Optimisation of Stingless Bee Honey Nanoemulsions Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Formulation and Preparation of SBH NEs
2.2. Characterisation of SBH NEs
2.2.1. Particle Size and Polydispersity Index Analysis
2.2.2. Stability
2.2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.4. Transmission Electron Microscopy (TEM)
2.3. Response Surface Methodology (RSM)
3. Results and Discussion
3.1. Identification of Optimum SBH NE Formation
3.1.1. The Physical Observation of SBH NEs
3.1.2. Stability Analysis and Optimisation for Best SBH NE Formulation
3.2. Characterisation of SBH and SBH NEs
3.2.1. Fourier-Transform Infrared Spectroscopy (FTIR) Patterns of SBH and SBH NEs
3.2.2. Investigation of the SBH NEs’ Morphology Using Transmission Electron Microscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Visweswara, P.; Thevan, K.; Salleh, N.; Hua, S. Biological and therapeutic effects of honey produced by honey bees and stingless bees : A comparative review. Rev. Bras. Farmacogn. 2016, 26, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Shadan, A.F.; Mahat, N.A.; Wan Ibrahim, W.A.; Ariffin, Z.; Ismail, D. Provenance Establishment of Stingless Bee Honey Using Multi-element Analysis in Combination with Chemometrics Techniques. J. Forensic Sci. 2018, 63, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Department of Agriculture. Industrial Crop Statistics; Department of Agriculture: Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Nascimento, A.; Marchini, L.; Carvalho, C.; Araújo, D.; Olinda, R.; Silveira, T. Physical-Chemical Parameters of Honey of Stingless Bee (Hymenoptera: Apidae). Am. Chem. Sci. J. 2015, 7, 139–149. [Google Scholar] [CrossRef]
- Abu Bakar, M.F.; Sanusi, S.B.; Abu Bakar, F.I.; Cong, O.J.; Mian, Z. Physicochemical and antioxidant potential of raw unprocessed honey from malaysian stingless bees. Pakistan J. Nutr. 2017, 16, 888–894. [Google Scholar] [CrossRef] [Green Version]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic properties of stingless bee honey in comparison with european bee honey. Adv. Pharmacol. Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M.; Shukor, N.I.A. Phytochemical compositions and antioxidant activities of malaysian stingless bee honey. Pertanika J. Sci. Technol. 2019, 27, 15–28. [Google Scholar]
- Calligaris, S.; Plazzotta, S.; Valoppi, F.; Anese, M. Combined high-power ultrasound and high-pressure homogenization nanoemulsification: The effect of energy density, oil content and emulsifier type and content. Food Res. Int. 2018, 107, 700–707. [Google Scholar] [CrossRef] [Green Version]
- De Azevedo Ribeiro, R.C.; Barreto, S.M.A.G.; Ostrosky, E.A.; Da Rocha-Filho, P.A.; Veríssimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) Mill extract as moisturizing agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Primozic, M.; Duchek, A.; Nickerson, M.; Ghosh, S. Formation, stability and in vitro digestibility of nanoemulsions stabilized by high-pressure homogenized lentil proteins isolate. Food Hydrocoll. 2018, 77, 126–141. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- Ceyhan, M.; Orhan, Z.; Domnori, E. Health service quality measurement from patient reviews in Turkish by opinion mining Classes Positive Negative Total t Train comments Test comments per dataset. In IFMBE Proceedings; Springer: Singapore, 2017; pp. 649–653. [Google Scholar] [CrossRef]
- Ali, A.; Le Potier, I.; Huang, N.; Rosilio, V.; Cheron, M.; Faivre, V.; Turbica, I.; Agnely, F.; Mekhloufi, G. Effect of high pressure homogenization on the structure and the interfacial and emulsifying properties of β-lactoglobulin. Int. J. Pharm. 2018, 537, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A. Can high-pressure homogenization cause thermal degradation to nutrients? J. Food Eng. 2019, 240, 133–144. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Villalobos-Carvajal, R.; Herrera-Lavados, C.; Moreno-Osorio, L.; Jarpa-Parra, M.; Pérez-Won, M. Physicochemical properties of high-pressure treated lentil protein-based nanoemulsions. LWT 2019, 101, 590–598. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Jo, W.; Kim, H.; Noh, J. Article effective droplet size reduction and excellent stability of limonene nanoemulsion formed by high-pressure homogenizer. Colloids Interfaces 2020, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Akbas, E.; Soyler, B.; Oztop, M.H. Formation of capsaicin loaded nanoemulsions with high pressure homogenization and ultrasonication. LWT 2018, 96, 266–273. [Google Scholar] [CrossRef]
- Majdi, H.; Esfahani, J.A.; Mohebbi, M. Optimization of convective drying by response surface methodology. Comput. Electron. Agric. 2019, 156, 574–584. [Google Scholar] [CrossRef]
- Mehmood, T. Optimization of the canola oil based vitamin E nanoemulsions stabilized by food grade mixed surfactants using response surface methodology. Food Chem. 2015, 183, 1–7. [Google Scholar] [CrossRef]
- Samiun, W.S.; Ashari, S.E.; Salim, N.; Ahmad, S. Optimization of processing parameters of nanoemulsion containing aripiprazole using response surface methodology. Int. J. Nanomed. 2020, 15, 1585–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadiyan, Z.; Basri, M.; Fard Masoumi, H.R.; Abedi Karjiban, R.; Salim, N.; Shameli, K. Modeling and optimization of nanoemulsion containing Sorafenib for cancer treatment by response surface methodology. Chem. Cent. J. 2017, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maringgal, B.; Hashim, N.; Amin Tawakkal, I.S.M.; Muda Mohamed, M.T.; Hazwan Hamzah, M.; Ali, M.M.; Abd Razak, M.F.H. Kinetics of quality changes in papayas (Carica papaya L.) coated with Malaysian stingless bee honey. Sci. Hortic. 2020, 267, 109321. [Google Scholar] [CrossRef]
- Rashid, M.R.; Nor Aripin, K.N.; Syed Mohideen, F.B.; Baharom, N.; Omar, K.; Md Taujuddin, N.M.S.; Mohd Yusof, H.H.; Addnan, F.H. The Effect of Kelulut Honey on Fasting Blood Glucose and Metabolic Parameters in Patients with Impaired Fasting Glucose. J. Nutr. Metab. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Yazan, L.S.; Zainal, N.A.; Ali, R.M.; Muhamad Zali, M.F.S.; Sze, O.Y.; Sim, T.Y.; Gopalsamy, B.; Ling, V.F.; Sapuan, S.; Esa, N.; et al. Antiulcer properties of kelulut honey against ethanol-induced gastric ulcer. Pertanika J. Sci. Technol. 2018, 26, 121–132. [Google Scholar]
- Chong, W.T.; Tan, C.P.; Cheah, Y.K.; Lajis, A.F.B.; Dian, N.L.H.M.; Kanagaratnam, S.; Lai, O.M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef] [Green Version]
- Ricaurte, L.; Perea-Flores, M.D.J.; Martinez, A.; Quintanilla-Carvajal, M.X. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov. Food Sci. Emerg. Technol. 2016, 35, 75–85. [Google Scholar] [CrossRef]
- Feng, J.; Shi, Y.; Yu, Q.; Sun, C.; Yang, G. Effect of emulsifying process on stability of pesticide nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 286–292. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Kato, E.T.M.; De Araujo, G.L.B.; Löbenberg, R.; Monteiro, L.M.; Lourenço, F.R.; Bou-Chacra, N.A. Olive oil nanoemulsion preparation using high-pressure homogenization and D-phase emulsification—A design space approach. J. Drug Deliv. Sci. Technol. 2019, 49, 622–631. [Google Scholar] [CrossRef]
- Chrastina, A.; Baron, V.T.; Abedinpour, P.; Rondeau, G.; Welsh, J.; Borgström, P. Plumbagin-Loaded Nanoemulsion Drug Delivery Formulation and Evaluation of Antiproliferative Effect on Prostate Cancer Cells. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Katsouli, M.; Polychniatou, V.; Tzia, C. LWT—Food Science and Technology Optimization of water in olive oil nano-emulsions composition with bioactive compounds by response surface methodology. LWT—Food Sci. Technol. 2018, 89, 740–748. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J. Dispersion process and effect of oleic acid on properties of cellulose sulfate-oleic acid composite film. Materials 2015, 8, 2346–2360. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Montañez, G.; Ragazzo-Sanchez, J.A.; Picart-Palmade, L.; Calderón-Santoyo, M.; Chevalier-Lucia, D. Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam). Innov. Food Sci. Emerg. Technol. 2017, 40, 35–41. [Google Scholar] [CrossRef]
- Tan, S.F.; Masoumi, H.R.F.; Karjiban, R.A.; Stanslas, J.; Kirby, B.P.; Basri, M.; Basri, H. Bin Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason. Sonochem. 2016, 29, 299–308. [Google Scholar] [CrossRef]
- Almeida, E.D.P.; Dipieri, L.V.; Rossetti, F.C.; Marchetti, J.M.; Bentley, M.V.L.; Nunes, R.D.S.; Sarmento, V.H.V.; Valerio, M.E.G.; Rodrigues Júnior, J.J.; Montalvão, M.M.; et al. Skin permeation, biocompatibility and antitumor effect of chloroaluminum phthalocyanine associated to oleic acid in lipid nanoparticles. Photodiagn. Photodyn. Ther. 2018, 24, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Sravanthi, V.; Pallavi, M.C.P.; Bonam, S.R.; Sathyabama, S.; Sampath Kumar, H.M. Oleic acid nanoemulsion for nasal vaccination: Impact on adjuvanticity based immune response. J. Drug Deliv. Sci. Technol. 2015, 28, 56–63. [Google Scholar] [CrossRef]
- Anjos, O.; Iglesias, C.; Peres, F.; Martínez, J.; García, Á.; Taboada, J. Neural networks applied to discriminate botanical origin of honeys. Food Chem. 2015, 175, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.A.; Bakhtiar, H.; Bidin, N.; Ghoshal, S.K. Antibacterial activity of decahedral cinnamon nanoparticles prepared in honey using PLAL technique. Mater. Lett. 2018, 232, 183–186. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Hamzah, M.H.; Mohamed, M.T.M. Biosynthesis of CaO nanoparticles using Trigona sp. Honey: Physicochemical characterization, antifungal activity, and cytotoxicity properties. J. Mater. Res. Technol. 2020, 9, 11756–11768. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Yu, H.; Lu, C.; Han, K. Investigation on thermal degradation properties of oleic acid and its methyl and ethyl esters through TG-FTIR. Energy Convers. Manag. 2017, 149, 495–504. [Google Scholar] [CrossRef]
- Mail, M.H.; Ab Rahim, N.; Amanah, A.; Khawory, M.H.; Shahudin, M.A.; Seeni, A. FTIR and elementary analysis of Trigona honey, Apis honey and adulterated honey mixtures. Biomed. Pharmacol. J. 2019, 12, 2011–2017. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V.; Rubini, D.; Nithyanand, P. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella Typhimurium. Food Biosci. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, characterization, and antimicrobial activity of nanoemulsions incorporating citral essential oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou, E.S.; Galhoum, G.F.; Mohamed, E.N. Curcumin loaded nanoemulsions/pectin coatings for refrigerated chicken fillets. Food Hydrocoll. 2018, 83, 445–453. [Google Scholar] [CrossRef]
- Md Saari, N.H.; Chua, L.S.; Hasham, R. Process Optimization of Curcumin-Loaded Coconut Oil and Honey Nanoemulsion for Better Skin Permeation. Int. J. Nanosci. 2020, 19, 1–9. [Google Scholar] [CrossRef]
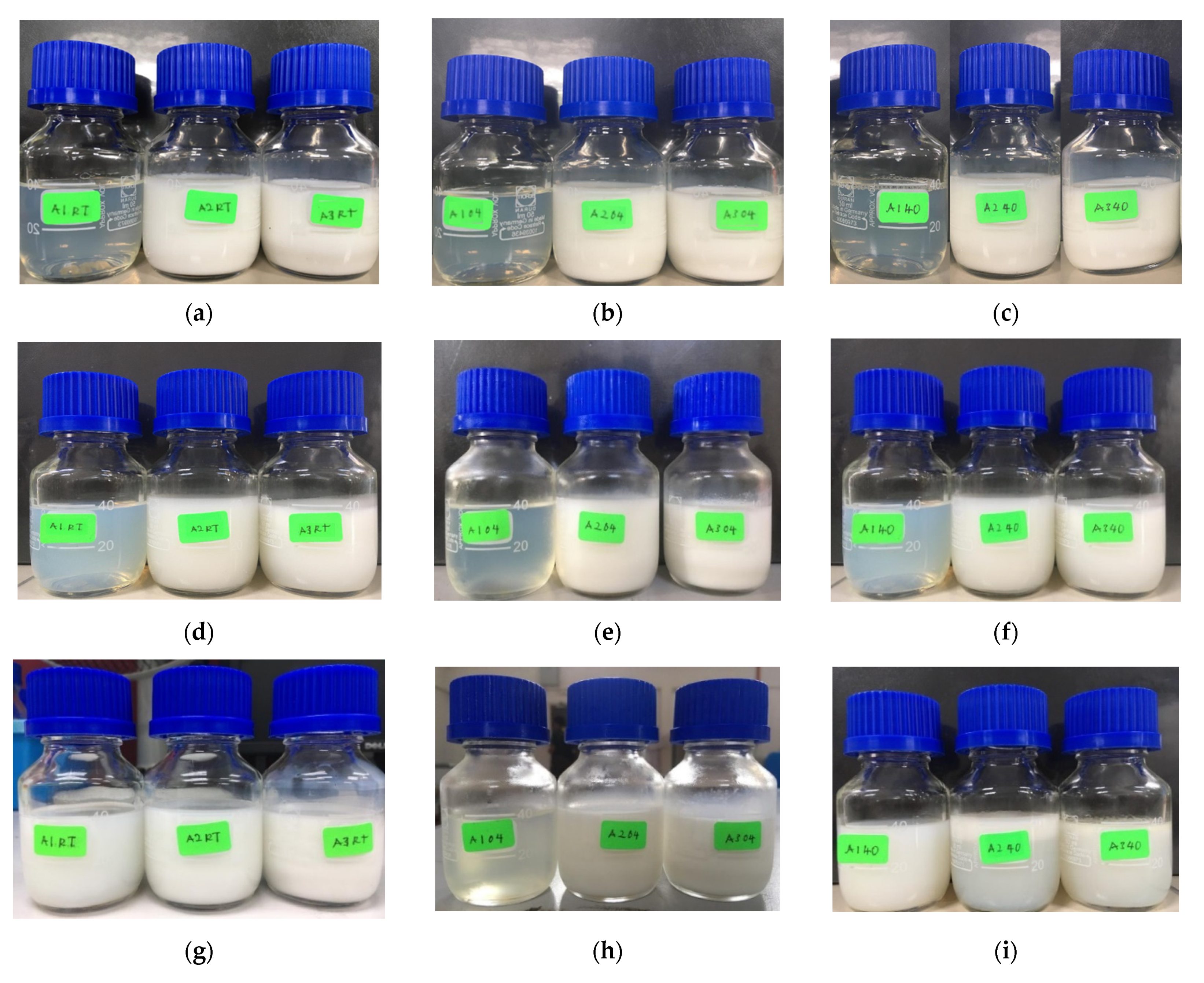
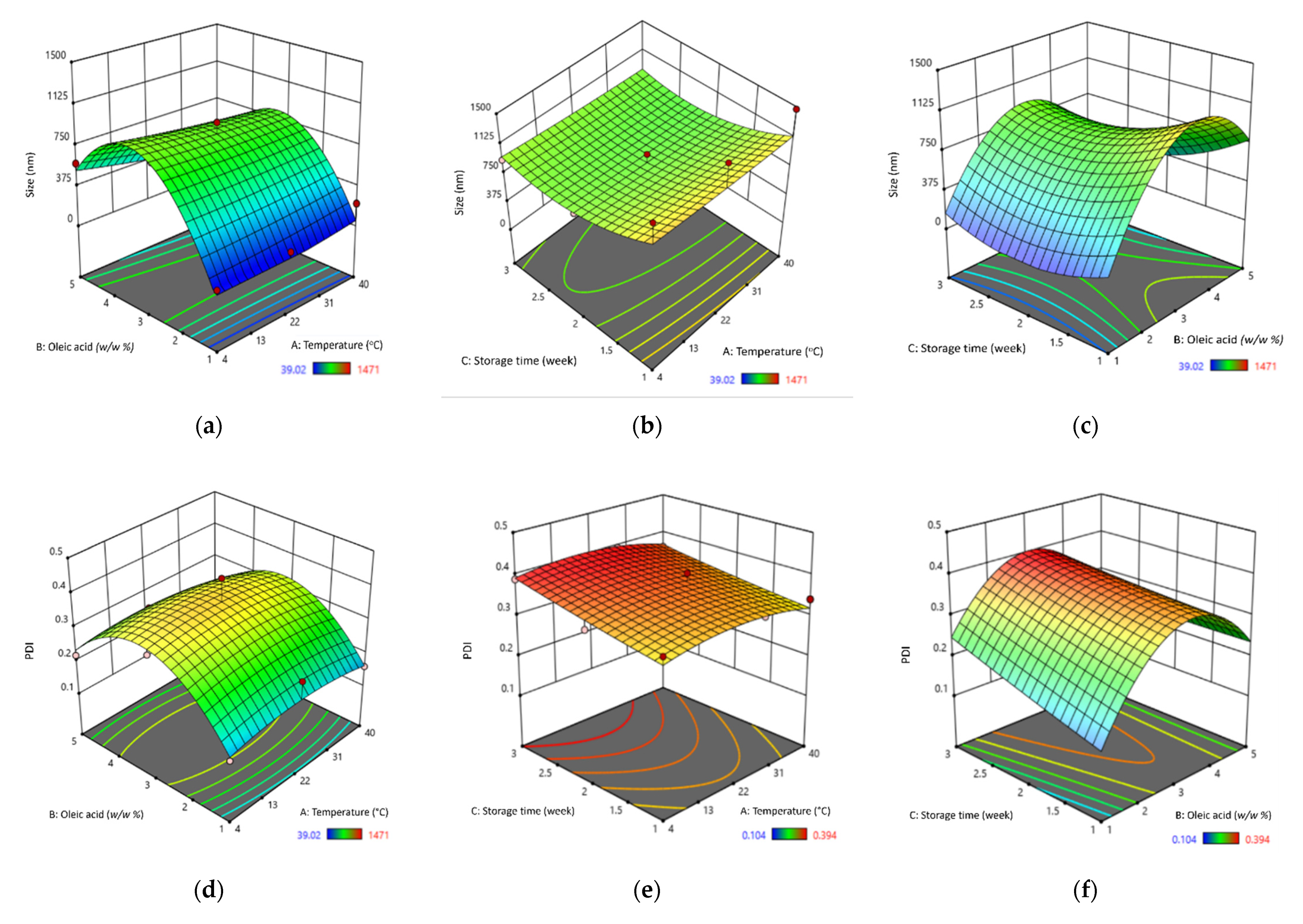
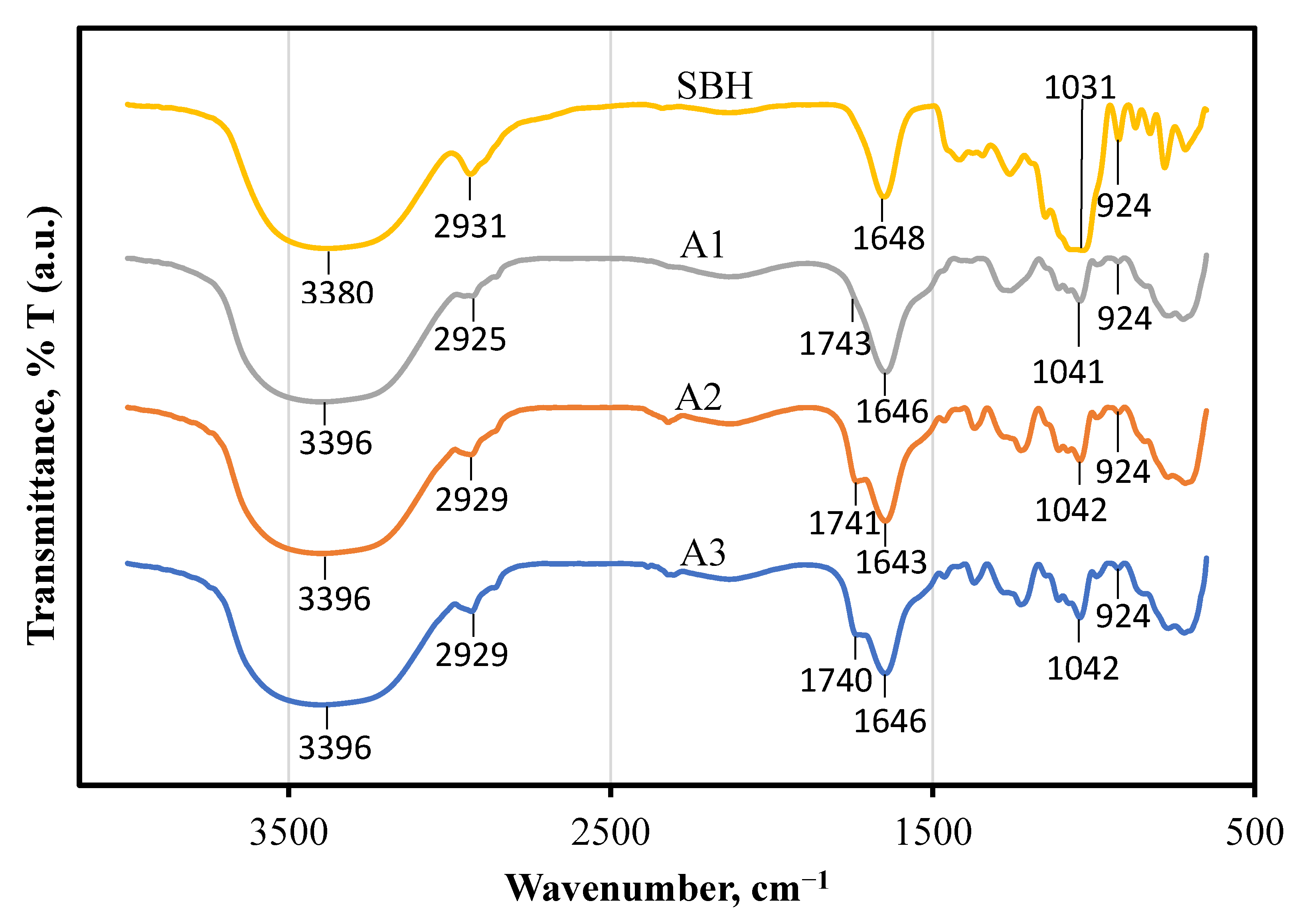
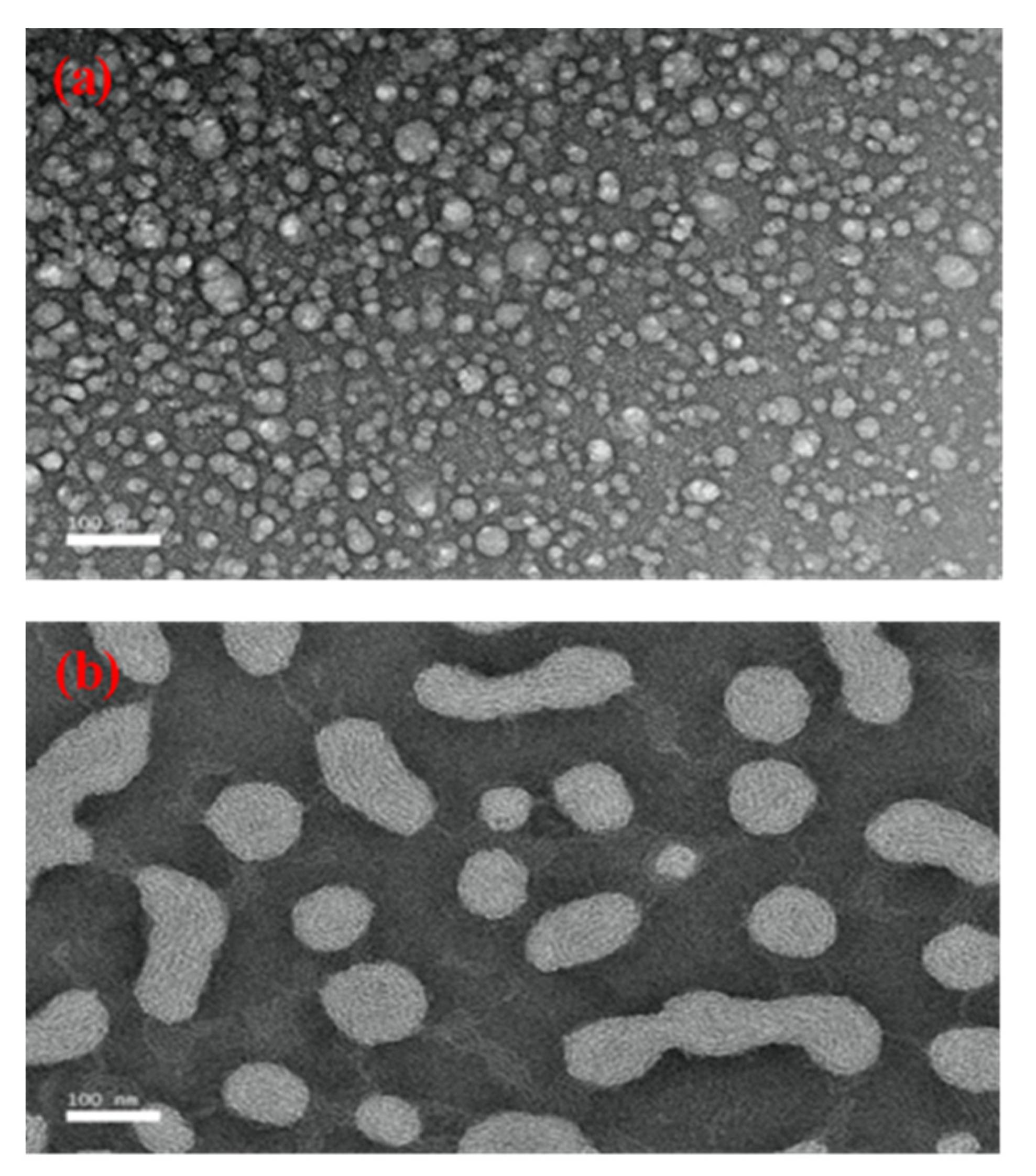
| Materials (w/w %) | Formulation | ||
|---|---|---|---|
| A1 | A2 | A3 | |
| Oleic acid | 1 | 3 | 5 |
| Glycerol | 8 | 8 | 8 |
| Tween 80 | 5 | 5 | 5 |
| Distilled water | 71 | 69 | 67 |
| SBH | 15 | 15 | 15 |
| Code | Independent Variables | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Temperature (°C) | 4 | 28 | 40 |
| B | Oleic acid (w/w %) | 1 | 3 | 5 |
| C | Storage time (weeks) | 1 | 2 | 3 |
| Run | Independent Variables | Response Variables | |||
|---|---|---|---|---|---|
| Temperature | Oleic Acid | Storage Time | PDI | Size | |
| 1 | −1 | −1 | 1 | 0.21 | 39.13 |
| 2 | −1 | −1 | 0 | 0.15 | 36.49 |
| 3 | 0 | −1 | 1 | 0.22 | 81.73 |
| 4 | 1 | −1 | 1 | 0.25 | 411.70 |
| 5 | 1 | −1 | −1 | 0.10 | 256.14 |
| 6 | −1 | 1 | 0 | 0.22 | 460.10 |
| 7 | 0 | 0 | −1 | 0.34 | 849.26 |
| 8 | 1 | 0 | 0 | 0.32 | 592.17 |
| 9 | 1 | 0 | −1 | 0.34 | 1031.00 |
| 10 | 0 | 0 | 0 | 0.38 | 570.70 |
| 11 | −1 | 0 | 1 | 0.39 | 551.76 |
| 12 | 1 | 1 | 0 | 0.16 | 245.80 |
| 13 | 1 | 1 | −1 | 0.22 | 518.86 |
| 14 | 0 | −1 | −1 | 0.10 | 35.35 |
| 15 | 1 | 0 | 1 | 0.36 | 609.83 |
| 16 | 0 | 0 | 1 | 0.39 | 499.43 |
| 17 | −1 | −1 | −1 | 0.10 | 35.35 |
| 18 | 1 | 1 | 1 | 0.20 | 195.30 |
| 19 | 0 | −1 | 0 | 0.24 | 51.43 |
| 20 | 0 | 1 | −1 | 0.22 | 518.86 |
| 21 | 0 | 1 | 0 | 0.25 | 294.76 |
| 22 | −1 | 1 | −1 | 0.22 | 709.22 |
| 23 | −1 | 1 | 1 | 0.27 | 413.03 |
| 24 | 0 | 1 | 1 | 0.20 | 263.76 |
| 25 | −1 | 0 | −1 | 0.34 | 1031.00 |
| 26 | −1 | 0 | 0 | 0.33 | 608.43 |
| 27 | 1 | −1 | 0 | 0.16 | 176.76 |
| Particle Size | |||||
|---|---|---|---|---|---|
| Source | Sum of Square | Degree of Freedom | Mean Aquare | F-Value | p-Value |
| Model | 5.006 × 106 | 9 | 5.562 × 105 | 28.42 | <0.0001 |
| A—Temperature | 300.78 | 1 | 300.78 | 0.015 | 0.9028 |
| B—Oleic acid | 6.895 × 105 | 1 | 6.895 × 105 | 35.24 | <0.0001 |
| C—Storage time | 3.655 × 105 | 1 | 3.655 × 105 | 18.68 | 0.0005 |
| Residual | 3.327 × 105 | 17 | 19,568.57 | ||
| Corrected total | 5.338 × 106 | 26 | |||
| PDI | |||||
| Source | Sum of Square | Degree of Freedom | Mean Square | F-Value | p-Value |
| Model | 0.1962 | 9 | 0.0218 | 31.17 | <0.0001 |
| A—Temperature | 0.0007 | 1 | 0.0007 | 1.07 | 0.3157 |
| B—Oleic acid | 0.0106 | 1 | 0.0106 | 15.11 | 0.0012 |
| C—Storage time | 0.0155 | 1 | 0.0155 | 22.20 | 0.0002 |
| Residual | 0.0119 | 17 | 0.0007 | ||
| Corrected total | 0.2081 | 26 | |||
| Optimum Condition | Coded Level | Actual Level |
|---|---|---|
| Oleic acid (w/w %) | −1 | 1 |
| Storage time (days) | 0 | 14 |
| Results | Particle Size (nm) | PDI |
| Predicted | 46.43 | 0.219 |
| Actual | 49.56 | 0.201 |
| Error (%) | 6.74 | 8.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman, A.S.; Hashim, N.; Maringgal, B.; Abdan, K. Optimisation of Stingless Bee Honey Nanoemulsions Using Response Surface Methodology. Foods 2021, 10, 2133. https://doi.org/10.3390/foods10092133
Rozman AS, Hashim N, Maringgal B, Abdan K. Optimisation of Stingless Bee Honey Nanoemulsions Using Response Surface Methodology. Foods. 2021; 10(9):2133. https://doi.org/10.3390/foods10092133
Chicago/Turabian StyleRozman, Azri Shahir, Norhashila Hashim, Bernard Maringgal, and Khalina Abdan. 2021. "Optimisation of Stingless Bee Honey Nanoemulsions Using Response Surface Methodology" Foods 10, no. 9: 2133. https://doi.org/10.3390/foods10092133
APA StyleRozman, A. S., Hashim, N., Maringgal, B., & Abdan, K. (2021). Optimisation of Stingless Bee Honey Nanoemulsions Using Response Surface Methodology. Foods, 10(9), 2133. https://doi.org/10.3390/foods10092133







