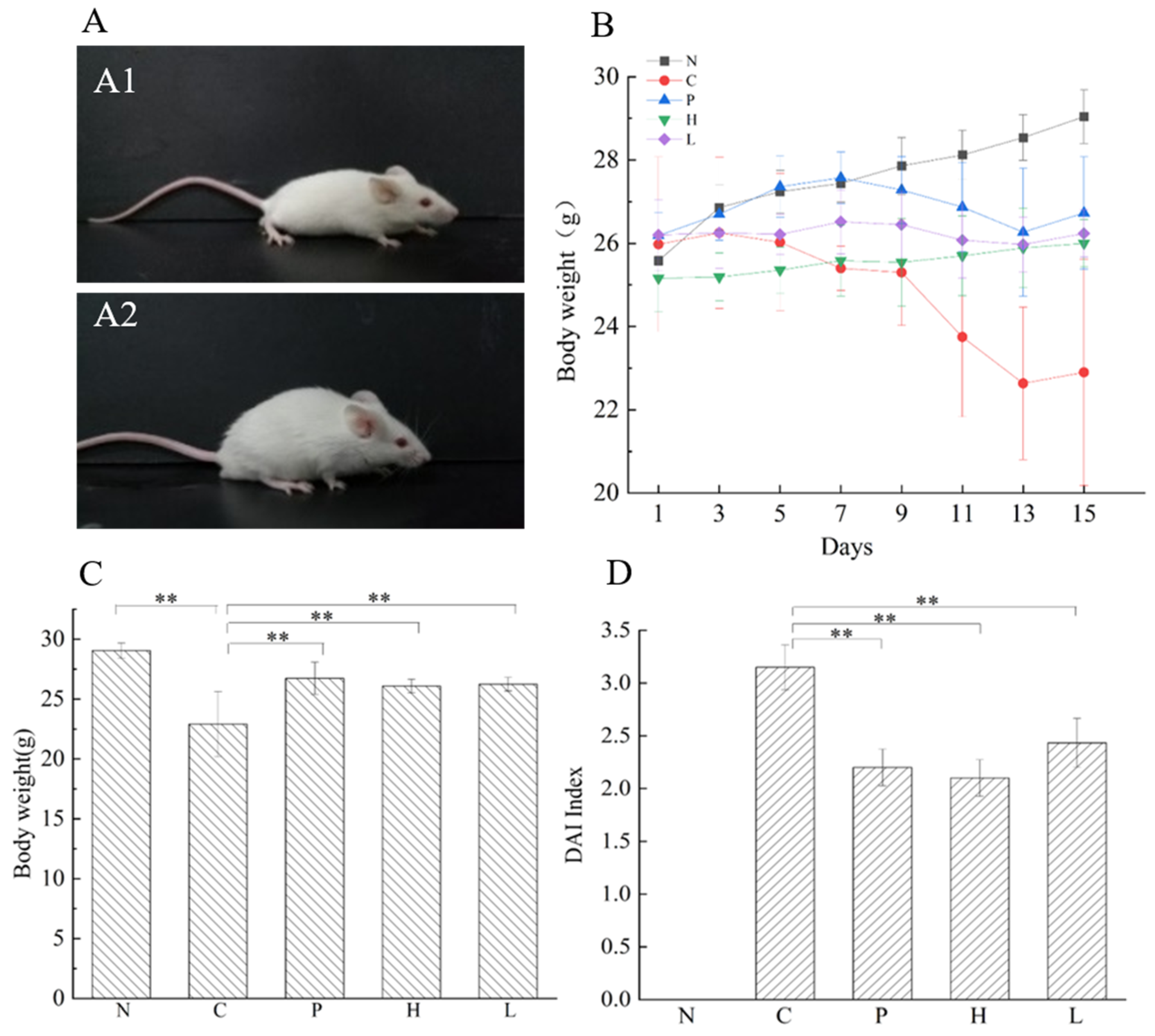
3.4.1. Effect of the Free Polyphenol Extract of Cherry on the Body Weight and DAI Index of UC Mice
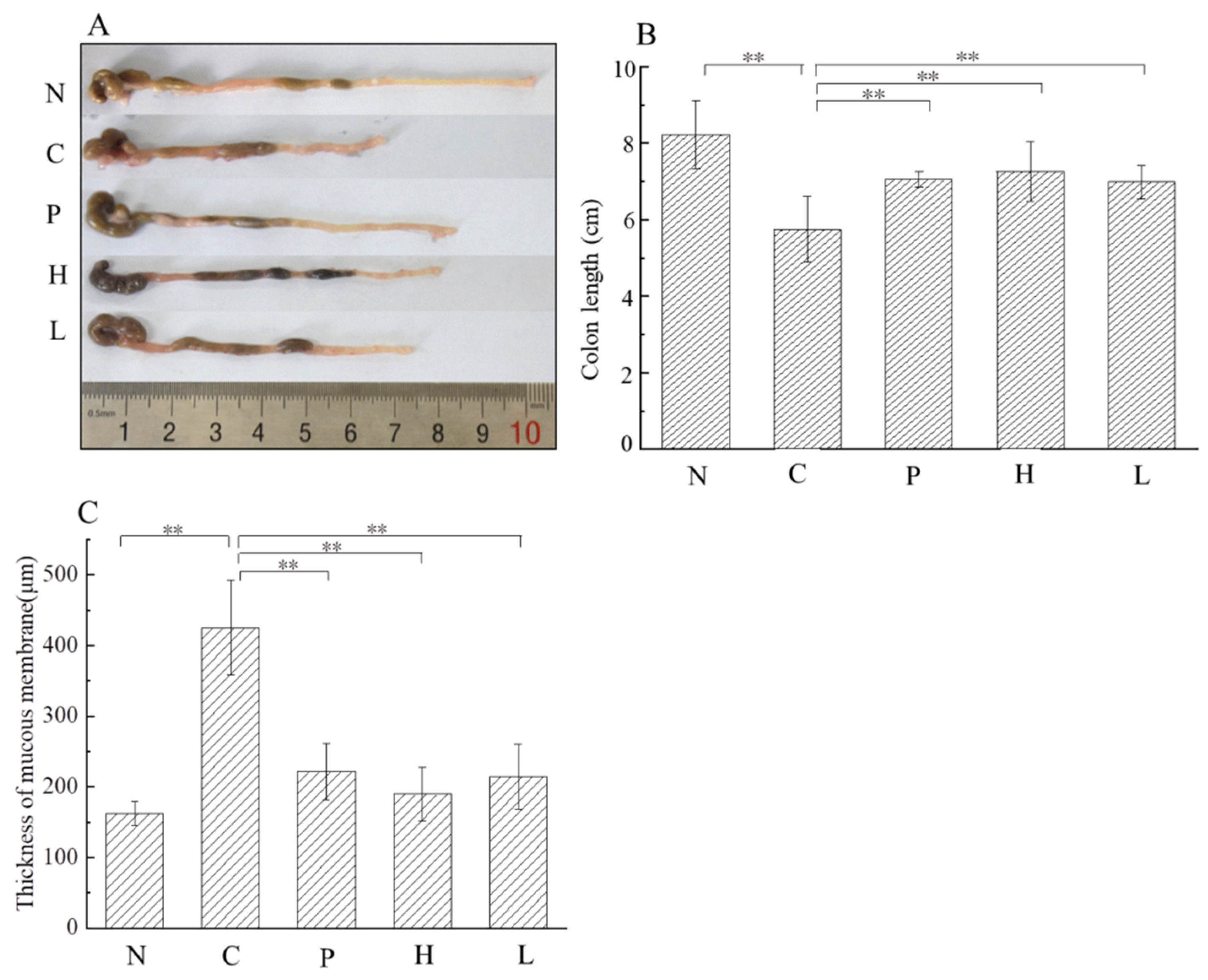
3.4.2. Effect of the Free Polyphenol Extract of Cherry on Colon Length, and the Thickness of Colonic Mucous Layer of UC Mice
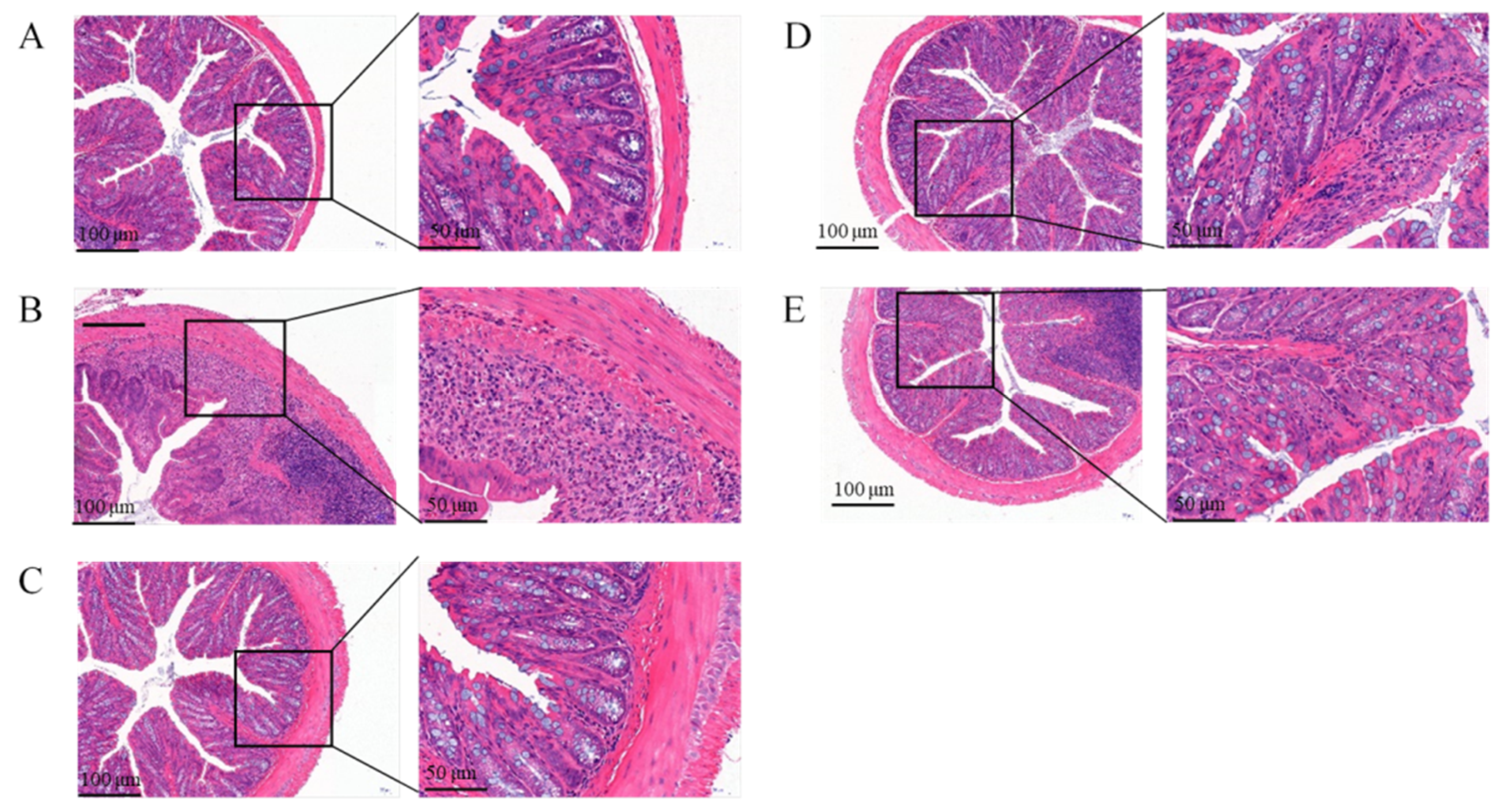
3.4.3. Effect of the Free Polyphenol Extract of Cherry on the Histological Properties of Colon of UC Mice
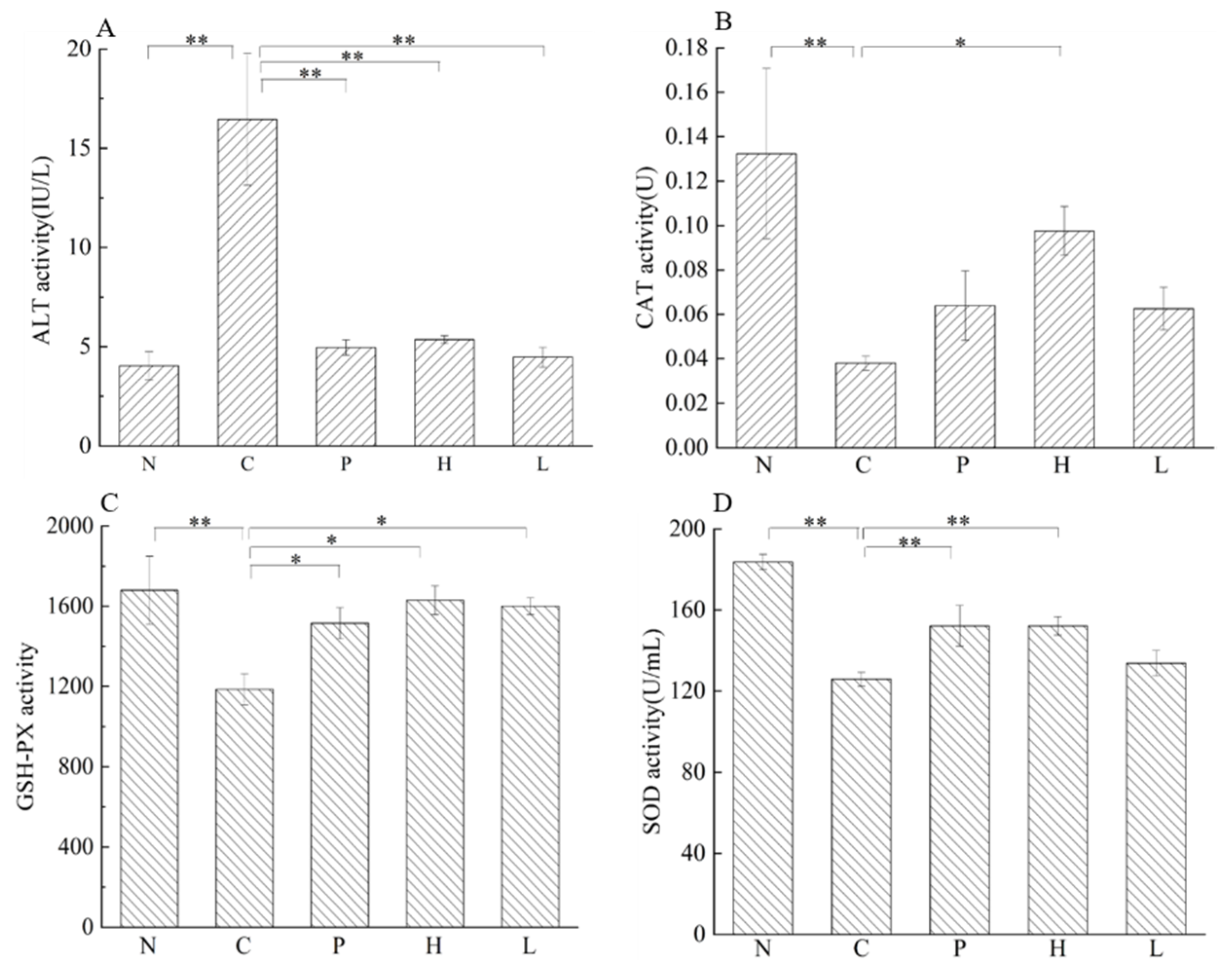
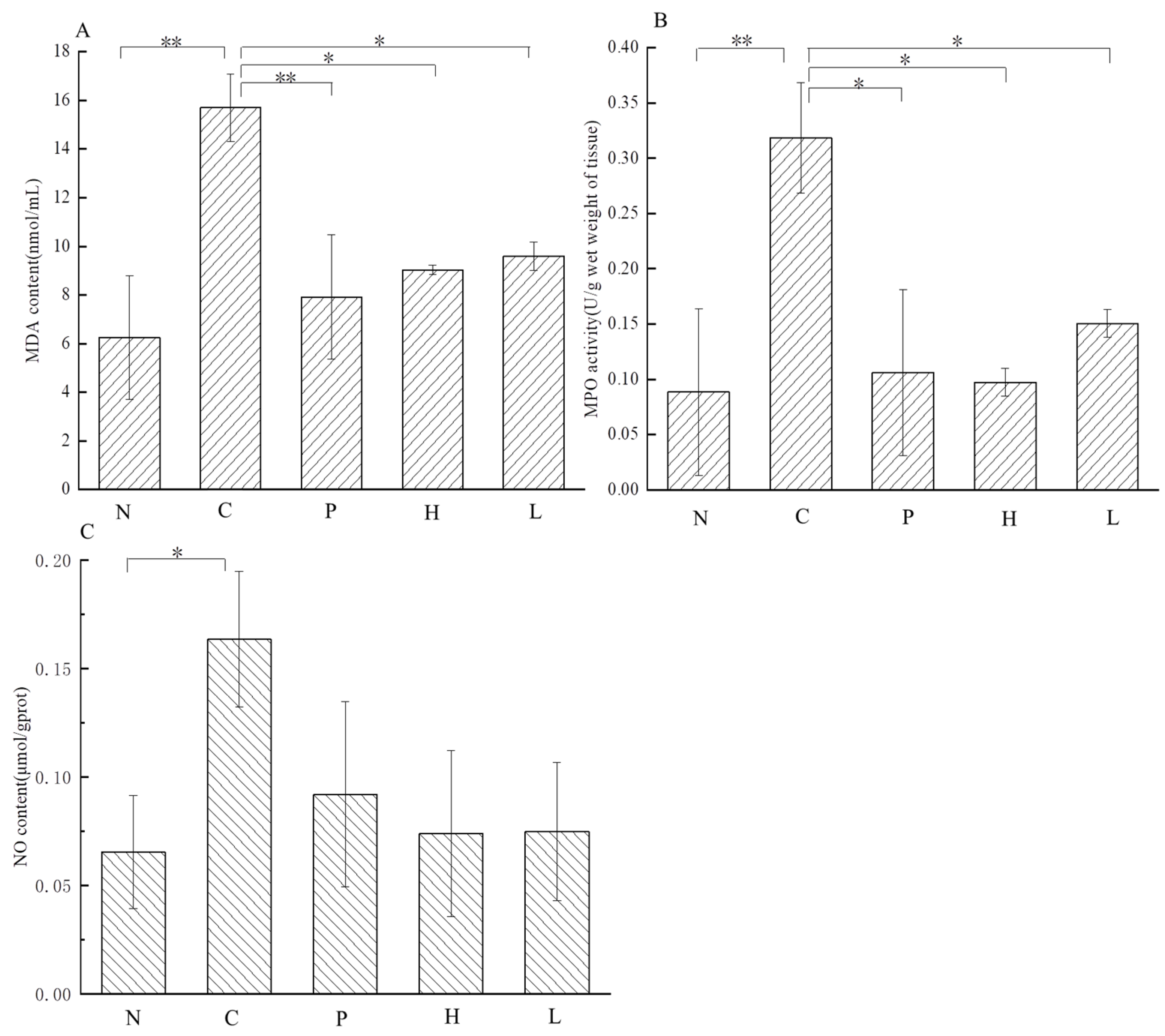
3.4.4. Effect of the Free Polyphenol Extract of Cherry on the Activity of Serum Enzymes, and the Levels of Inflammatory Cytokines of UC Mice
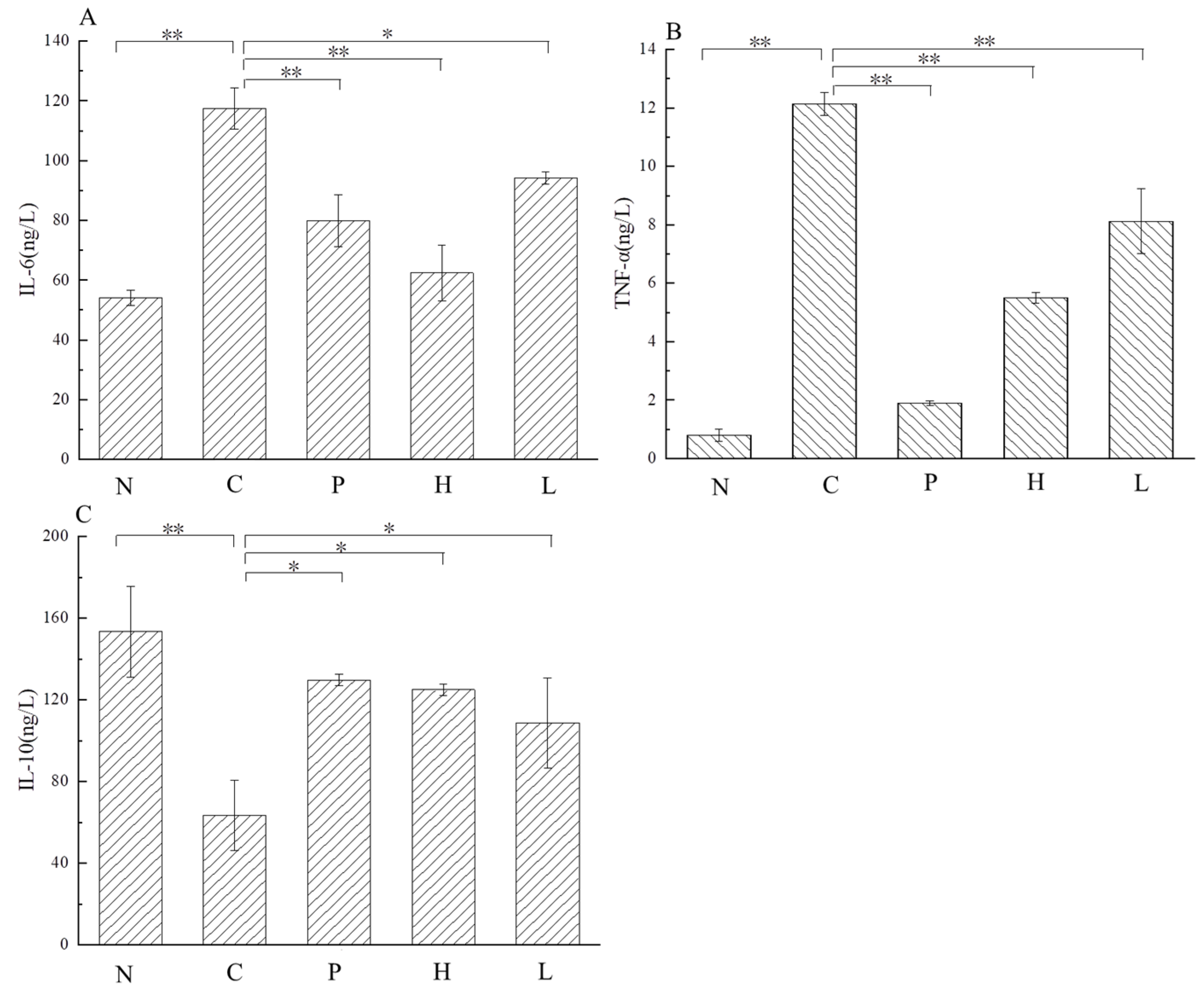
3.4.5. Effect of the Free Polyphenol Extract of Cherry on Levels of Inflammatory Factors of UC Mice
3.5. The Possible Mechanisms of the Free Polyphenol Extract of Cherry on DSS-Induced Ulcerative Colitis in Mice
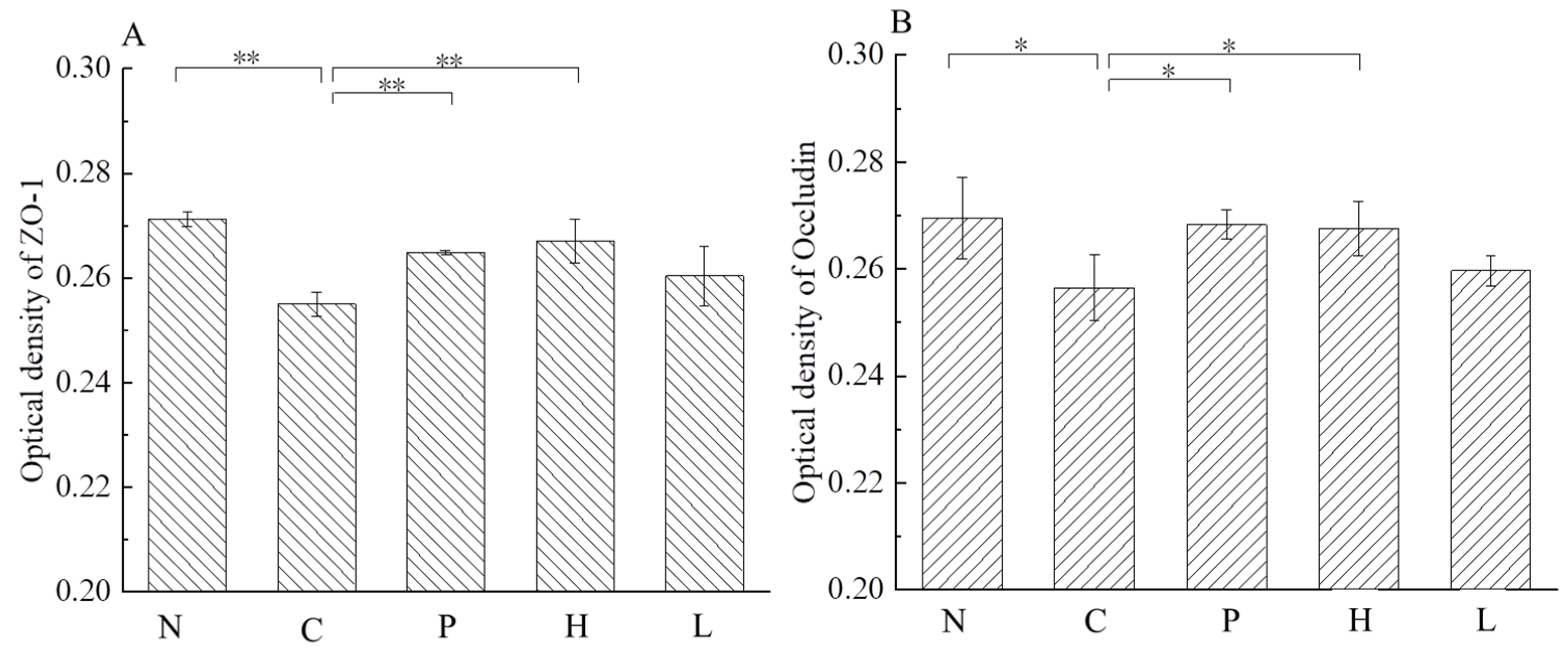
3.5.1. Effect of the Free Polyphenol Extract of Cherry on Tight Junction (TJ) Proteins
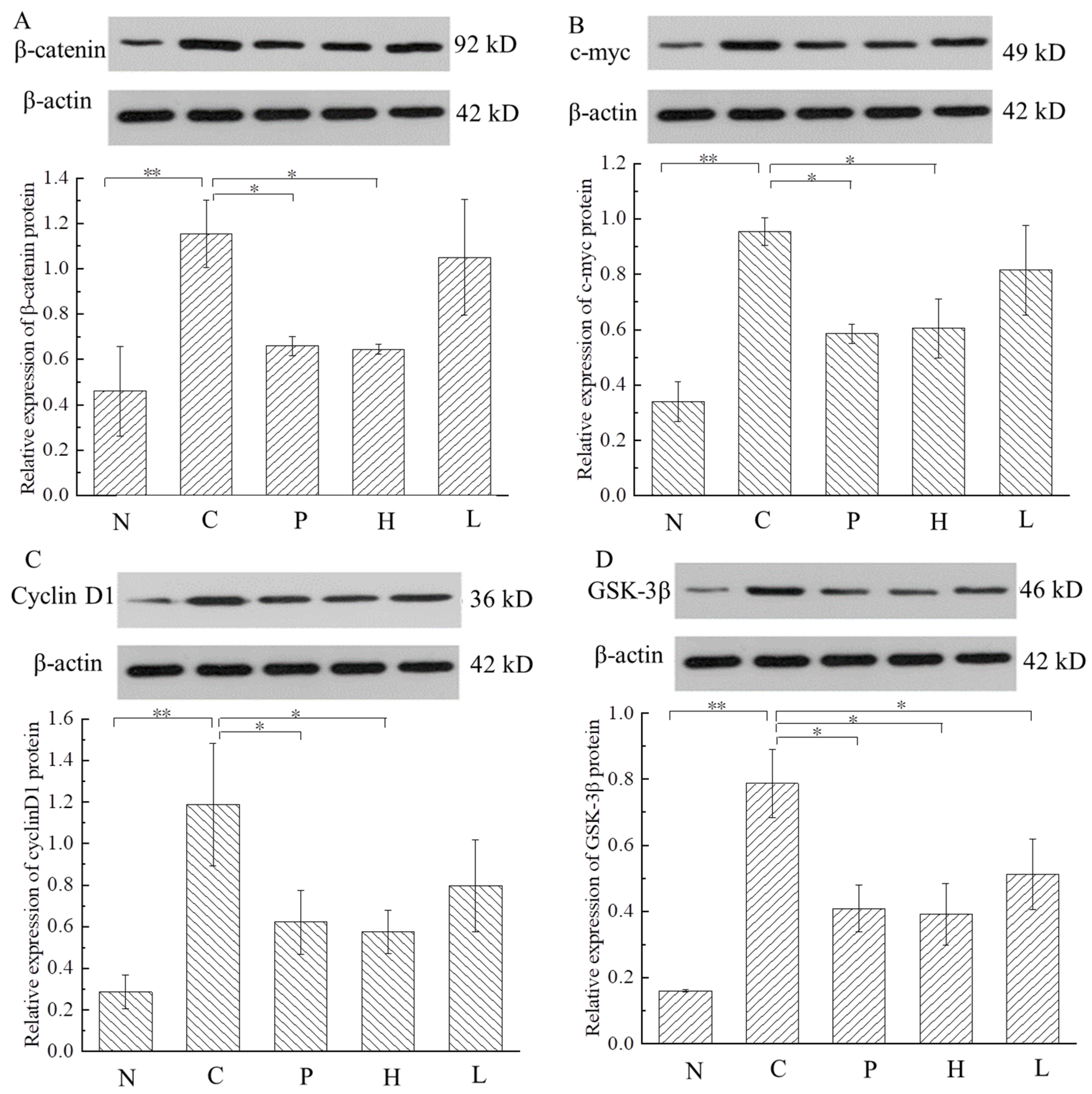
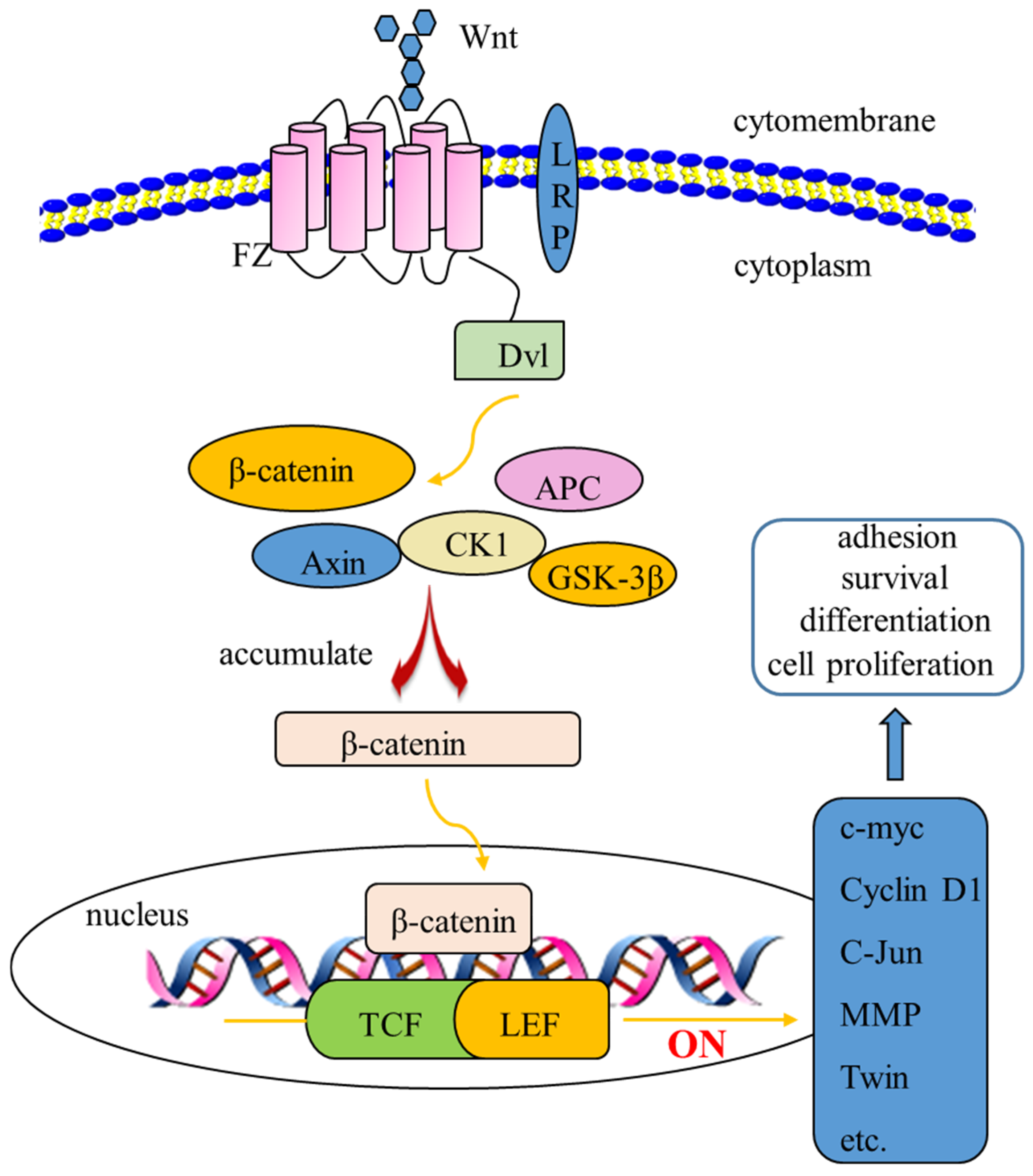
3.5.2. The Free Polyphenol Extract of Cherry Inhibited the Wnt/β-Catenin Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaulmann, A.; Bohn, T. Bioactivity of polyphenols: Preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [Green Version]
- D’Argenio, G.; Mazzone, G.; Tuccillo, C.; Ribecco, M.T.; Graziani, G.; Gravina, A.G.; Caserta, S.; Guido, S.; Fogliano, V.; Caporaso, N.; et al. Apple polyphenols extract (APE) improves colon damage in a rat model of colitis. Digest. Liver Dis. 2012, 44, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Roblin, X.; Michiels, C.; Presles, E.; Del Tedesco, E.; Hebuterne, X.; Filippi, J. Predictors of response to infliximab in patients with ulcerative colitis in remission after at least 6 months of combination therapy (infliximab plus azathioprine). Gastroenterology 2011, 140, S592–S593. [Google Scholar] [CrossRef]
- Iskandar, H.N.; Dhere, T.; Farraye, F.A. Ulcerative colitis: Update on medical management. Curr. Gastroenterol. Rep. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paiotti, A.P.R.; Miszputen, S.J.; Oshima, C.T.F.; Costa, H.D.; Ribeiro, D.A.; Franco, M. Effect of COX-2 inhibitor after TNBS-induced colitis in wistar rats. J. Mol. Histol. 2009, 40, 317–324. [Google Scholar] [CrossRef]
- Li, S.Y.; Wu, B.N.; Fu, W.Y.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [Green Version]
- Saldanha, E.; Saxena, A.; Kaur, K.; Kalekhan, F.; Venkatesh, P.; Fayad, R.; Rao, S.; George, T.; Baliga, M.S. Chapter 23—Polyphenols in the Prevention of Ulcerative Colitis: A Revisit. In Dietary Interventions in Gastrointestinal Diseases; Ronald, R.W., Victor, R.P., Eds.; Academic Press: Salt Lake City, UT, USA, 2019; pp. 277–287. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 56, 56–66. [Google Scholar] [CrossRef]
- Sobczak, M.; Fabisiak, A.; Murawska, N.; Wesolowska, E.; Wierzbicka, P.; Wlazlowski, M.; Wojcikowska, M.; Zatorski, H.; Zwolinska, M.; Fichna, J. Current overview of extrinsic and intrinsic factors in etiology and progression of inflammatory bowel diseases. Pharmacol. Rep. 2014, 66, 766–775. [Google Scholar] [CrossRef]
- Malik, T.A. Inflammatory bowel disease: Historical perspective, epidemiology, and risk factors. Surg. Clin. N. Am. 2015, 95, 1105–1122. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef] [Green Version]
- Shigeshiro, M.; Tanabe, S.; Suzuki, T. Dietary polyphenols modulate intestinal barrier defects and inflammation in a murine model of colitis. J. Funct. Foods 2013, 5, 949–955. [Google Scholar] [CrossRef]
- Xue, B.; Liu, X.L.; Dong, W.W.; Liang, L.L.; Chen, K.Y. EGCG maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-kappa B signaling pathway in rats. Can. J. Gastroenterol. 2017, 2017, 3057268. [Google Scholar] [CrossRef] [Green Version]
- Maurer, L.H.; Cazarin, C.B.B.; Quatrin, A.; Minuzzi, N.M.; Costa, E.L.; Morari, J.; Velloso, L.A.; Leal, R.F.; Rodrigues, E.; Bochi, V.C.; et al. Grape peel powder promotes intestinal barrier homeostasis in acute TNBS-colitis: A major role for dietary fiber and fiber-bound polyphenols. Food Res. Int. 2019, 123, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Graziani, G.; D’Argenio, G.; Tuccillo, C.; Loguercio, C.; Ritieni, A.; Morisco, F.; Blanco, C.D.; Fogliano, V.; Romano, M. Apple polyphenol extracts prevent damage to human gastric epithelial cells in vitro and to rat gastric mucosa in vivo. Gut 2005, 54, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ricciardiello, L.; Bazzoli, F.; Fogliano, V. Phytochemicals and colorectal cancer prevention-myth or reality? Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.P.; Alibas, I. The effect of different drying techniques on color parameters, ascorbic acid content, anthocyanin and antioxidant capacities of cornelian cherry. Food Chem. 2021, 364, 130358. [Google Scholar] [CrossRef]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. 2011, 51, 1–12. [Google Scholar] [CrossRef]
- He, Y.H.; Zhou, J.; Wang, Y.S.; Xiao, C.; Tong, Y.; Tang, J.C.O.; Chan, A.S.C.; Lu, A.P. Anti-inflammatory and anti-oxidative effects of cherries on Freund’s adjuvant induced arthritis in rats. Scand. J. Rheumatol. 2006, 35, 356–358. [Google Scholar] [CrossRef]
- Matias, A.A.; Rosado-Ramos, R.; Nunes, S.L.; Figueira, I.; Serra, A.T.; Bronze, M.R.; Santos, C.N.; Duarte, C.M.M. Protective effect of a (poly)phenol-rich extract derived from sweet cherries culls against oxidative cell damage. Molecules 2016, 21, 406. [Google Scholar] [CrossRef]
- Faienza, F.M.; Corbo, F.; Carocci, A.; Catalano, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.H.; D’Amato, G.; Muraglia, M.; Franchini, C. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.Z.; Liu, R.H. Antioxidant and anti proliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.; Wu, X.Z.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.P.; Yu, Z.F.; Yue, T.L.; Quek, S.Y. Optimization of polyphenol removal from kiwifruit juice using a macroporous resin. J. Sci. Food Agric. 2017, 97, 2498–2507. [Google Scholar] [CrossRef]
- Xi, L.S.; Mu, T.H.; Sun, H.N. Preparative purification of polyphenols from sweet potato (Ipomoea batatas L.) leaves by AB-8 macroporous resins. Food Chem. 2015, 172, 166–174. [Google Scholar] [CrossRef]
- Kriengsak, T.; Unaroj, B.; Kevin, C.; Luis, C.Z.; David, H.B. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2002, 19, 669–675. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Oszmianski, J. Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int. J. Mass Spectrom. 2015, 392, 154–163. [Google Scholar] [CrossRef]
- Nawirska-Olszanska, A.; Kolniak-Ostek, J.; Oziemblowski, M.; Ticha, A.; Hyspler, R.; Zadak, Z.; Zidova, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef]
- Acero, N.; Gradillas, A.; Beltran, M.; Garcia, A.; Mingarro, D.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef]
- Boussenna, A.; Cholet, J.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Fraisse, D.; Vasson, M.P.; Texier, O.; Felgines, C. Polyphenol-rich grape pomace extracts protect against dextran sulfate sodium-induced colitis in rats. J. Sci. Food Agric. 2016, 96, 1260–1268. [Google Scholar] [CrossRef] [Green Version]
- Perez-Jimenez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary intake of 337 polyphenols in French adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julia, C.; Touvier, M.; Lassale, C.; Fezeu, L.; Galan, P.; Hercberg, S.; Kesse-Guyot, E. Cluster analysis of polyphenol intake in a French middle-aged population (aged 35-64 years). J. Nutr. Sci. 2016, 5, e28. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, C.; Kishimoto, Y.; Fukushima, Y.; Kondo, K.; Yamakawa, M.; Wada, K.; Nagata, C. Dietary intake of total polyphenols and the risk of all-cause and specific-cause mortality in Japanese adults: The Takayama study. Eur. J. Nutr. 2020, 59, 1263–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Wu, X.Y.; Cao, S.Y.; Cromie, M.; Shen, Y.H.; Feng, Y.M.; Yang, H.; Li, L. Chlorogenic acid ameliorates experimental colitis by promoting growth of Akkermansia in mice. Nutrients 2017, 9, 677. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.Y.; Zhuang, T.T.; Ping, Y.; Zhang, Y.Z.; Wang, X.C.; Yu, P.; Duan, X.Z. Elevated systemic immune inflammation index level is associated with disease activity in ulcerative colitis patients. Clin. Chim. Acta 2021, 517, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Yan, Y.M.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.W.; Mi, J.; Lu, L.; Zhang, Z.J.; Li, X.Y.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Biol. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.H.; Chen, Q.B.; Luo, L.Y.; Ma, M.J.; Xiao, B.; Zeng, L. Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol. Nutr. Food Res. 2020, 64, 1900943. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.L.; Yao, L.; Liang, H.W.; Wang, D.; He, Y.Q.; Wei, Y.; Ye, L.; Wang, K.; Li, L.M.; Chen, J.N. Intestinal epithelial PKM2 serves as a safeguard against experimental colitis via activating β-catenin signaling. Mucosal Immunol. 2019, 12, 1280–1290. [Google Scholar] [CrossRef]
- Chen, S.N.; Zhao, H.A.; Cheng, N.; Cao, W. Rape bee pollen alleviates dextran sulfate sodium (DSS)-induced colitis by neutralizing IL-1β and regulating the gut microbiota in mice. Food Res. Int. 2019, 122, 241–251. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Jiang, Q.; Lin, J.; Li, X.; Sun, C.; Chen, K. Physicochemical characterisation of four cherry species (Prunus spp.) grown in China. Food Chem. 2015, 173, 855–863. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.R.; Panaccione, R.; Higgins, P.D.R.; Vermeire, S.; Gassull, M.; Chowers, Y.; Hanauer, S.B.; Herfarth, H.; Hommes, D.W.; Kamm, M.; et al. The London position statement of the world congress of gastroenterology on biological therapy for IBD with the European crohn’s and colitis organization: When to start, when to stop, which drug to choose, and how to predict response. Am. J. Gastroenterol. 2011, 106, 199–212. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.Y.; Wu, Z.Q.; Yao, L.J.; Wu, Y.H.; Huang, L.; Liu, K.; Zhou, X.; Gou, D.M. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264.7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.A.; Smyth, J.A.; Liu, Z.H.; Bolling, B.W. Aronia berry (Aronia mitschurinii ‘Viking’) inhibits colitis in mice and inhibits T cell tumour necrosis factor-α secretion. J. Funct. Foods 2018, 44, 48–57. [Google Scholar] [CrossRef]
- van Dekken, H.; Wink, J.C.; Vissers, K.J.; Franken, P.F.; Schouten, W.R.; Hop, W.C.J.; Kuipers, E.J.; Fodde, R.; van der Woude, C.J. Wnt pathway-related gene expression during malignant progression in ulcerative colitis. Acta Histochem. 2007, 109, 266–272. [Google Scholar] [CrossRef]
- Mao, R.R.; Zou, F.Y.; Yang, L.Y.; Lin, S.C.; Li, Y.Q.; Ma, M.X.; Yin, P.H.; Liang, J.W. The loss of MiR-139-5p promotes colitis associated tumorigenesis by mediating PI3K/AKT/Wnt signaling. Int. J. Biochem. Cell Biol. 2015, 69, 153–161. [Google Scholar] [CrossRef]
- Soubh, A.A.; Abdallah, D.M.; El-Abhar, H.S. Geraniol ameliorates TNBS-induced colitis: Involvement of Wnt/β-catenin, p38MAPK, NFκB, and PPARγ signaling pathways. Life Sci. 2015, 136, 142–150. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Q.; Zhou, Q.; Wen, K.; Wu, B.S.; Sun, X.L.; Wang, X.P.; Chen, Y.G. Huangkui lianchang decoction ameliorates DSS-induced ulcerative colitis in mice by inhibiting the NF-kappaB signaling pathway. Evid. -Based Complementary Altern. Med. 2019, 2019, 1040847. [Google Scholar] [CrossRef] [Green Version]
- Abd Elmaksoud, H.A.; Motawea, M.H.; Desoky, A.A.; Elharrif, M.G.; Ibrahimi, A. Hydroxytyrosol alleviate intestinal inflammation, oxidative stress and apoptosis resulted in ulcerative colitis. Biomed. Pharmacother. 2021, 142, 112073. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K. Febuxostat attenuates ulcerative colitis by the inhibition of NF-κB, proinflammatory cytokines, and oxidative stress in mice. Int. Immunopharmacol. 2019, 76, 105884. [Google Scholar] [CrossRef]
- El-Akabawy, G.; El-Sherif, N.M. Zeaxanthin exerts protective effects on acetic acid-induced colitis in rats via modulation of pro-inflammatory cytokines and oxidative stress. Biomed. Pharmacother. 2019, 111, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; de lbarguen, L.S.; Cardeno, A.; de la Lastra, C.A. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef]
- Guan, Q.D.; Zhang, J.G. Recent advances: The imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediat. Inflamm. 2017, 2017, 4810258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.Z.; Zhao, S.N.; Zhou, J.F.; Yan, J.K.; Wang, L.Y.; Du, X.X.; Li, H.; Chen, Y.W.; Cai, W.; Wu, J. Curcumin alleviates DSS-induced colitis via inhibiting NLRP3 inflammsome activation and IL-1β production. Mol. Immunol. 2018, 104, 11–19. [Google Scholar] [CrossRef]
- Amirshahrokhi, K.; Khalili, A.R. Methylsulfonylmethane is effective against gastric mucosal injury. Eur. J. Pharmacol. 2017, 811, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Cader, M.Z.; Kaser, A. Recent advances in inflammatory bowel disease: Mucosal immune cells in intestinal inflammation. Gut 2013, 62, 1653–1664. [Google Scholar] [CrossRef]
- Niu, X.F.; Zhang, H.L.; Li, W.F. Protective effect of cavidine on acetic acid-induced murine colitis via regulating antioxidant, cytokine profile and NF-κB signal transduction pathways. Chem.-Biol. Interact. 2015, 239, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T.L. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef]
- Gao, J.G.; Yu, M.S.; Zhang, M.M.; Gu, X.W.; Ren, Y.; Zhou, X.X.; Chen, D.; Yan, T.L.; Li, Y.M.; Jin, X. Adipose-derived mesenchymal stem cells alleviate TNBS-induced colitis in rats by influencing intestinal epithelial cell regeneration, Wnt signaling, and T cell immunity. World J. Gastroenterol. 2020, 26, 3750. [Google Scholar] [CrossRef]
- Hu, C.A.A.; Hou, Y.Q.; Yi, D.; Qiu, Y.S.; Wu, G.Y.; Kong, X.F.; Yin, Y.L. Autophagy and tight junction proteins in the intestine and intestinal diseases. Anim. Nutr. 2015, 1, 123–127. [Google Scholar] [CrossRef]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. Biofactors 2014, 40, 586–595. [Google Scholar] [CrossRef]
- Zeng, S.; Chen, L.; Sun, Q.; Zhao, H.; Yang, H.; Ren, S.; Liu, M.L.; Meng, X.L.; Xu, H.B. Scutellarin ameliorates colitis-associated colorectal cancer by suppressing Wnt/β-catenin signaling cascade. Eur. J. Pharmacol. 2021, 906, 174253. [Google Scholar] [CrossRef]
- Hwang, I.; Seo, E.Y.; Ha, H. Wnt/β-catenin signaling: A novel target for therapeutic intervention of fibrotic kidney disease. Arch. Pharm. Res. 2009, 32, 1653–1662. [Google Scholar] [CrossRef]
- Goretsky, T.; Bradford, E.M.; Ryu, H.; Tahir, M.; Moyer, M.P.; Gao, T.Y.; Li, L.H.; Barrett, T.A. A cytosolic multiprotein complex containing p85α is required for β-catenin activation in colitis and colitis-associated cancer. J. Biol. Chem. 2016, 291, 4166–4177. [Google Scholar] [CrossRef] [Green Version]
- Tian, Q.; Feetham, M.C.; Tao, W.A.; He, X.C.; Li, L.H.; Aebersold, R.; Hood, L. Proteomic analysis identifies that 14-3-3ζ interacts with β-catenin and facilitates its activation by Akt. Proc. Nat. Acad. Sci. USA 2004, 101, 15370–15375. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.Y.; Liao, Y.L.; Ma, K.W.; Wang, Y.L.; Zhang, G.X.; Yang, R.C.; Deng, J. PI3K is required for the physical interaction and functional inhibition of NF-κB by β-catenin in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013, 434, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, R.J.; Hodgkin, M.N.; Akhtar, N.; Morse, M.A.; Fuller, K.J.; Saqib, K.; Thompson, N.T.; Wakelam, M.J.O. The p85 subunit of phosphoinositide 3-kinase is associated with β-catenin in the cadherin-based adhesion complex. Biochem. J. 2001, 360, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.R.; Fabris, F.M.; Silva, C.M.G.; Rodrigues, M.R.; Sato, D.T.; Ribeiro, M.L.; Pereira, J.A. Oxidative stress and changes in the content and pattern of tissue expression of β-catenin protein in diversion colitis. J. Coloproctol. 2012, 32, 343–358. [Google Scholar] [CrossRef]
| Compounds | TR a (min) | Molecular Formula | (M + H)+ | MS2 ion Fragments (m/z) | Error (ppm) | Ref | |
|---|---|---|---|---|---|---|---|
| Free anthocyanins | |||||||
| 1 | Cyanidin 3-O-glucoside | 1.169 | C21H20O11 | 449.1103 | 287.0569 (100%) | 3.4 | [30] |
| 2 | Cyanidin 3-O-rutinoside | 1.014 | C27H30O15 | 595.1691 | 287.0561 (100%) | 5.6 | [30] |
| 3 | Peonidin 3-O-rutinoside | 1.288 | C28H33O15 | 610.1888 | 302.0759 (100%), 301.0759 (87%) | −0.6 | [30] |
| 4 | Malvidin-hexoside | 3.659 | C23H25O12 | 493.1361 | 331.0817 (100%) | 3.1 | [41] |
| 5 | Pelargonidin 3-rutinoside | 1.588 | C27H30O14 | 579.1537 | 271.0613 (100%), 127.0389 (79%), 139.0388 (45%) | 6.9 | [41] |
| Bound anthocyanins | |||||||
| 6 | Delphinidin 3-O-rutinoside | 1.648 | C27H30O16 | 611.1632 | 303.0498 (100%) | 3.2 | [30] |
| 7 | Delphinidin-hexoside | 1.920 | C21H20O12 | 465.1038 | 303.0514 (100%) | 2.3 | [30] |
| Free hydroxybenzoic acid | |||||||
| 8 | Protocatechuic acid | 3.096 | C7H6O4 | 155.0348 | 68.9973 (100%), 109.0284 (13%) | 2.3 | [30] |
| Free hydroxycinnamic acids | |||||||
| 9 | Caffeoylquinic acid | 1.502 | C16H18O9 | 355.1048 | 163.0398 (100%), 145.0289 (28%), 117.0337 (15%) | 1.7 | [30] |
| 10 | 3-p-Coumarylquinic acid | 1.584 | C16H18O8 | 339.1083 | 147.0443 (100%), 119.0488 (30%) | 2.7 | [30] |
| 11 | Feruloylquinic acid | 2.111 | C17H20O9 | 369.1193 | 145.0292 (100%), 177.0549 (91%) | 3.5 | [41] |
| 12 | di-Caffeoylquinic acid | 2.731 | C25H24O12 | 517.1368 | 163.0394 (100%) | 5.3 | [41] |
| Bound hydroxycinnamic acid | |||||||
| 13 | p-Coumaric acid | 2.698 | C9H8O3 | 165.0551 | 91.0538 (100%), 119.0485 (57%) | 2.7 | [41,42] |
| Free flavonols | |||||||
| 14 | Quercetin 3-O-rutinoside | 1.698 | C27H30O16 | 611.1648 | 303.0518 (100%) | 4.1 | [30] |
| 15 | Quercetin | 1.744 | C15H10O7 | 303.0510 | 303.0514 (100%) 257.0450 (15%), 229.0497 (33%), 153.0180 (40%) | 3.6 | [30] |
| 16 | Quercetin 3-O-hexoside | 1.741 | C21H20O12 | 465.1048 | 303.0509 (100%) | 3.1 | [30] |
| 17 | Quercetin-7-O-glucoside-3-O-rutinoside | 1.551 | C33H41O21 | 773.2135 | 303.0519 (100%), 465.0962 (65%) | −0.7 | [30] |
| Bound flavonol | |||||||
| 18 | Kaempferol 3-glucoside | 2.441 | C21H20O11 | 449.1099 | 287.0570 (100%) | 4.6 | [21] |
| Free flavanols | |||||||
| 19 | Catechin | 1.755 | C15H14O6 | 291.0872 | 139.0388 (100%), 123.0435 (80%), 147.0439 (32%) | 3.1 | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yan, H.; Jiang, L.; Zhao, J.; Lei, X.; Ming, J. Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/β-Catenin Signaling Pathway. Foods 2022, 11, 49. https://doi.org/10.3390/foods11010049
Li F, Yan H, Jiang L, Zhao J, Lei X, Ming J. Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/β-Catenin Signaling Pathway. Foods. 2022; 11(1):49. https://doi.org/10.3390/foods11010049
Chicago/Turabian StyleLi, Fuhua, Huiming Yan, Ling Jiang, Jichun Zhao, Xiaojuan Lei, and Jian Ming. 2022. "Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/β-Catenin Signaling Pathway" Foods 11, no. 1: 49. https://doi.org/10.3390/foods11010049
APA StyleLi, F., Yan, H., Jiang, L., Zhao, J., Lei, X., & Ming, J. (2022). Cherry Polyphenol Extract Ameliorated Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice by Suppressing Wnt/β-Catenin Signaling Pathway. Foods, 11(1), 49. https://doi.org/10.3390/foods11010049