The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking
Abstract
:1. Introduction
2. Materials and Methods
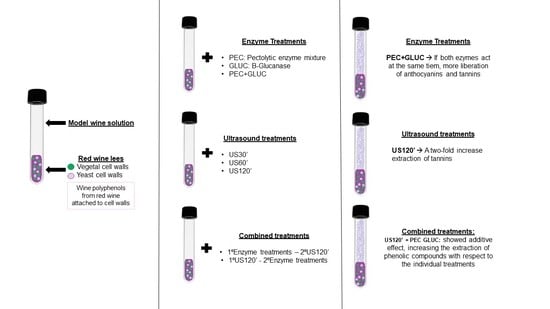
2.1. The Preparation of the Lees
2.2. Enzyme and Ultrasound Treatments
2.3. Tannin Analysis by Liquid Chromatography Using the Phloroglucinolysis Method
2.4. Tannin Analysis by Size Exclusion Chromatography (SEC)
2.5. Anthocyanin Analysis by High Performance Liquid Chromatography
2.6. Soluble Polysaccharide Analysis by Size Exclusion Chromatography (SEC)
2.7. Statistical Analysis
3. Results and Discussion
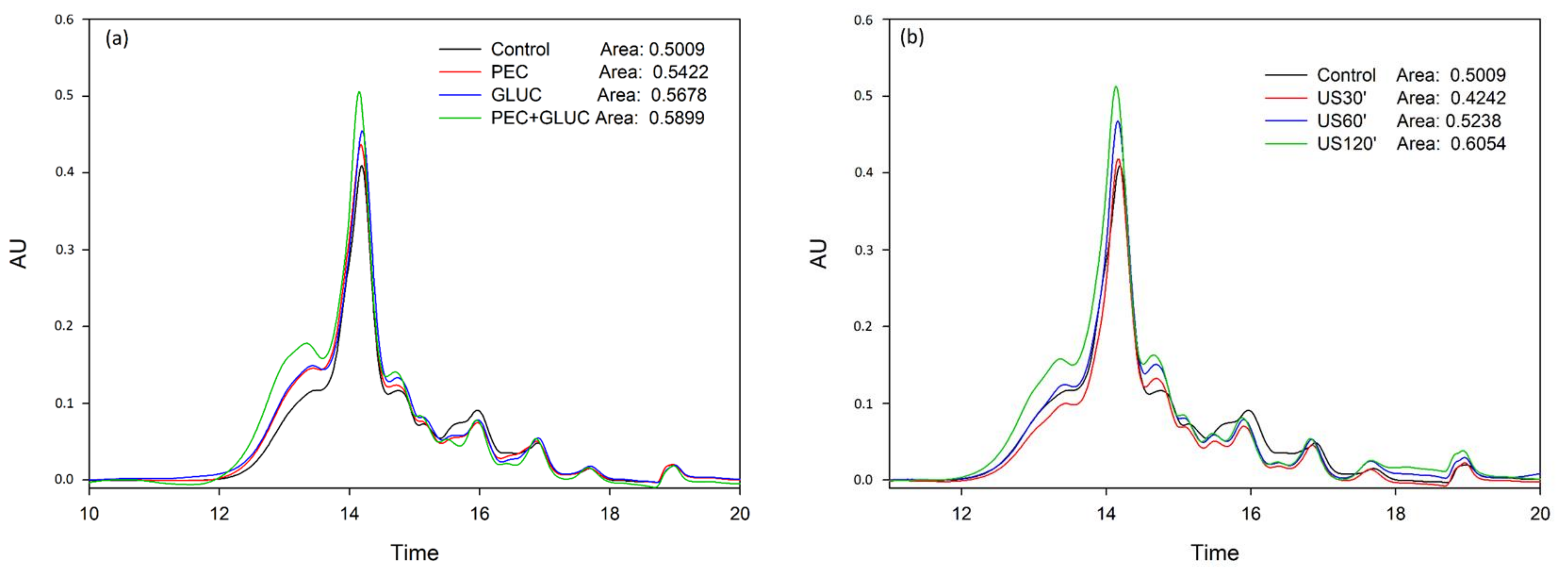
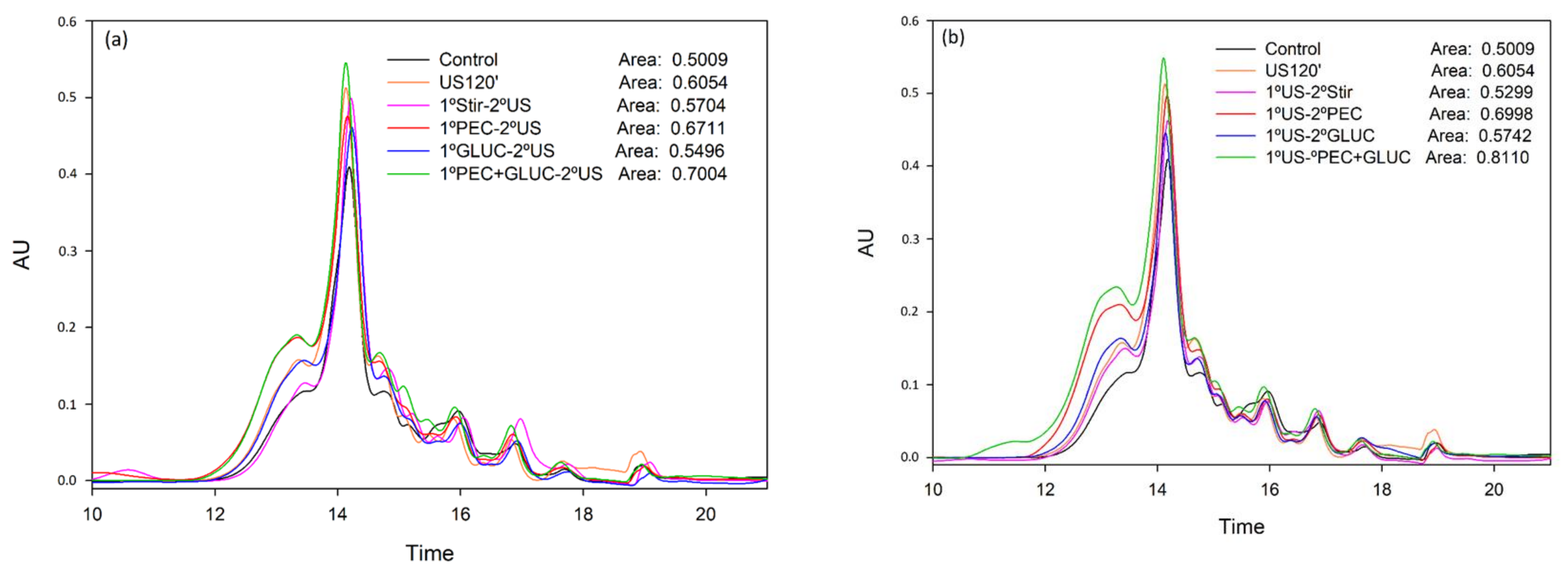
3.1. Determination of the Anthocyanins Liberated from the Lees
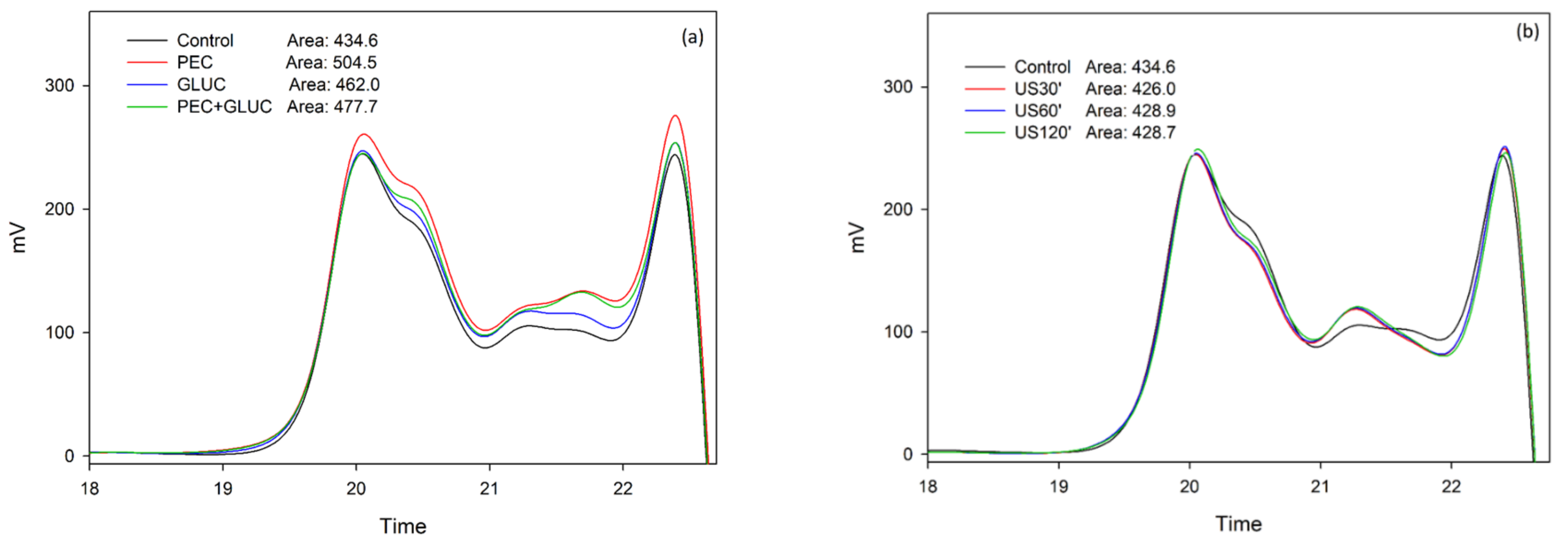
3.2. Tannin Analysis by High Performance Liquid Chromatography and Size Exclusion Chromatography
3.3. Analysis of Soluble Polysaccharides by Size Exclusion Chromatography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef]
- Molano, J.; Bowers, B.; Cabib, E. Distribution of chitin in the yeast cell wall. An ultrastructural and chemical study. Int. J. Cell Biol. 1980, 85, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Fleet, G.H.; Phaff, H.J. Fungal glucans—Structure and metabolism. In Plant Carbohydrates II, 1st ed.; Tanner, W., Loewus, F.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; Volume 13B, pp. 416–440. [Google Scholar]
- Ballou, C. Structure and biosynthesis of the mannan component of the yeast cell envelope. In Advances in Microbial Physiology, 1st ed.; Rose, A.H., Tempest, D.W., Eds.; Academic Press: London, UK, 1976; Volume 14, pp. 93–158. [Google Scholar]
- Charpentier, C.; Freyssinet, M. The mechanism of yeast autolysis in wine. Yeast 1989, 5, 181–186. [Google Scholar]
- Feuillat, M. Yeast macromolecules: Origin, composition, and enological interest. Am. J. Enol. Vitic. 2003, 54, 211–213. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Junior, W.J.F.L.; Nadai, C.; Rolle, L.; da Silva Gulao, E.; da Rocha Leãoe, M.H.M.; Giacomini, A.; Corich, V.; Vincenzi, S. Influence of the mannoproteins of different strains of Starmerella bacillaris used in single and sequential fermentations on foamability, tartaric and protein stabilities of wines. Oeno One 2020, 54, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Manjón, E.; Recio-Torrado, A.; Ramos-Pineda, A.M.; García-Estévez, I.; Escribano-Bailón, M.T. Effect of different yeast mannoproteins on the interaction between wine flavanols and salivary proteins. Int. Food Res. J. 2021, 143, 110279. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodriguez, A.J.; Carrascosa, A.V.; Polo, M.C. Release of nitrogen compounds to the extracellular medium by three strains of Saccharomyces cerevisiae during induced autolysis in a model wine system. Int. J. Food Microbiol. 2001, 68, 155–160. [Google Scholar] [CrossRef]
- Pueyo, E.; Martínez-Rodríguez, A.; Polo, M.C.; Santa-María, G.; Bartolomé, B. Release of lipids during yeast autolysis in a model wine system. J. Agric. Food Chem. 2000, 48, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Gómez-Cordovés, C.; Martín-Álvarez, P.J. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008, 109, 149–158. [Google Scholar] [CrossRef]
- Larue, F.; Geneix, C.; Lafon-Lafourcade, S.; Bertrand, A.; Ribéreau-Gayon, P. Premières observations sur le mode d’action des écorces de levure. Oeno One 1984, 18, 155–163. [Google Scholar] [CrossRef]
- Vasserot, Y.; Caillet, S.; Maujean, A. Study of anthocyanin adsorption by yeast lees. Effect of some physicochemical parameters. Am. J. Enol. Vitic. 1997, 48, 433–437. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- De Nobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; Van Den Ende, H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef]
- Mekoue-Nguela, J.; Vernhet, A.; Sieczkowski, N.; Brillouet, J.M. Interactions of condensed tannins with Saccharomyces cerevisiae yeast cells and cell walls: Tannin location by microscopy. J. Agric. Food Chem. 2015, 63, 7539–7545. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Pérez-Porras, P.; Bautista-Ortín, A.B. Revisiting the use of pectinases in enology: A role beyond facilitating phenolic grape extraction. Food Chem. 2022, 372, 131282. [Google Scholar] [CrossRef]
- Salmon, J.M.; Vuchot, P.; Doco, T.; Moutounet, M. Maintenance and protection of yeast morphology by contact with wine polyphenols during simulation of wine aging on lees. J. Food Sci. 2003, 68, 1782–1787. [Google Scholar] [CrossRef]
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; González, M.C.; Suárez-Lepe, J.A. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007, 105, 838–846. [Google Scholar] [CrossRef]
- Masino, F.; Montevecchi, G.; Arfelli, G.; Antonelli, A. Evaluation of the combined effects of enzymatic treatment and aging on lees on the aroma of wine from Bombino bianco grapes. J. Agric. Food Chem. 2008, 56, 9495–9501. [Google Scholar] [CrossRef]
- Nighojkar, A.; Patidar, M.K.; Nighojkar, S. Pectinases: Production and applications for fruit juice beverages. In Processing and Sustainability of Beverages, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 2, pp. 235–273. [Google Scholar]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.; Moore, J.; Ros-García, J.M.; Bautista-Ortín, A.B. The impact of carbohydrate-active enzymes on mediating cell wall polysaccharide-tannin interactions in a wine-like matrix. Food Res. Int. 2020, 129, 108889. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Gómez-Plaza, E.; Martínez-Pérez, P.; Weiller, F.; Schückel, J.; Willats, W.; Moore, J.; Ros-García, J.M.; Bautista-Ortín, A.B. The influence of hydrolytic enzymes on tannin adsorption-desorption onto grape cell walls in a wine-like matrix. Molecules 2021, 26, 770. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, P.; Cacciola, V.; Batllo, I.F.; Celotti, E. Ultrasounds application in winemaking: Grape maceration and yeast lysis. Ital. J. Food Sci. 2013, 25, 160–168. [Google Scholar]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.E.; Gómez-Plaza, E. Combined use of pectolytic enzymes and ultrasounds for improving the extraction of phenolic compounds during vinification. Food Bioproc. Tech. 2019, 12, 1330–1339. [Google Scholar] [CrossRef]
- Beaver, J.W.; Miller, K.V.; Medina-Plaza, C.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.; Oberholster, A. The effects of temperature and ethanol on proanthocyanidin adsorption to grape cell wall material in the presence of anthocyanins. Molecules 2020, 25, 4139. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Jiménez-Martínez, M.D.; Jurado, R.; Iniesta, J.A.; Terrades, S.; Andrés, A.; Gómez-Plaza, E. Application of high-power ultrasounds during red wine vinification. Int. J. Food Sci. 2017, 52, 1314–1323. [Google Scholar] [CrossRef]
- Perez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gomez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT 2021, 138, 110645. [Google Scholar] [CrossRef]
- Cacciola, V.; Batllò, I.F.; Ferraretto, P.; Vincenzi, S.; Celotti, E. Study of the ultrasound effects on yeast lees lysis in winemaking. Eur. Food Res. Technol. 2013, 236, 311–317. [Google Scholar] [CrossRef]
- Kulkarni, P.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Use of non-Saccharomyces yeast strains coupled with ultrasound treatment as a novel technique to accelerate ageing on lees of red wines and its repercussion in sensorial parameters. LWT Food Sci. Technol. 2015, 64, 1255–1262. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Loira, I.; Morata, A.; González, C.; Suárez-Lepe, J.A.; Cuerda, R. Application of ultrasound to improve lees ageing processes in red wines. Food Chem. 2018, 261, 157–163. [Google Scholar] [CrossRef]
- Zhang, Q.A.; Wang, T.T. Effect of ultrasound irradiation on the evolution of color properties and major phenolic compounds in wine during storage. Food Chem. 2017, 234, 372–380. [Google Scholar] [CrossRef]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.J.; Matias, A.A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [Green Version]
- De Iseppi, A.; Marangon, M.; Vincenzi, S.; Lomolino, G.; Curioni, A.; Divol, B. A novel approach for the valorization of wine lees as a source of compounds able to modify wine properties. LWT 2021, 136, 110274. [Google Scholar] [CrossRef]
- Liu, L.; Loira, I.; Morata, A.; Suárez-Lepe, J.A.; González, M.C.; Rauhut, D. Shortening the ageing on lees process in wines by using ultrasound and microwave treatments both combined with stirring and abrasion techniques. Eur. Food Res. Technol. 2016, 242, 559–569. [Google Scholar] [CrossRef]
- Del Rio, J.L.P.; Kennedy, J.A. Development of proanthocyanidins in Vitis vinifera L. cv. Pinot noir grapes and extraction into wine. Am. J. Enol. Vitic. 2006, 57, 125–132. [Google Scholar]
- Kennedy, J.A.; Taylor, A.W. Analysis of proanthocyanidins by high-performance gel permeation chromatography. J. Chromatogr. A 2003, 995, 99–107. [Google Scholar] [CrossRef]
- Castro-López, L.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin—Cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Busse-Valverde, N.; Gomez-Plaza, E.; Lopez-Roca, J.M.; Gil-Munoz, R.; Bautista-Ortin, A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef]
- Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Ortega-Regules, A.; Gómez-Plaza, E. Elimination of suspended cell wall material in musts improves the phenolic content and color of red wines. Am. J. Enol. Vitic. 2019, 70, 201–204. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Martínez-Hernández, A.; Ruiz-García, Y.; Gil-Muñoz, R.; Gómez-Plaza, E. Anthocyanins influence tannin–cell wall interactions. Food Chem. 2016, 206, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Medina-Plaza, C.; Beaver, J.W.; Miller, K.V.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Cell wall—Anthocyanin interactions during red wine fermentation-like conditions. Am. J. Enol. Vitic. 2020, 71, 149–156. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Garcia, Y.; Smith, P.A.; Bindon, K.A. Selective extraction of polysaccharide affects the adsorption of proanthocyanidin by grape cell walls. Carbohydr. Polym. 2014, 114, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fangel, J.U.; Willats, W.G.; Vivier, M.A.; Moore, J.P. Dissecting the polysaccharide-rich grape cell wall matrix using recombinant pectinases during winemaking. Carbohydr. Polym. 2016, 152, 510–519. [Google Scholar] [CrossRef] [PubMed]

| Del (µg/g) | Cian (µg/g) | Pet (µg/g) | Peond (µg/g) | Malv (µg/g) | ∑Mono (µg/g) | VitA (µg/g) | ∑Acyl (µg/g) | ∑Ant (µg/g) | ∑Ant (mg/L) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Enzyme treatments | ||||||||||
| Control | 40.2 ± 1.9 a 1 | 10.4 ± 0.2 a | 91.4 ± 2.4 a | 49.8 ± 1.4 a | 665.4 ± 9.8 a | 857.3 ± 11.2 a | 9.4 ± 0.2 a | 36.9 ± 5.1 a | 903.7 ± 15.8 a | 56.5 ± 1.0 a |
| PEC | 42.7 ± 1.3 a | 10.8 ± 0.3 ab | 97.1 ± 2.3 a | 53.0 ± 1.0 b | 676.3 ± 5.7 ab | 879.9 ± 9.9 a | 6.5 ± 0.4 a | 60.4 ± 3.5 b | 949.9 ± 13.8 b | 59.4 ± 0.9 b |
| GLUC | 55.0 ± 7.9 b | 11.4 ± 0.2 b | 108.9 ± 7.1 b | 54.0 ± 0.5 b | 692.9 ± 7.7 c | 922.1 ± 16.0 b | 10.6 ± 0.8 ab | 61.0 ± 6.3 b | 993.8 ± 17.3 c | 62.1 ± 1.1 c |
| PEC+GLUC | 59.3 ± 9.6 b | 11.3 ± 0.5 b | 111.4 ± 7.1 b | 52.9 ± 1.5 b | 682.4 ± 9.1 bc | 917.1 ± 25.0 b | 11.2 ± 0.9 b | 102.1 ± 7.3 c | 1030.7 ± 32.9 c | 64.4 ± 2.1 c |
| Ultrasound treatments | ||||||||||
| Control | 40.2 ± 1.9 a | 10.4 ± 0.3 a | 91.4 ± 2.4 a | 49.8 ± 1.4 a | 665.4 ± 9.8 b | 857.3 ± 11.2 b | 9.4 ± 0.2 a | 36.9 ± 5.1 a | 903.7 ± 15.8 a | 56.5 ± 1.0 a |
| US30′ | 61.9 ± 5.1 b | 11.1 ± 0.7 a | 104.4 ± 5.7 b | 49.1 ± 3.1 a | 573.4 ± 31.4 a | 799.9 ± 45.9 ab | 10.3 ± 0.6 ab | 65.5 ± 17.1 b | 875.7 ± 62.4 a | 54.7 ± 3.9 a |
| US60′ | 60.6 ± 1.4 b | 11.2 ± 0.7 a | 102.9 ± 4.5 b | 48.7 ± 2.5 a | 571.0 ± 31.1 a | 794.3 ± 39.7 a | 10.1 ± 0.5 ab | 68.3 ± 13.2 b | 872.6 ± 29.8 a | 54.5 ± 1.9 a |
| US120′ | 60.1 ± 1.9 b | 11.0 ± 0.7 a | 101.3 ± 3.2 b | 47.8 ± 0.9 a | 551.6 ± 9.5 a | 771.7 ± 16.2 a | 10.4 ± 0.4 b | 84.8 ± 5.3 b | 866.9 ± 21.9 a | 54.2 ± 1.4 a |
| Combined treatments | ||||||||||
| Control | 40.2 ± 1.9 a | 10.4 ± 0.3 a | 91.4 ± 2.4 a | 49.8 ± 1.4 a | 665.4 ± 9.8 b | 857.3 ± 11.2 b | 9.4 ± 0.2 a | 36.9 ± 5.1 a | 903.7 ± 15.8 a | 56.5 ± 1.0 a |
| US120′ | 60.1 ± 1.9 cd | 11.0 ± 0.7 a | 101.3 ± 3.2 b | 47.8 ± 0.9 a | 551.6 ± 9.5 a | 771.7 ± 16.2 a | 10.4 ± 0.4 b | 84.8 ± 5.3 c | 866.9 ± 21.9 a | 54.2 ± 1.4 a |
| 1ºStir-2ºUS | 66.1 ± 3.1 de | 13.3 ± 0.2 c | 124.2 ± 1.3 cd | 57.8 ± 0.5 b | 747.0 ± 6.4 cde | 1008.3 ± 2.3 d | 12.3 ± 0.6 d | 56.3 ± 0.3 b | 1076.9 ± 1.5 bc | 68.7 ± 2.5 cd |
| 1ºUS-2ºStir | 47.3 ± 1.4 b | 11.9 ± 0.3 b | 106.1 ± 1.1 b | 57.4 ± 1.1 b | 731.3 ± 8.6 cde | 954.0 ± 11.4 c | 10.4 ± 0.2 b | 70.2 ± 8.6 bc | 1034.7 ± 15.0 b | 64.7 ± 0.9 b |
| 1ºPEC-2ºUS | 67.0 ± 6.8 e | 14.2 ± 0.4 d | 125.9 ± 3.4 b | 49.3 ± 2.2 bc | 754.1 ± 8.6 de | 1020.6 ± 23.8 de | 11.9 ± 0.9 d | 112.8 ± 13.5 d | 1145.3 ± 28.8 d | 71.6 ± 1.8 d |
| 1ºUS-2ºPEC | 47.2 ± 2.8 b | 12.1 ± 0.7 b | 105.7 ± 5.9 b | 56.9 ± 3.2 b | 711.9 ± 30.8 c | 933.7 ± 49.4 c | 10.4 ± 0.7 b | 110.2 ± 4.8 d | 1054.3 ± 53.0 b | 65.9 ± 3.3 bc |
| 1ºGLUC-2ºUS | 59.1 ± 3.7 c | 13.8 ± 0.5 cd | 122.0 ± 5.1 cd | 62.0 ± 1.9 c | 802.0 ± 36.9 f | 1059.0 ± 32.0 e | 12.0 ± 0.9 d | 71.0 ± 16.7 bc | 1142.0 ± 48.4 d | 71.4 ± 3.0 d |
| 1ºUS-2ºGLUC | 46.7 ± 2.7 b | 11.8 ± 0.1 b | 105.7 ± 2.9 b | 56.9 ± 0.2 b | 723.9 ± 22.8 cd | 945.1 ± 7.9 c | 10.6 ± 0.1 bc | 77.9 ± 11.1c | 1033.6 ± 18.6 b | 64.6 ± 1.2 b |
| 1ºPEC+GLUC-2ºUS | 48.3 ± 5.2 c | 12.5 ± 0.5 b | 118.4 ± 5.7 c | 59.6 ± 2.5 bc | 761.8 ± 2.4 e | 1010.6 ± 36.4 d | 11.4 ± 0.2 cd | 108.5 ± 5.7 d | 1130.5 ± 41.1 cd | 70.7 ± 2.6 d |
| 1ºUS-2ºPEC+GLUC | 47.6 ± 2.7 b | 12.2 ± 0.1 b | 107.1 ± 3.4 b | 57.3 ± 1.2 b | 719.7 ± 15.0 cd | 943.7 ± 22.3 c | 10.5 ± 0.3 b | 109.9 ± 12.0 d | 1064.1 ± 34.2 b | 66.5 ± 2.1 bc |
| Samples | TT (mg/L) | mPD | %Gal |
|---|---|---|---|
| Enzyme treatments | |||
| Control | 48.7 ± 2.5 a 1 | 2.95 ± 0.12 a | 18.0 ± 1.8 b |
| PEC | 63.8 ± 3.0 b | 3.04 ± 0.03 a | 15.9 ± 0.8 b |
| GLUC | 59.1 ± 4.8 b | 2.99 ± 0.11 a | 15.4 ± 2.2 b |
| PEC + GLUC | 91.8 ± 9.0 c | 3.23 ± 0.10 b | 11.5 ± 0.5 a |
| Ultrasound treatments | |||
| Control | 48.7 ± 2.5 a | 2.95 ± 0.12 a | 18.0 ± b |
| US30′ | 66.8 ± 14.6 ab | 3.00 ± 0.05 ab | 11.1 ± a |
| US60′ | 79.3 ± 12.5 bc | 3.05 ± 0.03 ab | 11.5 ± ab |
| US120′ | 88.0 ± 1.2 c | 3.11 ± 0.03 b | 12.8 ± a |
| Combined treatments | |||
| Control | 48.7 ± 2.5 a | 2.95 ± 0.12 a | 18.0 ± 1.8 de |
| US120′ | 88.0 ± 1.2 c | 3.11 ± 0.03 ab | 12.8 ± 0.5 abc |
| 1ºStir-2ºUS | 65.0 ± 7.5 b | 2.98 ± 0.09 a | 16.1 ± 2.5 cd |
| 1ºUS-2ºStir | 64.8 ± 4.3 b | 3.30 ± 0.18 cd | 15.6 ± 2.1 bcd |
| 1ºPEC-2ºUS | 103.9 ± 13.7 d | 3.36 ± 0.15 cde | 11.9 ± 0.9 ab |
| 1ºUS-2ºPEC | 99.9 ± 5.3 cd | 3.52 ± 0.13 ef | 10.5 ± 0.5 a |
| 1ºGLUC-2ºUS | 73.8 ± 12.9 b | 3.22 ± 0.12 bc | 19.7 ± 4.8 e |
| 1ºUS-2ºGLUC | 72.1 ± 7.6 b | 3.39 ± 0.07 cde | 15.2 ± 1.9 bcd |
| 1ºPEC+GLUC-2ºUS | 104.9 ± 5.9 d | 3.44 ± 0.06 de | 15.0 ± 0.6 bcd |
| 1ºUS-2ºPEC+GLUC | 104.3 ± 11.4 d | 3.66 ± 0.10 f | 12.0 ± 0.8 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osete-Alcaraz, A.; Bautista-Ortín, A.B.; Pérez-Porras, P.; Gómez-Plaza, E. The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking. Foods 2022, 11, 19. https://doi.org/10.3390/foods11010019
Osete-Alcaraz A, Bautista-Ortín AB, Pérez-Porras P, Gómez-Plaza E. The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking. Foods. 2022; 11(1):19. https://doi.org/10.3390/foods11010019
Chicago/Turabian StyleOsete-Alcaraz, Andrea, Ana Belén Bautista-Ortín, Paula Pérez-Porras, and Encarna Gómez-Plaza. 2022. "The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking" Foods 11, no. 1: 19. https://doi.org/10.3390/foods11010019
APA StyleOsete-Alcaraz, A., Bautista-Ortín, A. B., Pérez-Porras, P., & Gómez-Plaza, E. (2022). The Application of Ultrasound and Enzymes Could Be Promising Tools for Recovering Polyphenols during the Aging on Lees Process in Red Winemaking. Foods, 11(1), 19. https://doi.org/10.3390/foods11010019